Assessing Walking Strategies Using Insole Pressure Sensors for Stroke Survivors
Abstract
:1. Introduction
- Mastery experiences: Gaining confidence in achieving and accomplishing tasks.
- Modelling: The observation of similar individuals achieving and accomplishing tasks through direct observation or through written and visual material.
- Interpreting physiological signs: Having the ability and confidence to interpret symptoms and changes in symptoms such as, mobility, weakness, stiffness, and fatigue.
- Feedback and persuasion: The provision of some recognition of their performance and progress from personal achievement as well as significant others, i.e., family members.
2. Method
2.1. Aim
2.2. Inclusion Criteria
- A definite diagnosis of stroke (self-reported);
- Able to give informed consent;
- Able to walk (with or without walking aids); and
- Individuals with self-reported walking difficulties as a result of their stroke.
- Unable to speak or comprehend written English;
- Unable to give informed consent; and
- Medically unstable, or other neurological, neuromuscular, or orthopaedic disorders that would interfere with task performance (self-reported).
- 45 years of age or over;
- No conditions effecting their walking, e.g., back pain, hip conditions, and arthritis in the knees;
- Able to give informed consent;
- Able to speak and comprehend written English;
- No history of stroke; and
- Medically stable, and no other neurological, neuromuscular, or orthopaedic disorders that would interfere with task performance (self-reported).
2.3. Recruitment
2.4. Setting
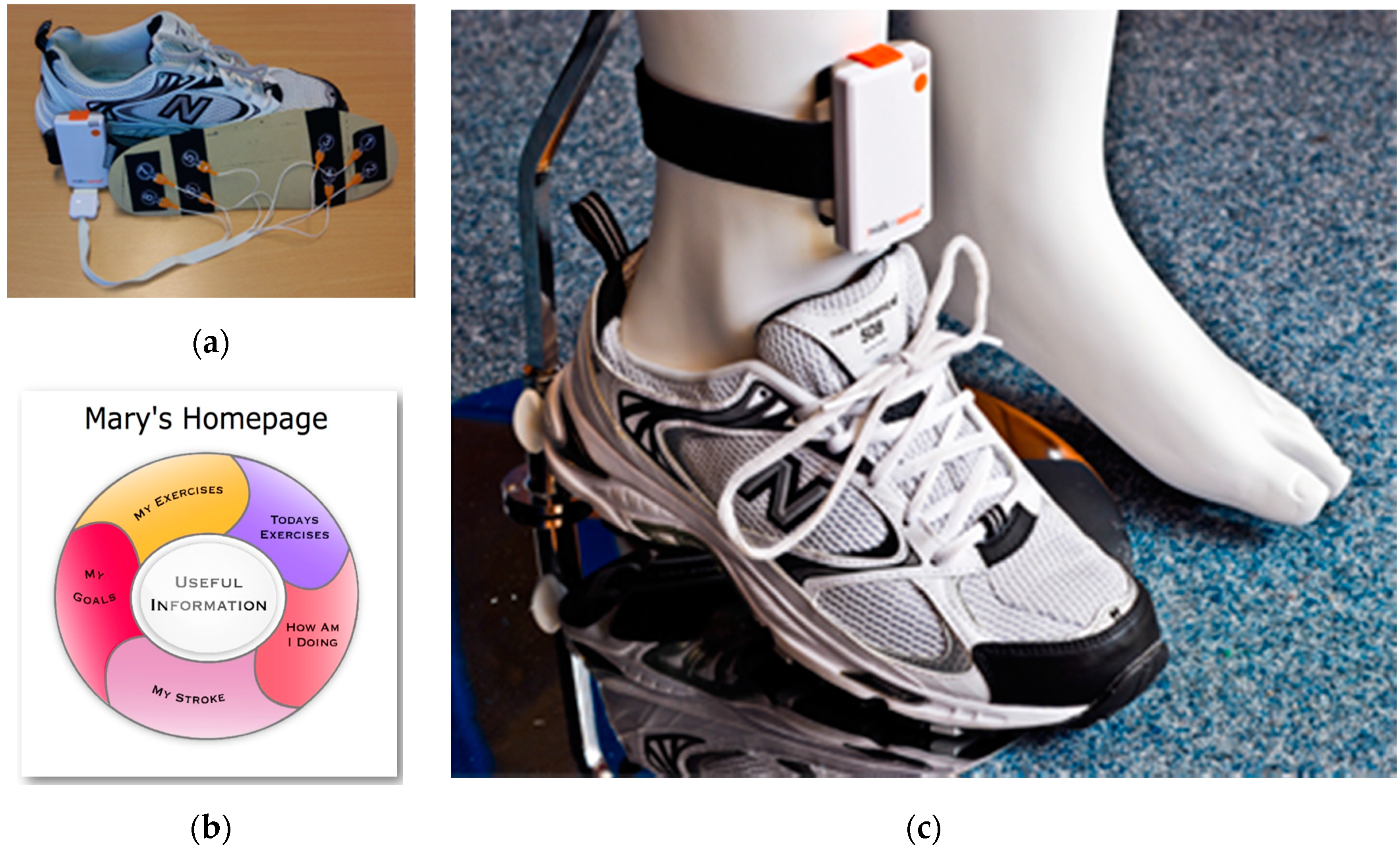
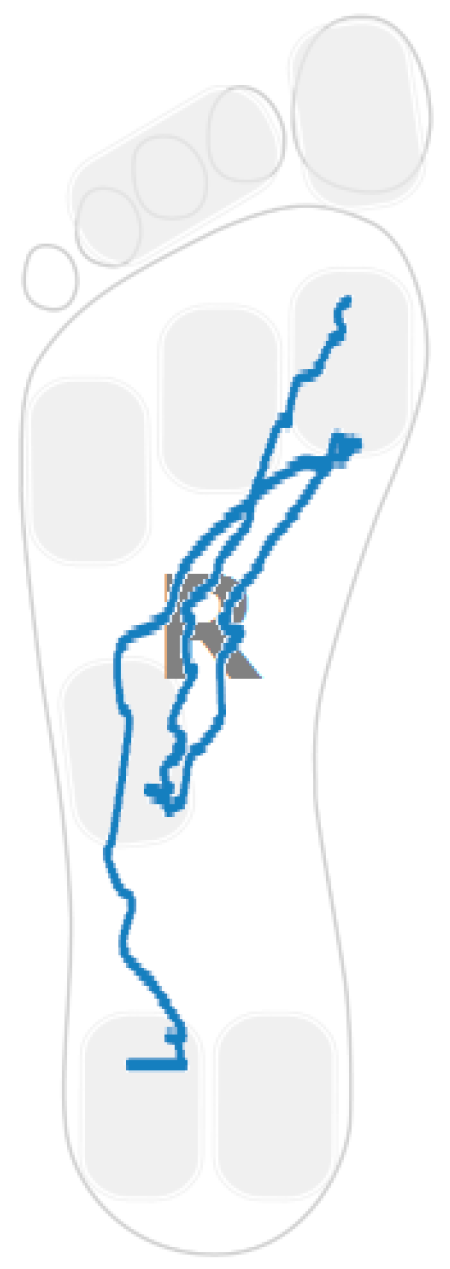
2.5. Procedures
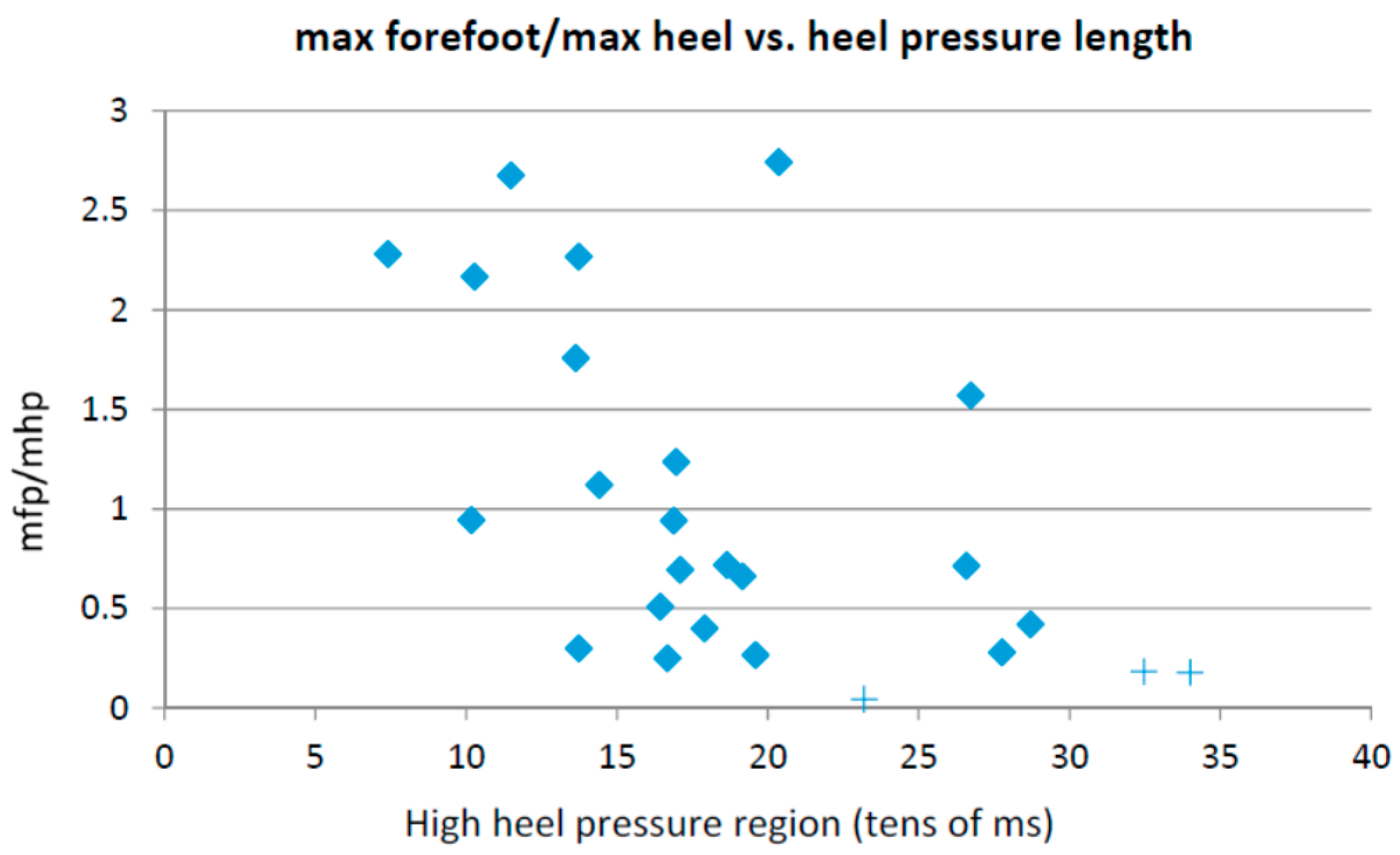
- Time in which the average pressure on the heel is bigger than the 80% of the maximum heel pressure;
- Maximum forefoot pressure;
- Ratio between the forefoot pressure at the point of maximum heel pressure and the maximum forefoot pressure;
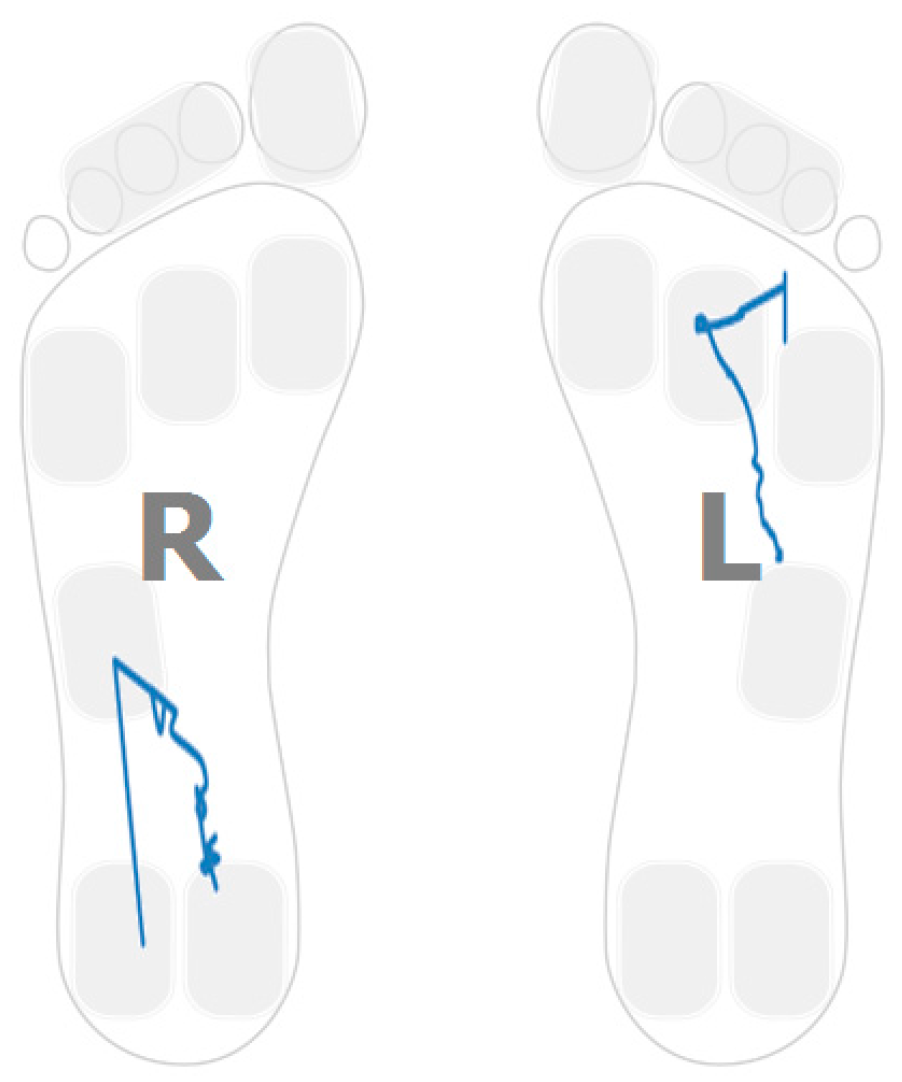
- Anterior–posterior pressure pattern length;
- Ratio between the maximum forefoot pressure and the maximum heel pressure;
- Anterior–posterior pressure length and location; and
- Lateral pressure length and location.
2.6. Ethical Considerations
2.7. Consent
2.8. Stroke Survivor Participants Demographics
2.9. Control Participants Demographics
3. Walking Strategies and Selected Parameters
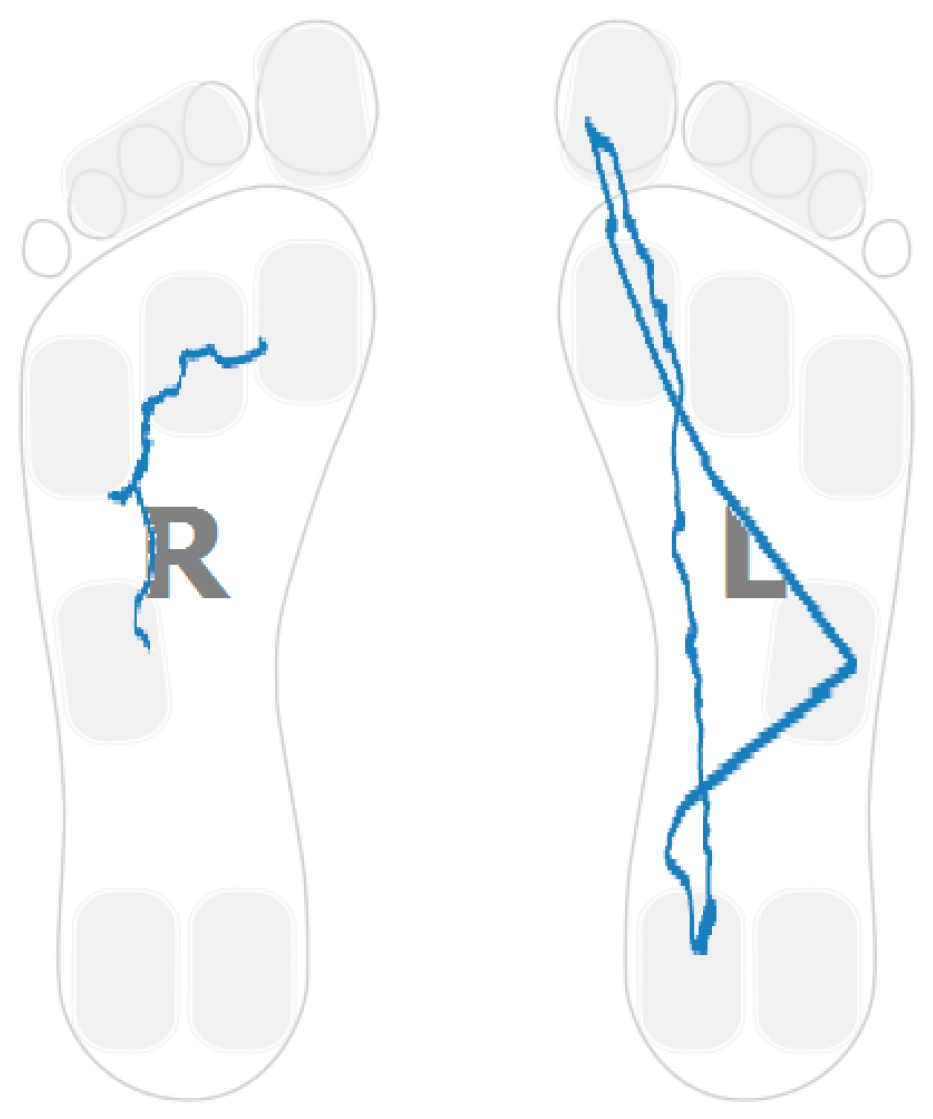
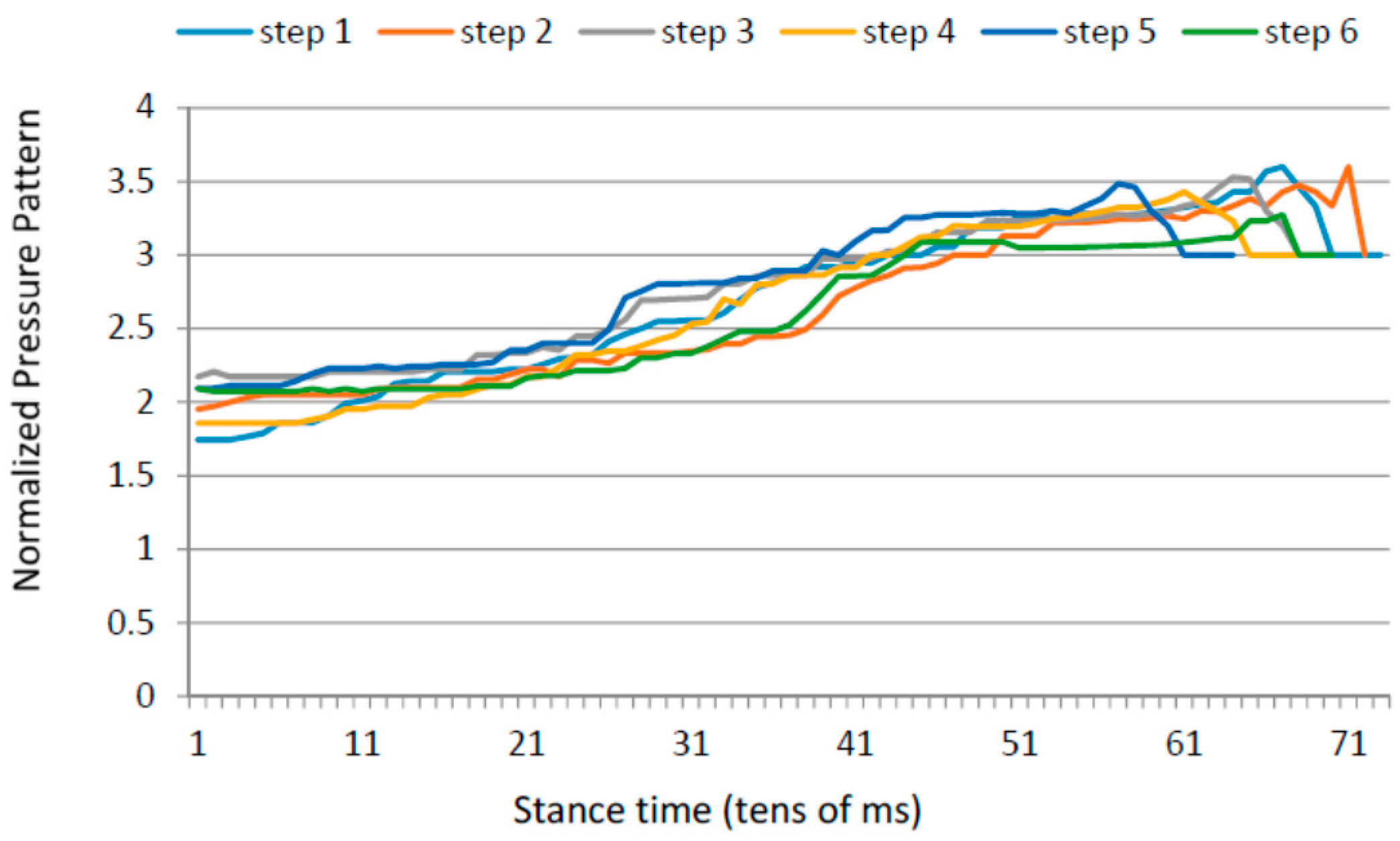
3.1. Heel Walking Strategy
- Time in which the average pressure on the heel is bigger than the 80% of the maximum heel pressure; and
- Maximum forefoot pressure.
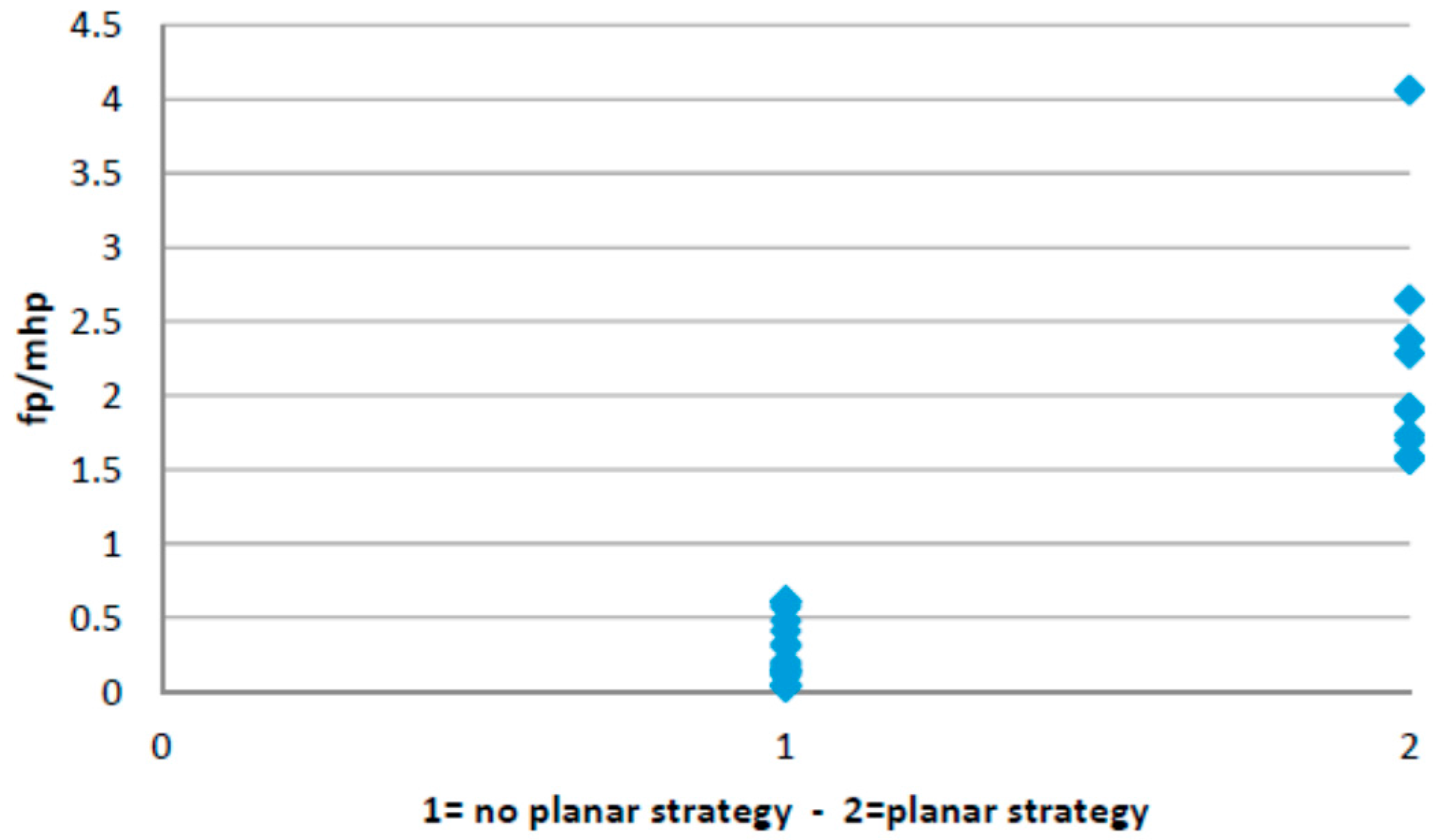
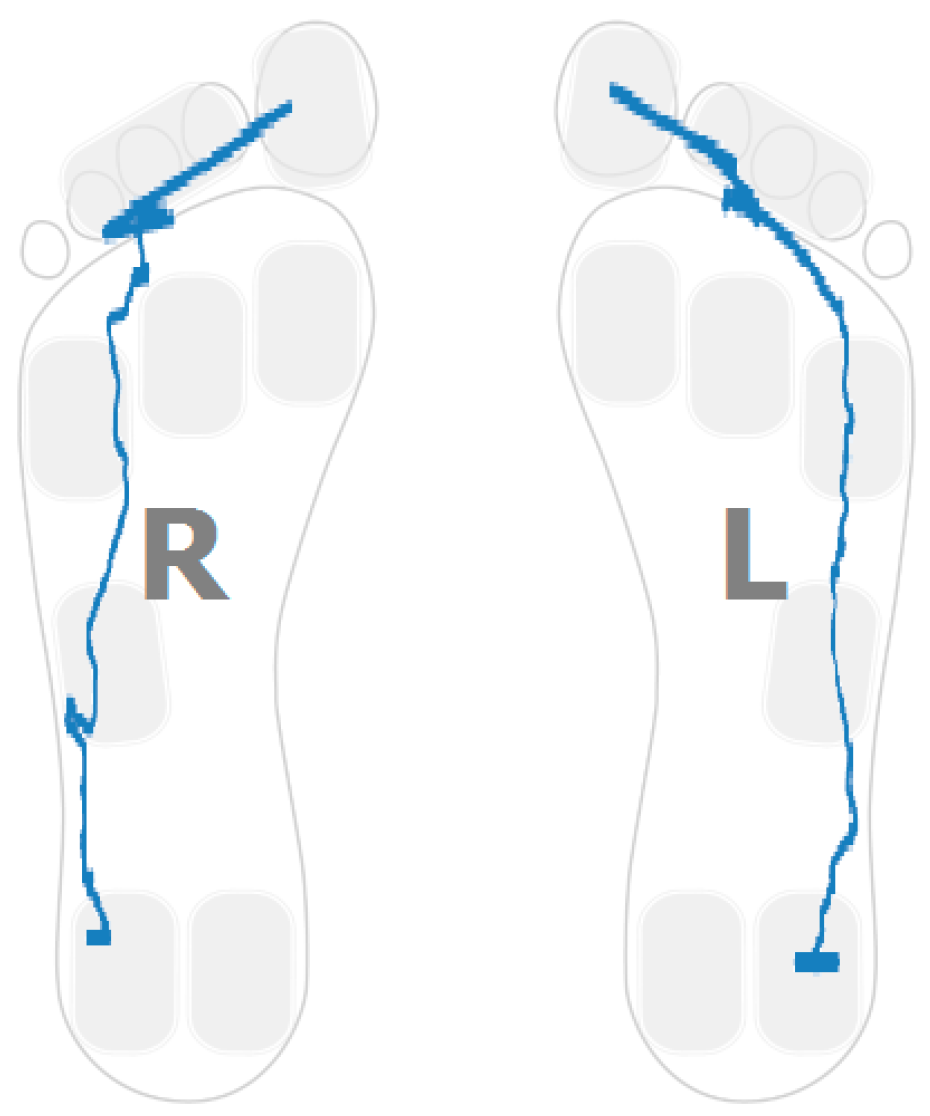
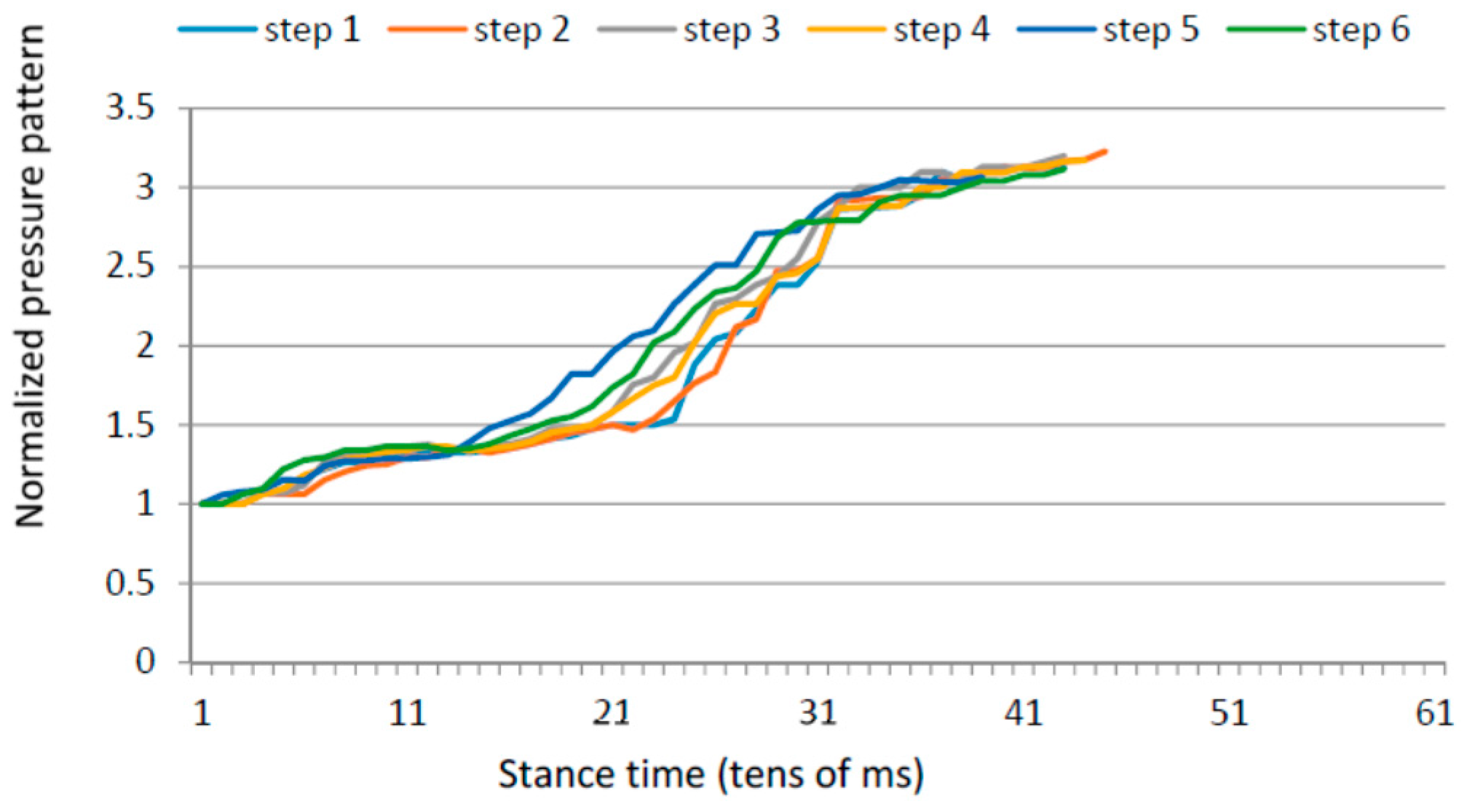
3.2. Planar Stride Strategy
- Ratio between the forefoot pressure at the point of maximum heel pressure and the maximum forefoot pressure; and
- Anterior/posterior pressure pattern length.
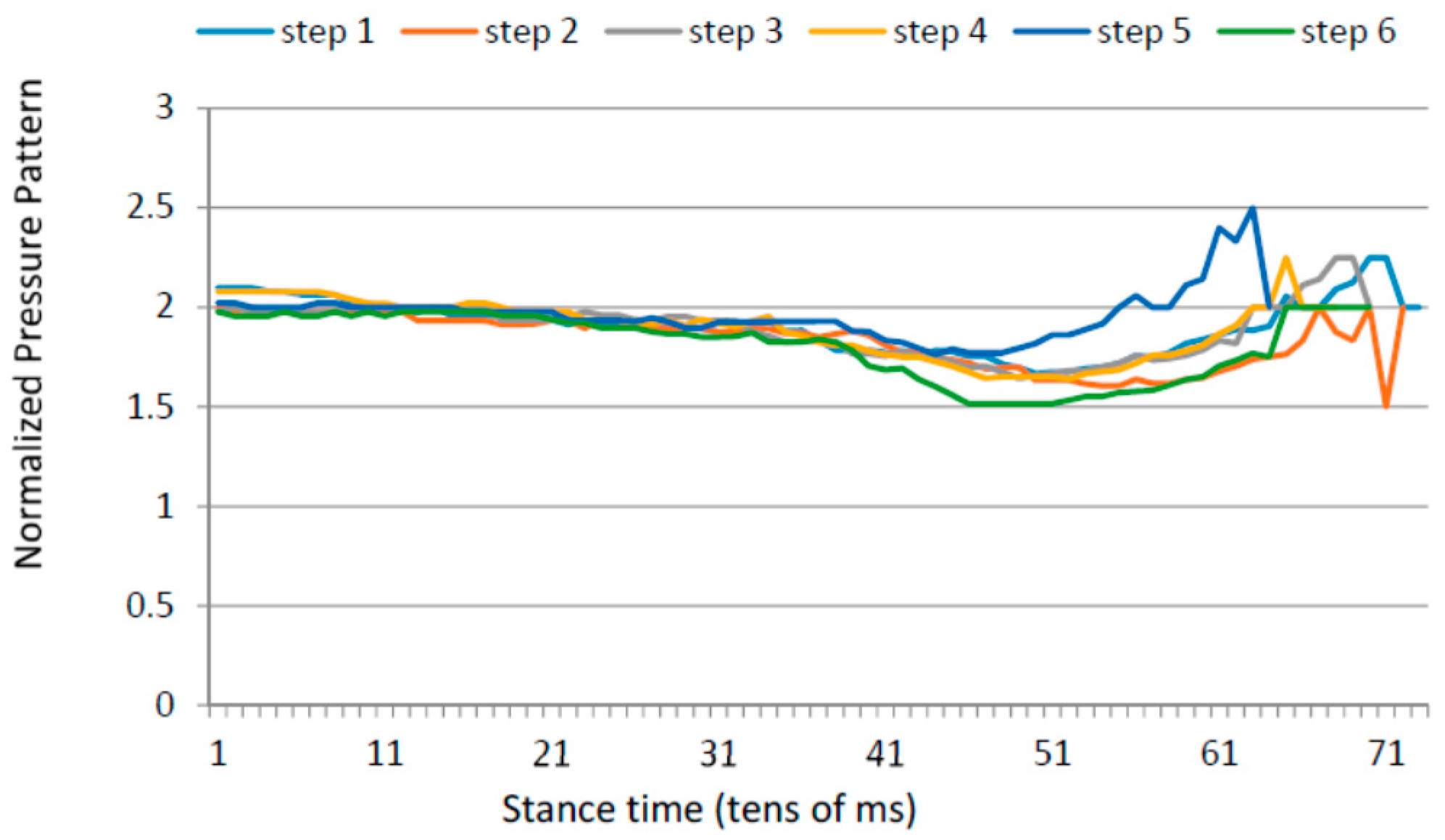
3.3. Low Heel Pressure Strategy
- Ratio between the maximum forefoot pressure and the maximum heel pressure; and
- Anterior/posterior pressure length and location.
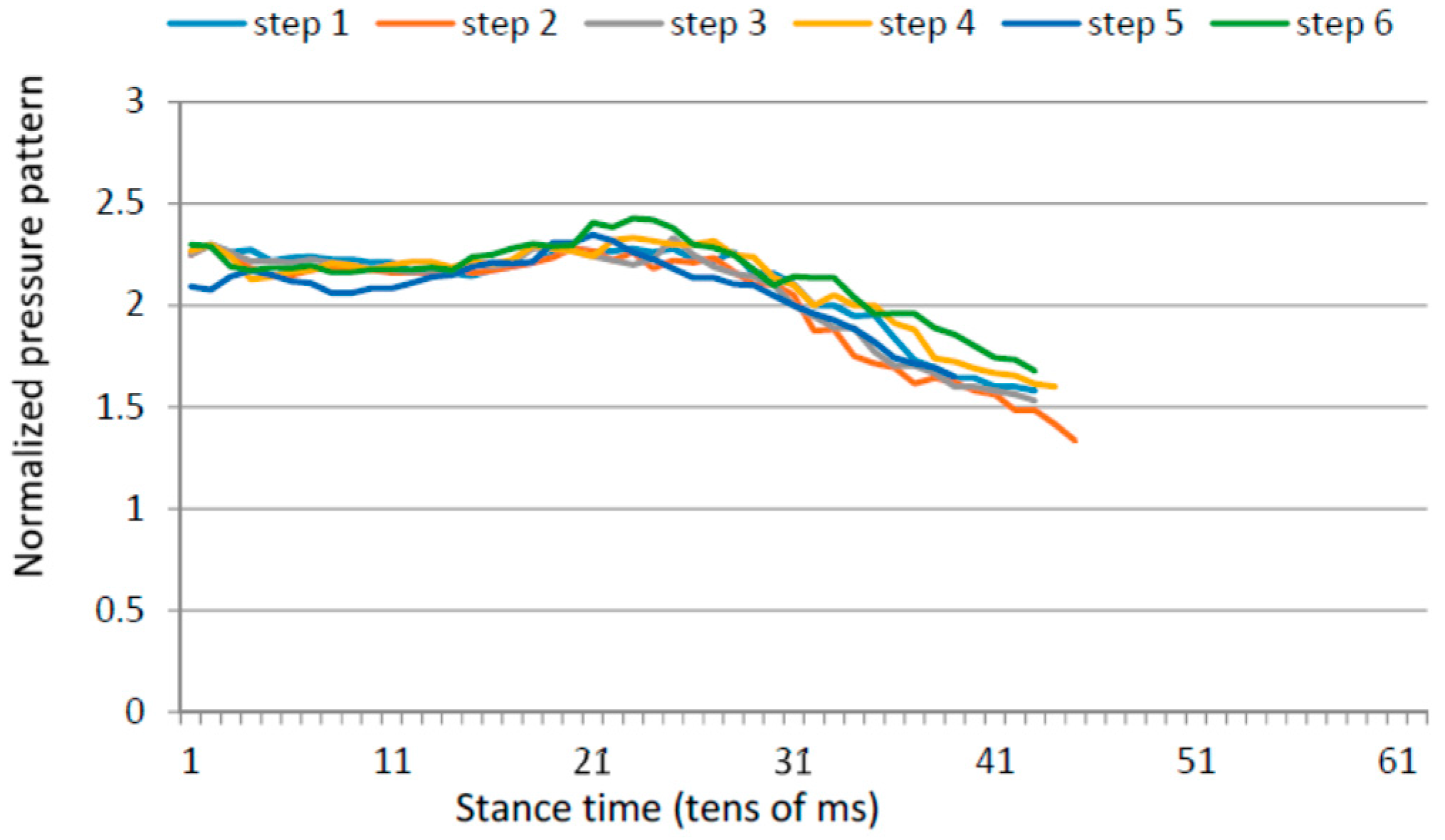
3.4. Gait Assymetries
3.5. Gait Variability over Time
4. Results
4.1. Heel Walking Strategy
4.2. Planar Stride Strategy
4.3. Low Heel Pressure Strategy
4.4. Gait Assymetries
4.5. Gait Variability over Time
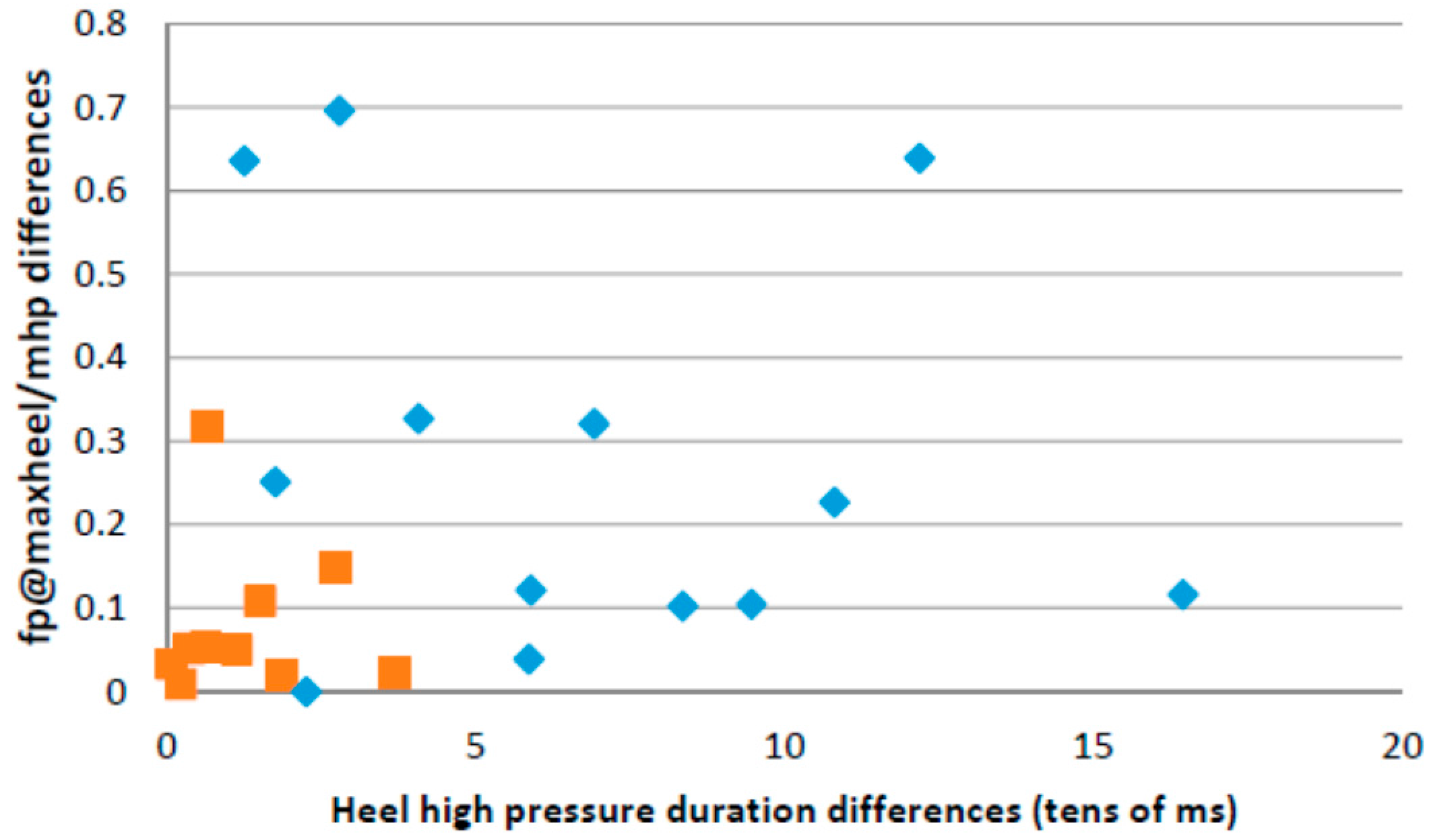
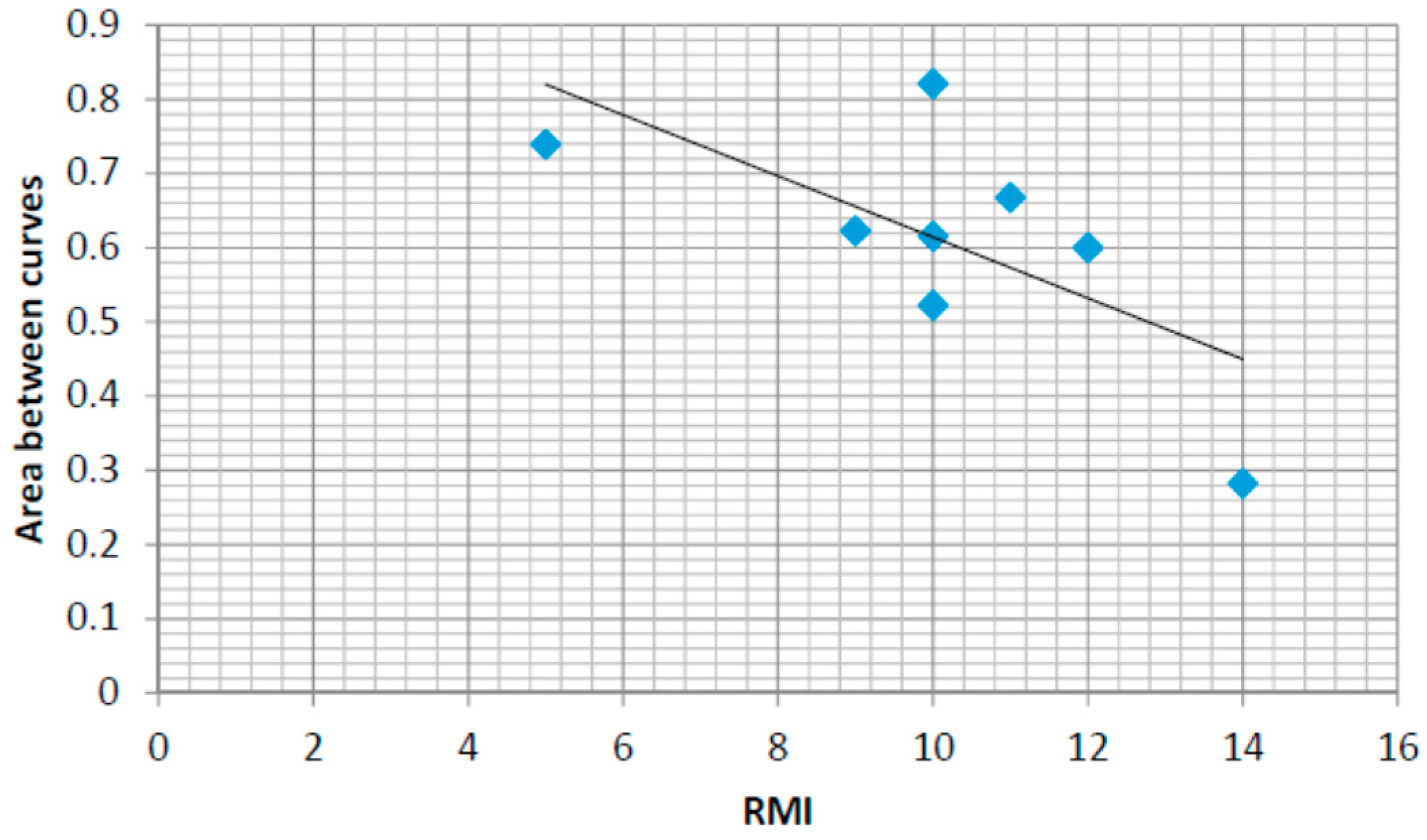
4.6. Correlations with the Rivermead Mobility Index
5. Discussions and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kanitthika, K.; Chan, K.S. Pressure sensor positions on insole used for walking analysis. In Proceedings of the 18th IEEE International Symposium on Consumer Electronics (ISCE 2014), Jeju, Korea, 22–25 June 2014; pp. 1–2.
- Abdul Razak, A.H.; Zayegh, A.; Begg, R.K.; Wahab, Y. Foot plantar pressure measurement system: A review. Sensors 2012, 12, 9884–9912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Kao, P.Y.; Lu, K.Y.; Wei, S.Y.; Hung, Y.P. Pressure Sensing Insoles for Learning Tai-Chi Chuan. In Proceedings of the Fourth International Conference on Information Science and Cloud Computing (ISCC2015), Guangzhou, China, 18–19 December 2015.
- Gerlach, C.; Krumm, D.; Illing, M.; Lange, J.; Kanoun, O.; Odenwald, S.; Hubler, A. Printed MWCNT-PDMS-composite pressure sensor system for plantar pressure monitoring in ulcer prevention. IEEE Sens. J. 2015, 15, 3647–3656. [Google Scholar] [CrossRef]
- Light, J.; Cha, S.; Chowdhury, M. Optimizing pressure sensor array data for a smart-shoe fall monitoring system. In Proceedings of the 2015 IEEE Sensors Conference, Busan, Korea, 1–4 November 2015. [CrossRef]
- Mustufa, A.; Barton, J.; O’Flynn, B.; Davies, R.; McCullagh, P.; Zheng, H. Design of a smart insole for ambulatory assessment of gait. In Proceedings of the 2015 IEEE 12th International Conference on Wearable and Implantable Body Sensor Networks (BSN), Cambridge, MA, USA, 9–12 June 2015.
- Strong, K.; Mathers, C.; Bonita, R. Preventing stroke: Saving lives around the world. Lancet Neurol. 2007, 6, 182–187. [Google Scholar] [CrossRef]
- The Stroke Association. State of the Nation. Stroke Statistics. Available online: https://www.stroke.org.uk/sites/default/files/stroke_statistics_2015.pdf (accessed on 27 September 2016).
- Jørgensen, H.; Nakayama, H.; Ho, R.; Olsen, T. Recovery of walking function in stroke patients: The Copenhagen stroke study. Arch. Phys. Med. Rehabil. 1995, 76, 27–32. [Google Scholar] [CrossRef]
- Duncan, P.W.; Zorowitz, R.; Bates, B.; Choi, J.Y.; Glasberg, J.I.; Graham, G.D.; Katz, R.C.; Lamberty, K.; Reker, D. Management of adult stroke rehabilitation care. A clinical practice guideline. Stroke 2005, 36, e100–e143. [Google Scholar] [CrossRef] [PubMed]
- Kafri, M.; Dickstein, R. External validity of post-stroke interventional gait rehabilitation studies. Top. Stroke Rehabil. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mawson, S.; Nasr, N.; Parker, J.; Davies, R.; Zheng, H.; Mountain, G. A Personalized Self-Management Rehabilitation System with an Intelligent Shoe for Stroke Survivors: A Realist Evaluation. JMIR Rehabil. Assistive Technol. 2016, 3, e1. [Google Scholar] [CrossRef]
- Qin, L.Y.; Ma, H.; Liao, W.H. Insole plantar pressure systems in the gait analysis of post-stroke rehabilitation. In Proceedings of the 2015 IEEE International Conference on Information and Automation, Yunnan, China, 8–10 August 2015; pp. 1784–1789.
- Howell, A.M.; Kobayashi, T.; Chou, T.R.; Daly, W.; Orendurff, M.; Bamberg, S.J. A laboratory insole for analysis of sensor placement to determine ground reaction force and ankle moment in patients with stroke. In Proceedings of the 2012 IEEE Annual International Conference on Engineering in Medicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; pp. 6394–6397.
- Nolan, K.J.; Yarossi, M.; Mclaughlin, P. Changes in center of pressure displacement with the use of a foot drop stimulator in individuals with stroke. Clin. Biomech. 2015, 30, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Itotani, K.; Murakami, M.; Itotani, M.; Nagai, A.; Imabori, Y.; Fujimoto, K.; Kato, J. Relationship between the weight-bearing ratio on the affected lower extremity and gait ability using a portable electronic foot sensor shoe (Step Aid®) in hemiplegic stroke patients. J. Phys. Therapy Sci. 2015, 27, 321. [Google Scholar] [CrossRef] [PubMed]
- Sazonov, E.S.; Hegde, N.; Tang, W. Development of SmartStep: An insole-based physical activity monitor. In Proceedings of the 2013 35th IEEE Annual International Conference on Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 7209–7212.
- Parker, J.; Mawson, S.; Mountain, G.; Nasr, N.; Zheng, H. Stroke patients’ utilisation of extrinsic feedback from computer-based technology in the home: A multiple case study realistic evaluation. BMC Med. Informatics Decis. Mak. 2014, 14, 46. [Google Scholar] [CrossRef] [PubMed]
- Khoo, I.H.; Marayong, P.; Krishnan, V.; Balagtas, M.N.; Rojas, O. Design of a biofeedback device for gait rehabilitation in post-stroke patients. In Proceedings of the 2015 IEEE 58th International Midwest Symposium on Circuits and Systems (MWSCAS), Fort Collins, CO, USA, 2–5 August 2015; pp. 1–4.
- Afzal, M.R.; Oh, M.K.; Lee, C.H.; Park, Y.S.; Yoon, J. A portable gait asymmetry rehabilitation system for individuals with stroke using a vibrotactile feedback. BioMed Res. Int. 2015, 2015, 375638. [Google Scholar] [CrossRef] [PubMed]
- Godi, M.; Turcato, A.M.; Schieppati, M.; Nardone, A. Test-retest reliability of an insole plantar pressure system to assess gait along linear and curved trajectories. J. Neuroeng. Rehabil. 2014, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- The SMART Consortium. Available online: http://www.catch.org.uk/current-project/smart-stroke/ (accessed on 28 September 2016).
- Zheng, H.; Nugent, C.; McCullagh, P.; Huang, Y.; Zhang, S.; Burns, W.; Davies, R.; Black, N.; Wright, P.; Mawson, S.; et al. Smart self-management: Assistive technology to support people with chronic disease. J. Telemed. Telecare 2010, 16, 224–227. [Google Scholar] [CrossRef] [PubMed]
- McCullagh, P.J.; Nugent, C.D.; Zheng, H.; Burns, W.P.; Davies, R.J.; Black, N.D.; Wright, P.; Hawley, C.; Eccleston, M.S.; Mawson, S.J.; et al. Promoting Behaviour Change in Long Term Conditions Using a Self-Management Platform. In Designing Inclusive Interactions; Langdon, P., Clarkson, J., Robinson, P., Eds.; Springer: London, UK, 2010; pp. 229–238. [Google Scholar]
- Mawson, S.; Nasr, N.; Parker, J.; Zheng, H.; Davies, R.; Mountain, G. Developing a personalised self-management system for post stroke rehabilitation: Utilising a user-centred design methodology. Disabil. Rehabil. Assist. Technol. 2013, 9, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.H.; Shepherd, R.B. Enhancing physical activity and brain reorganization after stroke. Neurol. Res. Int. 2011, 2011, 515938. [Google Scholar] [CrossRef] [PubMed]
- Kleim, J.A.; Jones, T.A. Principles of experience-dependent neural plasticity: Implications for rehabilitation after brain damage. J. Speech Lang. Hear. Res. 2008, 51, S225–S239. [Google Scholar] [CrossRef]
- Krakauer, J.W. Motor learning: Its relevance to stroke recovery and neurorehabilitation. Curr. Opin. Neurol. 2006, 19, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, S.H.; Hennerici, M.G.; Bäzner, H. Pathophysiology of stroke rehabilitation: The natural course of clinical recovery, use-dependent plasticity and rehabilitative outcome. Cerebrovasc. Dis. 2007, 23, 243–255. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Mawson, S.; Mountain, G.; Nasr, N.; Davies, R.; Zheng, H. The provision of feedback through computer-based technology to promote self-managed post-stroke rehabilitation in the home. Disabil. Rehabil. Assist. Technol. 2013, 9, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Mawson, S.; Nasr, N.; Parker, J.; Davies, R.; Zheng, H.; Mountain, G. A Realist Evaluation of an Information Communication Technology based Personalised Self Management Rehabilitation System for Stroke Survivors. JMIR Rehabil. Assist. Technol. 2016, 3. [Google Scholar] [CrossRef]
- Jones, F. Strategies to enhance chronic disease self-management: How can we apply this to stroke? Disabil. Rehabil. 2006, 28, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Bandura, A. The Nature and Structure of Self-Efficacy. Self-Efficacy: The Exercise of Control; WH Freeman and Company: New York, NY, USA, 1997; pp. 36–78. [Google Scholar]
- Chen, H.M.; Hsieh, C.L.; Sing, K.L.; Liaw, L.J.; Chen, L.M.; Lin, J.H. The test-retest reliability of 2 mobility performance tests in patients with chronic stroke. Neurorehabil. Neural Repair 2007, 21, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kinematix Company. Insole Pressure Sensors. Available online: http://kinematix.pt/tune (accessed on 27 June 2016).
- Waikato Environment for Knowledge Analysis (Weka). Available online: http://www.cs.waikato.ac.nz/ml/weka/ (accessed on 13 September 2016).
- Hall, M.; Frank, E.; Holmes, G.; Pfahringer, B.; Reutemann, P.; Witten, I.H. The WEKA data mining software: An update. ACM SIGKDD Explor. Newsl. 2009, 11, 10–18. [Google Scholar] [CrossRef]
- Anderson, D.R. Information Theory and Entropy; Springer New York: New York, NY, USA, 2008; pp. 51–82. [Google Scholar]
- National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRCs). Yorkshire and Humber. Avaliable online: www.clahrc-yh.nihr.ac.uk (accessed on 29 September 2016).





| Unique ID | Gender | Age (years) | Affected Side | Insole Size | Weight (kg) |
|---|---|---|---|---|---|
| 11 | F | 64 | Right | L | 105 |
| 12 | M | 61 | Right | L | 85 |
| 13 | F | 66 | Right | M | 75 |
| 15 | M | 50 | Left | XL | 90 |
| 16 | F | 79 | Left | S | 64.8 |
| 21 | F | 72 | Right | M | 73 |
| 22 | M | 64 | Left | XL | 90.8 |
| 23 | F | 75 | Right | L | 114.3 |
| 24 | M | 75 | Left | L | 80 |
| 25 | F | 68 | Both sides | M | 95.3 |
| 26 | F | 69 | Left | M | 66 |
| 27 | M | 84 | Right | XL | 95.3 |
| 29 | M | 39 | Both sides | L | 84.1 |
| 210 | M | 64 | Right | XL | 87.3 |
| Unique ID | Gender | Age (years) | Insole Size | Weight (kg) |
|---|---|---|---|---|
| NKP1 | F | 45 | M | 63.5 |
| NKP4 | M | 44 | L | 69.9 |
| NKP5 | F | 46 | S | 64.8 |
| NKP6 | F | 55 | M | 64.1 |
| NKP7 | F | 54 | M | 75 |
| NKP9 | M | 45 | XL | 80 |
| NKPA | F | 52 | S | 64.2 |
| NKPB | F | 50 | M | 72 |
| NKPC | M | 46 | M | 70 |
| NKPD | F | 51 | S | 54 |
| Classified as | Heel Walking | No Heel Walking |
|---|---|---|
| Heel walking | 3 | 0 |
| No heel walking | 0 | 21 |
| Cluster | Mean | Standard Deviation |
|---|---|---|
| 1 | 2.0906 | 0.9036 |
| 2 | 0.279 | 0.2033 |
| Feature | p-Value |
|---|---|
| heel duration standard deviation | 0.0161 |
| Forefoot vs. heel maximum pressure standard deviation | 0.0138 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munoz-Organero, M.; Parker, J.; Powell, L.; Mawson, S. Assessing Walking Strategies Using Insole Pressure Sensors for Stroke Survivors. Sensors 2016, 16, 1631. https://doi.org/10.3390/s16101631
Munoz-Organero M, Parker J, Powell L, Mawson S. Assessing Walking Strategies Using Insole Pressure Sensors for Stroke Survivors. Sensors. 2016; 16(10):1631. https://doi.org/10.3390/s16101631
Chicago/Turabian StyleMunoz-Organero, Mario, Jack Parker, Lauren Powell, and Susan Mawson. 2016. "Assessing Walking Strategies Using Insole Pressure Sensors for Stroke Survivors" Sensors 16, no. 10: 1631. https://doi.org/10.3390/s16101631







