Ultra-Sensitive Nanofiber Fluorescence Detection in a Microfluidic Chip
Abstract
:1. Introduction
2. Experimental Section

3. Results and Discussion
3.1. R6G Evanescent Field Fluorescence Measurements


3.2. Quantum Dots-Labeled Steptavidin Evanescent Field Fluorescence Measurements
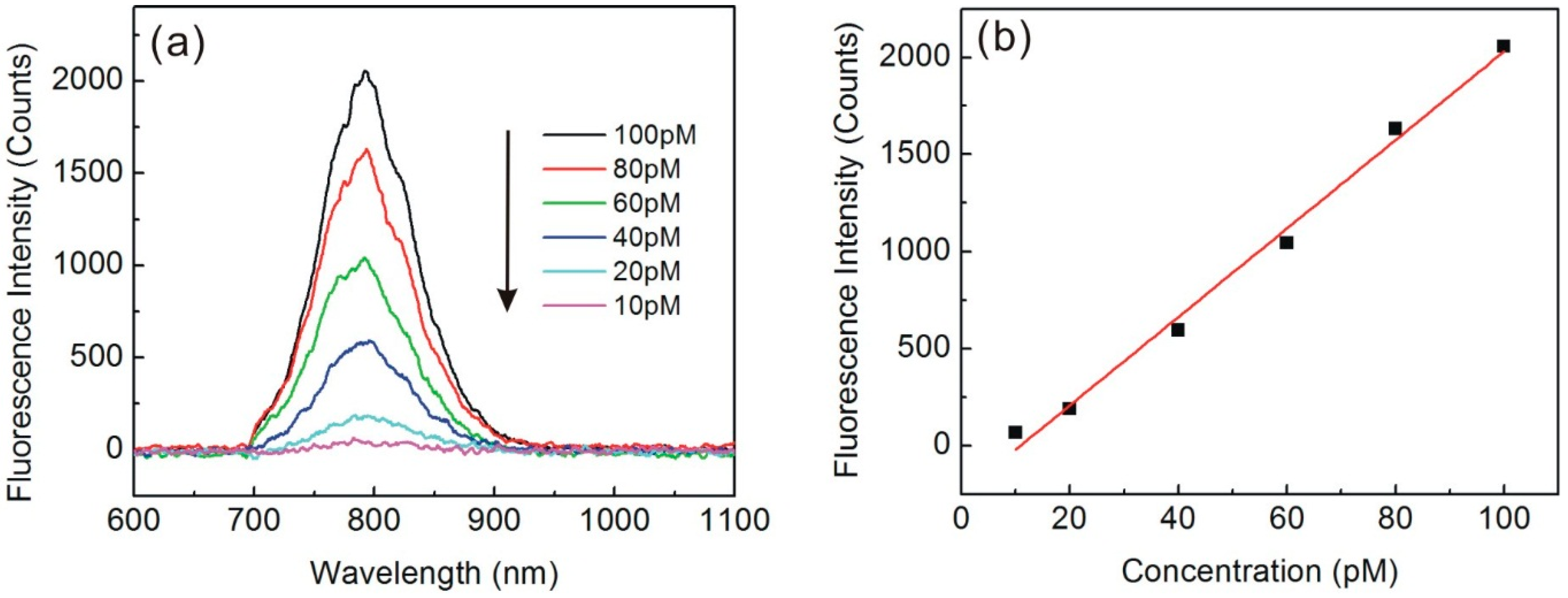
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tong, L.M.; Sumetsky, M. Subwavelength and Nanometer Diameter Optical Fibers, 1st ed.; Zhejiang University Press: Hangzhou, China, 2009; pp. 99–122. [Google Scholar]
- Brambilla, G. Optical fibre nanowires and microwires: A review. J. Opt. 2010, 12, 043001. [Google Scholar] [CrossRef]
- Wu, X.Q.; Tong, L.M. Optical microfibers and nanofibers. Nanophotonics 2013, 2, 407–428. [Google Scholar] [CrossRef]
- Guo, X.; Ying, Y.B.; Tong, L.M. Photonic nanowires: From subwavelength waveguides to optical sensors. Accounts Chem. Res. 2014, 47, 656–666. [Google Scholar] [CrossRef]
- Lou, J.Y.; Wang, Y.P.; Tong, L.M. Microfiber optical sensors: A review. Sensors 2014, 14, 5823–5844. [Google Scholar] [CrossRef] [PubMed]
- Polynkin, P.; Polynkin, A.; Peyghambarian, N.; Mansuripur, M. Evanescent field-based optical fiber sensing device for measuring the refractive index of liquids in microfluidic channels. Opt. Lett. 2005, 30, 1273–1275. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, J.; Monzon-Hernandez, D. Fast detection of hydrogen with nanofiber tapers coated with ultrathin palladium layers. Opt. Express 2005, 13, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Brambilla, G.; Lu, Y.Q. A microfluidic refractometric sensor based on gratings in optical fibre microwires. Opt. Express 2009, 17, 20866–20871. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, C.; Zhang, A.P.; Xiao, Y.; Yu, H.; Tong, L.M. Compact microfiber Bragg gratings with high-index contrast. Opt. Lett. 2011, 36, 3115–3117. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Horak, P.; Brambilla, G. Optical microfiber coil resonator refractometric sensor. Opt. Express 2007, 15, 7888–7893. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Xu, Y.H.; Tan, W.; Chen, X.F. Simulation of optical microfiber loop resonators for ambient refractive index sensing. Sensors 2007, 7, 689–696. [Google Scholar] [CrossRef]
- Guo, X.; Tong, L.M. Supported microfiber loops for optical sensing. Opt. Express 2008, 16, 14429–14434. [Google Scholar] [CrossRef] [PubMed]
- Lou, J.Y.; Tong, L.M.; Ye, Z.Z. Modeling of silica nanowires for optical sensing. Opt. Express 2005, 13, 2135–2140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Tong, L.M. Mach-Zehnder interferometers assembled with optical microfibers or nanofibers. Opt. Lett. 2008, 33, 303–305. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.H.; Sui, C.H.; Ye, B.Q. Modelling nanofiber Mach-Zehnder interferometers for refractive index sensors. J. Mod. Optic. 2009, 56, 2335–2339. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.X.; Lou, J.Y.; Yin, X.F.; Tong, L.M. Fast detection of humidity with a subwavelength-diameter fiber taper coated with gelatin film. Opt. Express 2008, 16, 13349–13353. [Google Scholar] [CrossRef] [PubMed]
- Monzon-Hernandez, D.; Luna-Moreno, D.; Escobar, D.M.; Villatoro, J. Optical microfibers decorated with PdAu nanoparticles for fast hydrogen sensing. Sens. Actuators B Chem. 2010, 151, 219–222. [Google Scholar] [CrossRef]
- Mignani, A.G.; Falciai, R.; Ciaccheri, L. Evanescent wave absorption spectroscopy by means of Bi-tapered multimode optical fibers. Appl. Spectrosc. 1998, 52, 546–551. [Google Scholar] [CrossRef]
- Warken, F.; Vetsch, E.; Meschede, D.; Sokolowski, M.; Rauschenbeutel, A. Ultra-sensitive surface absorption spectroscopy using sub-wavelength diameter optical fibers. Opt. Express 2007, 15, 11952–11958. [Google Scholar] [CrossRef] [PubMed]
- Stiebeiner, A.; Rehband, O.; Garcia-Fernandez, R.; Rauschenbeutel, A. Ultra-sensitive fluorescence spectroscopy of isolated surface-adsorbed molecules using an optical nanofiber. Opt. Express 2009, 17, 21704–21711. [Google Scholar] [CrossRef] [PubMed]
- Warren-Smith, S.C.; Afshar, S.; Monro, T.M. Fluorescence-based sensing with optical nanowires: A generalized model and experimental validation. Opt. Express 2010, 18, 9474–9485. [Google Scholar] [CrossRef] [PubMed]
- Taitt, C.R.; Anderson, G.P.; Ligler, F.S. Evanescent wave fluorescence biosensors. Biosens. Bioelectron. 2005, 20, 2470–2487. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Shankar, P.M.; Mutharasan, R. A review of fiber-optic biosensors. Sens. Actuators B Chem. 2007, 125, 688–703. [Google Scholar] [CrossRef]
- Jiang, X.S.; Song, Q.H.; Liu, X.; Fu, J.; Tong, L.M. Microfiber knot dye laser based on the evanescent-wave-coupled gain. Appl. Phys. Lett. 2007, 90, 233501. [Google Scholar] [CrossRef]
- Fujiwara, M.; Toubaru, K.; Noda, T.; Zhao, H.Q.; Takeuchi, S. Highly efficient coupling of photons from nanoemitters into single-mode optical fibers. Nano Lett. 2011, 11, 4362–4365. [Google Scholar] [CrossRef] [PubMed]
- Yalla, R.; le Kien, F.; Morinaga, M.; Hakuta, K. Efficient channeling of fluorescence photons from single quantum dots into guided modes of optical nanofiber. Phys. Rev. Lett. 2012, 109, 063602. [Google Scholar] [CrossRef] [PubMed]
- Wiejata, P.J.; Shankar, P.M.; Mutharasan, R. Fluorescent sensing using biconical tapers. Sens. Actuators B Chem. 2003, 96, 315–320. [Google Scholar] [CrossRef]
- Vienne, G.; Li, Y.H.; Tong, L.M. Effect of host polymer on microfiber resonator. IEEE Photon. Technol. Lett. 2007, 19, 1386–1388. [Google Scholar] [CrossRef]
- Lou, N.; Jha, R.; Domínguez-Juárez, J.L.; Finazzi, V.; Villatoro, J.; Badenes, G.; Pruneri, V. Embedded optical micro/nano-fibers for stable devices. Opt. Lett. 2010, 35, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Lorenzi, R.; Jung, Y.; Brambilla, G. In-line absorption sensor based on coiled optical microfiber. Appl. Phys. Lett. 2011, 98, 173504. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Xiao, Y.; Yu, H.; Tong, L.M. Ultra-sensitive microfibre absorption detection in a microfluidic chip. Lab Chip 2011, 11, 3720–3724. [Google Scholar] [CrossRef] [PubMed]
- Gu, F.X.; Zhang, L.; Yin, X.F.; Tong, L.M. Polymer single-nanowire optical sensors. Nano Lett. 2008, 8, 2757–2761. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Chen, B.G.; Meng, C.; Fang, W.; Xiao, Y.; Li, X.Y.; Hu, Z.F.; Xu, Y.X.; Tong, L.M.; Wang, H.; et al. Ultrafast all-optical graphene modulator. Nano Lett. 2014, 14, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Du, W.B.; Fang, Q.; He, Q.H.; Fang, Z.L. High-throughput nanoliter sample introduction microfluidic chip-based flow injection analysis system with gravity-driven flows. Anal. Chem. 2005, 77, 1330–1337. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Xu, Y.; Fang, W.; Tong, L.; Zhang, L. Ultra-Sensitive Nanofiber Fluorescence Detection in a Microfluidic Chip. Sensors 2015, 15, 4890-4898. https://doi.org/10.3390/s150304890
Li Z, Xu Y, Fang W, Tong L, Zhang L. Ultra-Sensitive Nanofiber Fluorescence Detection in a Microfluidic Chip. Sensors. 2015; 15(3):4890-4898. https://doi.org/10.3390/s150304890
Chicago/Turabian StyleLi, Zhiyong, Yingxin Xu, Wei Fang, Limin Tong, and Lei Zhang. 2015. "Ultra-Sensitive Nanofiber Fluorescence Detection in a Microfluidic Chip" Sensors 15, no. 3: 4890-4898. https://doi.org/10.3390/s150304890





