A Disposable Alkaline Phosphatase-Based Biosensor for Vanadium Chronoamperometric Determination
Abstract
: A chronoamperometric method for vanadium ion determination, based on the inhibition of the enzyme alkaline phosphatase, is reported. Screen-printed carbon electrodes modified with gold nanoparticles were used as transducers for the immobilization of the enzyme. The enzymatic activity over 4-nitrophenyl phosphate sodium salt is affected by vanadium ions, which results in a decrease in the chronoamperometric current registered. The developed method has a detection limit of 0.39 ± 0.06 μM, a repeatability of 7.7% (n = 4) and a reproducibility of 8% (n = 3). A study of the possible interferences shows that the presence of Mo(VI), Cr(III), Ca(II) and W(VI), may affect vanadium determination at concentration higher than 1.0 mM. The method was successfully applied to the determination of vanadium in spiked tap water.1. Introduction
Being considered as one of the important transition elements in biological systems, vanadium is an ultra-trace metal that can be found in some marine organisms, in the prosthetic group of bromoperoxidases in certain marine algae [1–3], as part of the nitrogenase system of some bacteria and plants [4,5], as well as in plasma and inside cells of mammals [6]. It participates in the synthesis of chlorophyll in photosynthetic organisms and is a micronutrient of marine and terrestrial species [7].
In the past, vanadium compounds were used as a therapeutic agent for diabetes, anemia, chlorosis, and even for tuberculosis. It is also a tonic, antiseptic and as a spirocheticide. Nevertheless vanadium, especially as vanadium pentoxide, has a broad spectrum of known toxic effects on the respiratory, circulatory and central nervous systems, digestive organs, kidneys and skin in humans. However confirmative mutagenicity and carcinogenicity studies are not consistent, though they should be given priority in long exposure studies [8,9]. In recent years, vanadium has been used in the development of novel materials in biochemistry and industrial processes [10–12]. Its metallic form is used as a carbide stabilizer in making steels. Vanadium pentoxide is used in ceramics, as a catalyst, and in the production of superconductive magnets, and vanadyl sulfate and sodium metavanadate have been used in dietary supplements [8].
Industries using fossil fuels like petroleum, coal and oil, cause most of the discharges of vanadium into the environment. Mining areas are other sources of this contamination, while distillation and purification of crude oils contribute less vanadium into the atmosphere [13].
Vanadate in aqueous solution influences numerous enzyme-catalyzed reactions. Its effects on living systems and the different responses to the influence of vanadium are well documented [14]. As it can assume many stable anionic forms in aqueous solution, depending on acidity and concentration [15], it has been described as an inhibitor of different enzymes. Lindquist in 1973 [16] described the inhibition of ribonuclease by vanadate in the presence of uridine, explaining in some way the origin of the biological influences of vanadium compounds. A year later, in 1974, Van Etten and coworkers [17], demonstrated the influence of vanadate, molybdate and tungstate on phosphohydrolases such as acid phosphatases which are relatively nonspecific enzymes that catalyze the hydrolysis of several alkyl and aryl phosphate esters at a pH between 4 and 6. Lopez et al. showed that alkaline phosphatase, which is a metalloproteinase, catalyzes the hydrolysis of a number of phosphate esters, and there are a few competitive inhibitors of alkaline phosphatase aside from inorganic phosphate and arsenate, such as oxovanadium (IV) VO2+. It is also possible that vanadium (V) might adopt a trigonal bipyramidal structure since crystalline hydrated metavanadates (VO3−·H2O) are five-coordinate with oxygen atoms, and the geometry is approximately trigonal bipyramidal like phosphate, which is one of the reasons why vanadate is a known inhibitor (and sometimes stimulator) of many phosphate-metabolizing enzymes [18]. This includes the inhibition of a regulatory protein phosphatase, which is likely to lead to activation of a protein kinase, the activity of which is key to the insulin-mimetic action of vanadate [17,18]. It also can inhibit hexokinase, adenylate kinase and phosphofructokinase [15]. Vanadate-dependent haloperoxidases have been shown to attain phosphatase activity, and this finding may have some impact on medical applications. Another important impetus to vanadium coordination chemistry has arisen from the observation that vanadate, peroxovanadate, vanadyl and several vanadium complexes exert an insulin-mimetic effect [6].
Electrochemical biosensors based on the principle of enzyme inhibition have been applied for a wide range of toxic analytes such as pesticides, derivatives of insecticides, heavy metals and glycoalkaloids [19]. Because of their excellent performance capabilities, such as rapid response, high specificity and sensitivity, relatively compact size, low cost and easy operation, these biosensors can be a good alternative for the detection of vanadium [20].
Alkaline phosphatases (ALPs), which catalyze the hydrolysis of phosphate esters, are widely distributed in mammalian tissues, and are present in high concentrations in bones, intestines, kidneys, placenta, and liver [21]. ALP is probably the most commonly used conjugated enzyme for immunoassays due to its high turnover number, broad substrate specificity and possibility of application. The determination of its activity has been carried out using various spectrophotometric and electrochemical methods [21–25]. In the development of sensitive electrochemical ALP-based assays stable substrates such as phenyl phosphate [26–28], naphthyl phosphate [28,29], ascorbic acid 2-phosphate [28,30], p-nitrophenyl phosphate [28,31] and rivoflavin-5-monophosphate [20] have been used. Among them, p-nitrophenyl phosphate is probably one of the most widely used substrate for ALP, since the enzymatically produced p-nitrophenol can be detected electrochemically [31].
Reversible inhibition of ALP by vanadium has been previously reported [18,20,25], although this interaction has been scarcely used for vanadium determination [20]. The presence of vanadium produces a decrease of the chronoamperometric reduction signal registered that can be related to the concentration of this species.
Thus, the aim of this work has been the development of a screen-printed based amperometric biosensor, easily usable in any analytical laboratory, for the detection of vanadium. ALP has been cross-linked to the working electrode of screen-printed carbon electrodes (SPCEs) previously modified by gold nanoparticles (ALP-AuNPs-SPCEs). In order to obtain a biosensor with improved conductivity and performance for vanadium detection, AuNPs were deposited onto the working electrode previous to the enzyme immobilization [32]. The use of AuNPs have been reported in order to enhance the chronoamperometric current response, yielding a sensor with an excellent electrocatalytic response, fast response time, long term stability and reproducibility [32–38]. The ALP-based biosensor has been characterized for the detection of vanadium in water samples. Figures of merit, such as precision or limit of detection, have been evaluated.
2. Results and Discussion
In a previous paper 5-riboflavin monophosphate was used as a substrate for an alkaline phosphatase biosensor because there was no report of such a substrate being used for a biosensor. Preechaworapun [28] presented a list of the substrates for this type of enzymes, and Fanjul [31] studied the detection of p-nitrophenol in alkaline phosphatase assays. In our case we proved several substrates recommended by Preechaworapun such as 3-indoxyphenyl phosphate, 1-naftyl phosphate and p-nitrophenyl phosphate, but the last one presented higher currents, and also ALP inhibition currents decreased significantly with vanadium additions.
As mentioned above, p-nitrophenyl phosphate is hydrolyzed by ALPs, under alkaline conditions, to 4-nitrophenol, which is electrochemically oxidized, originating a well-defined oxidation current [31]. Taking into account that the enzymatic activity of ALPs is inhibited by vanadium [18,20,25], the presence of this metal into the electrochemical cell results in a current decrease. In this way, the difference between the steady-state current in the absence of vanadium (I0) and the steady-state current in the presence of vanadium (I) (ΔI (I0–I)) can be quantitatively related to concentration of vanadium added.
In order to quantify this kind of electrochemical current, an ALP-based biosensor was built according to the procedure described in Section 3.3. This chronoamperometric current depends on experimental factors, such as pH of supporting electrolyte, substrate concentration, working potential or ionic strength of the medium (concentration of Cl− ions into the electrochemical cell). In order to maximize the registered inhibition current, the effect of these variables and their interactions in the chronoamperometric response was at first evaluated by the experimental design methodology [39–41]. The experimental domain was defined by the values shown in Table 1, corresponding to the high (+) and low (−) levels for each factor. Then, the 17 experiments corresponding to all those possible combinations, bearing in mind the three replicates in the central point necessary to estimate the residual value, were carried out. Once the oxidation current registered due to the enzymatically produced p-nitrophenol was stable, vanadium was added, quantifying the chronoamperometric current of a 1.8 μM solution as response variable for the analysis.
From this optimization process, the following optimum values for the experimental variables in the vanadium determination were used: supporting electrolyte pH 8.7, working potential of + 0.8 V vs. Ag/AgCl SPE, substrate concentration of 0.32 mM and Cl− concentration of 0.36 M. Easily quantifiable chronoamperometric signals are registered under these optimized conditions for vanadium (Figure 1).
Control experiments were carried out under the optimum conditions using bare SPCEs and AuNPs-SPCEs but without enzyme as reported in Section 3.3. No analytical signal was obtained, that is to say, the inhibition response registered after the addition of the substrate is only related to vanadium concentration. Therefore, vanadium can be determined by its inhibitory effect on the response of ALP to p-nitrophenyl phosphate. Figure 2 shows the amperometric signals of the substrate addition and vanadium additions 1 to 10 under optimal conditions. An insert figure shows a calibration curve for vanadium V.
The inhibitory effect of this metal in the enzymatic activity, when using p-nitrophenyl phosphate as substrate, was also studied by the kinetic parameters of the Lineweaver-Burk plot (Vmax and Km), in absence and presence of vanadium. In absence of the metal, Vmax and Km were 1.1 × 10−6 and 2.8 × 10−4, respectively. In presence of vanadium, it was observed that Vmax and Km increased: with 3.8 μM of vanadium, Vmax = 2.1 × 10−6, Km = 6.5 × 10−4, and with 11 μM of vanadium Vmax = 2.5 × 10−6 M and Km = 9.9 × 10−4 which suggest a mixed inhibition [42]. Thus, the inhibitory effect of vanadium on the ALP/p-nitrophenyl phosphate reaction has been confirmed for the higher affinity of ALP for p-nitrophenyl phosphate in the absence of this metal.
The detection of vanadium through the inhibition of ALP/p-nitrophenyl reaction (Calibration range from 0.8 μM to 30.0 μM) has resulted more sensitive than the reported one based on the inhibition of ALP/riboflavin-5-monophosphatase (calibration range from 1.8 μM to 15.0 μM) [15]. In this way, the limit of detection based on the standard deviation (Sy/x) of the responses for the blank injection in triplicate and the slope of the calibration curve was 0.39 ± 0.07 μM, one order lower than the previously one reported [20].
Precision of the developed procedure was studied in terms of repeatability (intra-biosensor) and reproducibility (inter-biosensors). Both figures of merit have been determined as the relative standard deviation (RSD) of the slopes of four calibration curves built under the optimum conditions of the experimental variables. Values of 7.7% and 8% (n = 4) were obtained for repeatability and reproducibility, respectively.
The performance of the developed procedure was checked by its accuracy and trueness. The accuracy of the proposed method was evaluated by means of the analysis of a vanadium certified sample (High Purity Standards(R) Vanadium Standard solution with a Certificate of Analysis confirmed against SRM 3165, lot 992706, certified value (1,000 ± 4) mg L−1). The vanadium mean concentration quantified, 1,055 ± 65 mg L−1 (n = 4; α = 0.05), matches the certified value of the sample. The method also showed a satisfactory value for trueness, evaluated by recovery studies, since the added vanadium concentration values (7.33 μM) were in good agreement with the found concentration value of 7.56 ± 0.14 μM (n = 4, α = 0.05). The average recovery for this analysis was 103.1 ± 3.6% with a RSD of 3.5%. Therefore, the proposed method is both accurate and suitable for the analysis of vanadium.
The possible interference from other metals, such as Ca(II), Sn(II), Al(III), Fe(III), Cr(III), As(V), Mo(VI) and W(VI), has been studied. Their effect was analyzed by measuring the inhibition current after consecutive additions of several solutions of each metal. It was observed that Al(III), As(V), Fe(III) and Sn(II) have a null influence on the vanadium chronoamperometric response. However, Cr(III), Mo(VI) and W(VI) present a higher inhibition current, so these metals must be taken into account in the analysis of vanadium.
Finally, the developed procedure was applied to the determination of vanadium in spiked tap water samples (1.96 μM), by standard addition methodology in quadruplicate. The concentration of vanadium found was 1.99 ± 0.23 μM (n = 4, α = 0.05, RSD = 6.8%), with an average recovery of 101%.
3. Experimental Section
3.1. Chemical Reagents
Several inks were used in the fabrication of the screen printed electrodes (SPEs), namely Electrodag PF-407 A (carbon ink), Electrodag 6037 SS (silver/silver chloride ink) and Electrodag 452 SS (dielectric ink) all supplied by Acheson Colloiden (Scheemda, The Netherlands). Analytical grade chemicals with no additional purification were used. All solutions were prepared in ultrapure water, conductivity of 0.05 μS/cm (Gen-Pure TKA, Niederelbert, Germany).
Hydrogen tetrachloroaurate (III) trihydrate (HAuCl4), ALP, bovine serum albumine (BSA) and glutaraldehyde (GA) were obtained from Sigma Chemical Co. (St. Louis, MO, USA). p-Nitrophenyl phosphate sodium salt was acquired from Fluka Analytical (Buchs, Switzerland). Ammonium metavanadate (Merck, Darmstadt, Germany) was used as stock solution of vanadium. 28 mM Tris(hydroxymethyl)aminomethane buffer (Aldrich Chemical Co., Buchs, Switzerland) was used together with 19 mM of MgCl2 (Merck) and 0.36 M total Cl−, (Merck) as supporting electrolyte. HCl (Merck) was used to adjust the pH value.
3.2. Apparatus
SPCEs were produced on a DEK 248 printing machine (DEK, Weymouth, UK) using polyester screens with appropriate stencil designs. Electrochemical measurements were made with an Autolab 128N electrochemical system with GPES software (Echo Chemie, Utrecht, The Netherlands). The pH measurements were performed using a Mettler-Toledo pHmeter S47-K (Columbus, OH, USA).
3.3. Manufacturing of ALP-AuNPs-SCPEs
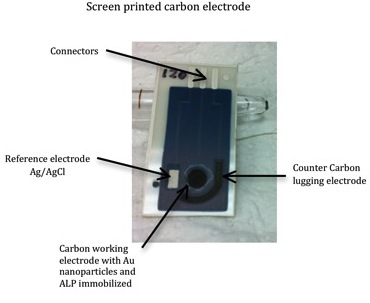
SPCEs were produce by sequential layer deposition of each component, that is conductive silver tracks, Ag/AgCl reference electrode (Ag/AgCl SPE), carbon counter and working electrodes and finally, dielectric ink, according to the procedure described anywhere else [43]. The different inks were cured according to the manufacturer's specifications. Screen-printed configurations of three electrodes (working, reference and counter electrode) were thus obtained (Figure 3).
The working electrode of these devices was electrochemically modified by AuNPs, using a 0.1 mM solution of HAuCl4 in 0.5 M H2SO4. The deposition was performed by applying a potential of + 0.18 V (vs. Ag/AgCl SPE) during 15 s under stirring conditions [20,44]. The enzyme was then immobilized by cross-linking on the surface of AuNPs modified SPCEs. To optimize an appropriate mixture of ALP enzyme, BSA and GA, several electrodes with different quantities of the enzyme from 20 μL to 80 μL, 10 μL–20 μL BSA and 20 μL–40 μL glutaraldehyde, were prepared and the best results were obtained with a mixture made up of 40 μL of ALP 0.6%, 20 μL of BSA 1.75% (w/v) and 40 μL of GA, 2.5% (w/v) which gave the best current response (Figure 4) by dropping 10 μL of a 2:1:2 mixture of a 0.6% of enzyme solution, 1.75% (w/v) of BSA solution and 2.5% (w/v) of GA solution onto the surface of a screen-printed working electrode [20]. Finally, the mixture was left to react at 4 °C during 1 h. ALP-AuNPs-SPCEs were stored at 4 °C. Under these storage conditions the developed biosensor showed a good stability for at least one week. Figure 5 shows calibration curves for vanadium prepared the same day but measured at different times from electrode preparation.
3.4. Measurement Procedure
Chronoamperometric measurements were performed at room temperature in a cell containing 5 mL of supporting electrolyte solution, of the desired pH, under constant mechanical stirring. An ALP- based biosensor was placed in the electrochemical cell containing 5 mL of supporting electrolyte solution. An adequate potential was applied and, once a steady-state current was set, a defined amount of p-nitrophenyl phosphate stock solution was added to the measuring cell. A large anodic current was observed due to the oxidation of the enzymatically produced p-nitrophenol. Then, once a plateau corresponding to the steady-state response was reached again, fixed portions of the vanadium stock solution were added consecutively. Enzyme electrodes were conditioned in a buffer solution for 5 min between each calibration setting.
4. Conclusions
The use of ALP based biosensors using AuNPs/SPCEs allows the selective chronoamperometric determination of vanadium. This biosensor offers better figures of merit compared with the previous work [20] using 5-monophosphate, lower limit of detection, wider linear range, but the same interferences, W(VI) and Mo(VI), which are the most significant at μM levels. The effect of vanadium in the ALP/p-nitrophenyl phosphate reaction results in a mixed inhibition, which allows the quantification of vanadium in tap water. The developed procedure has shown a limit of detection of 0.39 ± 0.06 μM, ten times lower than previously reported. The reproducibility and repeatability values of RSD for the slopes of several calibrations are lower than 10%.
Acknowledgments
Authors would like to acknowledge funding via Research Vicerrectory of Costa Rica University (Project 804-B0-058) and Spanish Ministry of Science and Innovation (TEC-2009/12029). This work was supported by the Spanish Ministry of Science and Innovation (MICINN) 410 and the European Regional Development Fund (FEDER) (INNPACTO SERIBIO 2011-411 2014) and TEC2009-12029, as well as through Junta de Castilla y León (BU212A12-2).
Conflicts of Interes
The authors declare no conflict of interest.
References
- Michibata, H.; Yamaguchi, N.; Uyama, T.; Ueki, T. Molecular biological approaches to the accumulation and reduction of vanadium by ascidians. Coordinat. Chem. Rev. 2003, 237, 41–51. [Google Scholar]
- Mukherjee, B.; Patra, B.; Mahapatra, S.; Banerjee, P.; Tiwari, A.; Chatterjee, M. Vanadium—An element of atypical biological significance. Toxicol. Lett. 2004, 150, 135–143. [Google Scholar]
- Ueki, T.; Michibata, H. Molecular mechanism of the transport and reduction pathway of vanadium in ascidians. Coordinat. Chem. Rev. 2011, 255, 2249–2257. [Google Scholar]
- Rehder, D. Vanadium nitrogenase. J. Inorg. Biochem. 2000, 80, 133–136. [Google Scholar]
- Janas, Z.; Sobota, P. Aryloxo and thiolato vanadium complexes as chemical models of the active site of vanadium nitrogenase. Coordinat. Chem. Rev. 2005, 249, 2144–2155. [Google Scholar]
- Rehder, D. The coordination chemistry of vanadium as related to its biological functions. Coordinat. Chem. Rev. 1999, 182, 297–322. [Google Scholar]
- Rodríguez-Mercado, J.J.; Altamirano-Lozano, M.A. Vanadio: Contaminación, metabolismo y genotoxicidad. Rev. Int. Contam. Ambient. 2010, 22, 173–189. [Google Scholar]
- Toxicological Profile For Vanadium U.S.; Department of Health And Human Services, United States Department of Health: Atlanta, GA, USA, 2012.
- Air Quality Guidelines for Europe; Vanadium, Chapter 6.12; World Health Organization, Regional Office for Europe: Copenhagen, Denmark, 2000.
- Guidelli, E.J.; Guerra, E.M.; Mulato, M. Ion sensing properties of vanadium/tungsten mixed oxides. Mater. Chem. Phys. 2011, 125, 833–837. [Google Scholar]
- Tsiafoulis, C.G.; Florou, A.B.; Trikalitis, P.N.; Bakas, T.; Prodromidis, M.I. Electrochemical study of ferrocene intercalated vanadium pentoxide xerogel/polyvinyl alcohol composite films: Application in the development of amperometric biosensors. Electrochem. Commun. 2005, 7, 781–788. [Google Scholar]
- Zhu, Z.; Sun, X.; Wang, Y.; Zeng, Y.; Sun, W.; Huang, X. Electrochemical horseradish peroxidase biosensor based on dextran–ionic liquid–V2O5 nanobelt composite material modified carbon ionic liquid electrode. Mater. Chem. Phys. 2010, 124, 488–492. [Google Scholar]
- Venkataraman, B.V.; Sudha, S. Vanadium toxicity. Asian J. Exp. Sci. 2005, 19, 127–134. [Google Scholar]
- Bhattacharyya, S.; Tracey, A.S. Vanadium (V) complexes in enzyme systems: aqueous chemistry, inhibition and molecular modeling in inhibitor design. J. Inorg. Biochem. 2001, 85, 9–13. [Google Scholar]
- Boyd, D.W.; Kustin, K.; Niwa, M. Do vanadate polyanions inhibit phosphotransferase enzymes? Biochim. Biophys. Acta 1985, 827, 472–475. [Google Scholar]
- Lindquist, R.N.; Lynn, J.L.; Lienhard, G.E. Possible transition-state analogs for ribonuclease. Complexes of uridine with oxovanadium (IV) ion and vanadium (V) ion. J. Am. Chem. Soc. 1973, 95, 8762–8768. [Google Scholar]
- Van Etten, R.L.; Waymack, P.P.; Rehkop, D.M. Transition metal ion inhibition of enzyme-catalyzed phosphate ester displacement reactions. J. Am. Chem. Soc. 1974, 96, 6782–6785. [Google Scholar]
- Lopez, V.; Stevens, T.; Lindquist, R.N. Vanadium ion inhibition of alkaline phosphatase-catalyzed phosphate ester hydrolysis. Arch. Biochem. Biophys. 1976, 175, 31–38. [Google Scholar]
- Amine, A.; Mohammadi, H.; Bourais, I.; Palleschi, G. Enzyme inhibition-based biosensors for food safety and environmental monitoring. Biosens. Bioelectron. 2006, 21, 1405–1423. [Google Scholar]
- Alvarado-Gámez, A.L.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Vanadium determination in water using alkaline phosphatase based screen-printed carbon electrodes modified with gold nanoparticles. J. Electroanal. Chem. 2013, 693, 51–55. [Google Scholar]
- Park, J.; Kim, Y. An improved fluorogenic substrate for the detection of alkaline phosphatase activity. Bioorg. Med. Chem. Lett. 2013, 23, 2332–2335. [Google Scholar]
- Berezhetskyy, A.L.; Sosovska, O.F.; Durrieu, C.; Chovelon, J.-M.; Dzyadevych, S.V.; Tran-Minh, C. Alkaline phosphatase conductometric biosensor for heavy-metal ions determination. ITBM-RBM 2008, 29, 136–140. [Google Scholar]
- Szydłowska, D.; Campàs, M.; Marty, J.-L.; Trojanowicz, M. Catechol monophosphate as a new substrate for screen-printed amperometric biosensors with immobilized phosphatases. Sens. Actuators B Chem. 2006, 113, 787–796. [Google Scholar]
- Zhang, J.; Cass, A.E. Electrochemical analysis of immobilised chemical and genetic biotinylated alkaline phosphatase. Analy. Chimica Acta 2000, 408, 241–247. [Google Scholar]
- Koncki, R.; Rudnicka, K.; Tymecki, Ł. Flow injection system for potentiometric determination of alkaline phosphatase inhibitors. Analy. Chimica Acta 2006, 577, 134–139. [Google Scholar]
- Ito, S.; Yamazaki, S.; Kano, K.; Ikeda, T. Highly sensitive electrochemical detection of alkaline phosphatase. Analy. Chimica Acta 2000, 424, 57–63. [Google Scholar]
- Serra, B.; Morales, M.D.; Reviejo, A.J.; Hall, E.H.; Pingarrón, J.M. Rapid and highly sensitive electrochemical determination of alkaline phosphatase using a composite tyrosinase biosensor. Analy. Biochem. 2005, 336, 289–294. [Google Scholar]
- Preechaworapun, A.; Dai, Z.; Xiang, Y.; Chailapakul, O.; Wang, J. Investigation of the enzyme hydrolysis products of the substrates of alkaline phosphatase in electrochemical immunosensing. Talanta 2008, 76, 424–431. [Google Scholar]
- Abad-Villar, E.M.; Fernández-Abedul, M.T.; Costa-García, A. Gold bands as a suitable surface for enzyme immunoassays. Biosens. Bioelectr. 2002, 17, 797–802. [Google Scholar]
- Kokado, A.; Arakawa, H.; Maeda, M. New electrochemical assay of alkaline phosphatase using ascorbic acid 2-phosphate and its application to enzyme immunoassay. Analy. Chim. Acta 2000, 407, 119–125. [Google Scholar]
- Fanjul-Bolado, P.; González-García, M.B.; Costa-García, A. Flow screen-printed amperometric detection of p-nitrophenol in alkaline phosphatase-based assays. Analy. Bioanaly. Chem. 2006, 385, 1202–1208. [Google Scholar]
- González-García, M.B.; Costa-García, A. Silver electrodeposition catalyzed by colloidal gold on carbon paste electrode: Application to biotin–streptavidin interaction monitoring. Biosens. Bioelectr. 2000, 15, 663–670. [Google Scholar]
- Siangproh, W.; Dungchai, W.; Rattanarat, P.; Chailapakul, O. Nanoparticle-based electrochemical detection in conventional and miniaturized systems and their bioanalytical applications: A review. Analy. Chim. Acta 2011, 690, 10–25. [Google Scholar]
- Liu, T.; Zhong, J.; Gan, X.; Fan, C.H.; Li, G.X.; Matsuda, N. Wiring electrons of cytochrome c with silver nanoparticles in layered films. Chem. Phys. Chem. 2003, 4, 1364–1366. [Google Scholar]
- Xiao, Y. Plugging into enzymes: Nanowiring of redox enzymes by a gold nanoparticle. Science 2003, 299, 1877–1881. [Google Scholar]
- Xu, J.Z.; Zhu, J.J.; Wang, H.; Chen, H.Y. Nano-sized copper oxide modified carbon paste electrodes as an amperometric sensor for amikacin. Analy. Lett. 2003, 36, 2723–2733. [Google Scholar]
- Zen, J.M.; Hsu, C.T.; Kumar, A.S.; Lyuu, H.J.; Lin, K.Y. Amino acid analysis using disposable copper nanoparticle plated electrodes. Analyst 2004, 129, 841–845. [Google Scholar]
- Kim, G.Y.; Shim, J.; Kang, M.S.; Moon, S.H. Preparation of a highly sensitive enzyme electrode using gold nanoparticles for measurement of pesticides at the ppt level. J. Environ. Monitor. 2008, 10, 632–637. [Google Scholar]
- Box, G.E.P. Estadística para Investigadores: Diseño, Innovación y Descubrimiento, 2nd ed.; Editorial Reverte S.A.: Barcelona, Spain, 2008. [Google Scholar]
- Lewis, G.A.; Mathieu, D.; Phan, R.T.L. Pharmaceutical Experimental Design; Marcel Dekker Inc.: New York, NY, USA, 1999. [Google Scholar]
- Massart, D.L.; Vandeginste, B.G.M.; Deming, S.M.; Michotte, Y.; Kaufman, L. Chemometrics: A Textbook; Elsevier: Amsterdam, The Netherlands, 2003. [Google Scholar]
- Turdean, G.L. Design and development of biosensors for the detection of heavy metal toxicity. Inter. J. Electrochem. 2011, 2011, 1–15. [Google Scholar]
- Del Torno-de Román, L.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. Gluconic acid determination in wine by electrochemical biosensing. Sens. Actuators B Chem. 2013, 176, 858–862. [Google Scholar]
- Domínguez Renedo, O.; Arcos Martínez, M.J. Anodic stripping voltammetry of antimony using gold nanoparticle-modified carbon screen-printed electrodes. Analy. Chim. Acta 2007, 589, 255–260. [Google Scholar]





| Low Level | High Level | |
|---|---|---|
| Supporting electrolyte pH | 7.0 | 9.6 |
| Working potential | + 0.5 V vs. Ag/AgCl SPE | + 1.0 V vs. Ag/AgCl SPE |
| Substrate concentration | 0.13 mM | 0.47 mM |
| Ionic strength | 0.26 M | 0.46 M |
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Alvarado-Gámez, A.L.; Alonso-Lomillo, M.A.; Domínguez-Renedo, O.; Arcos-Martínez, M.J. A Disposable Alkaline Phosphatase-Based Biosensor for Vanadium Chronoamperometric Determination. Sensors 2014, 14, 3756-3767. https://doi.org/10.3390/s140203756
Alvarado-Gámez AL, Alonso-Lomillo MA, Domínguez-Renedo O, Arcos-Martínez MJ. A Disposable Alkaline Phosphatase-Based Biosensor for Vanadium Chronoamperometric Determination. Sensors. 2014; 14(2):3756-3767. https://doi.org/10.3390/s140203756
Chicago/Turabian StyleAlvarado-Gámez, Ana Lorena, María Asunción Alonso-Lomillo, Olga Domínguez-Renedo, and María Julia Arcos-Martínez. 2014. "A Disposable Alkaline Phosphatase-Based Biosensor for Vanadium Chronoamperometric Determination" Sensors 14, no. 2: 3756-3767. https://doi.org/10.3390/s140203756