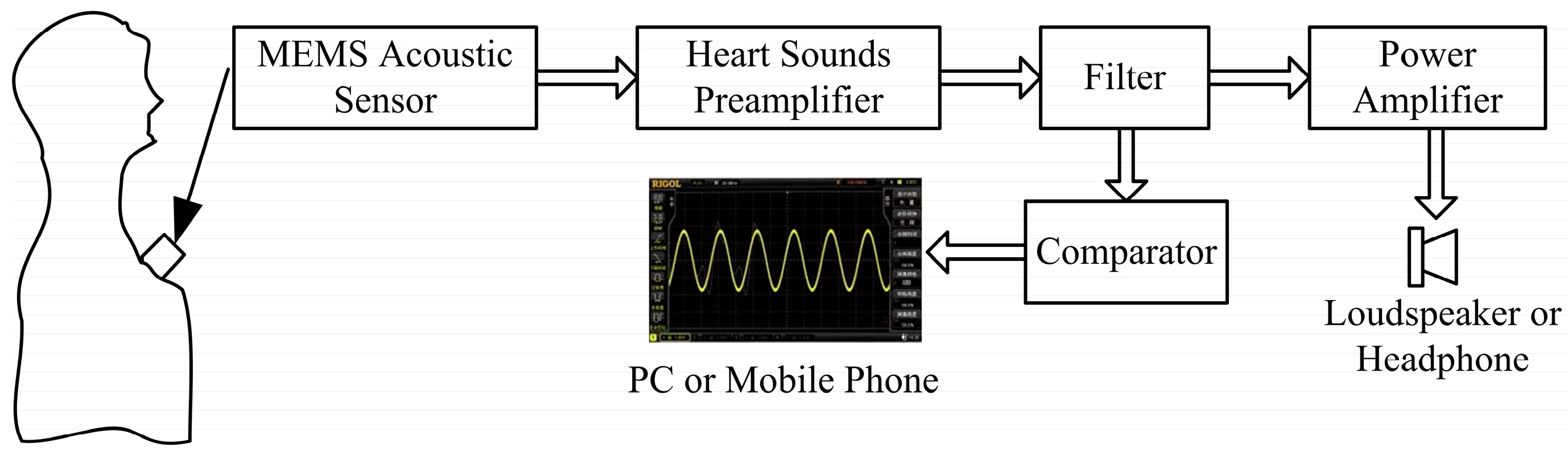
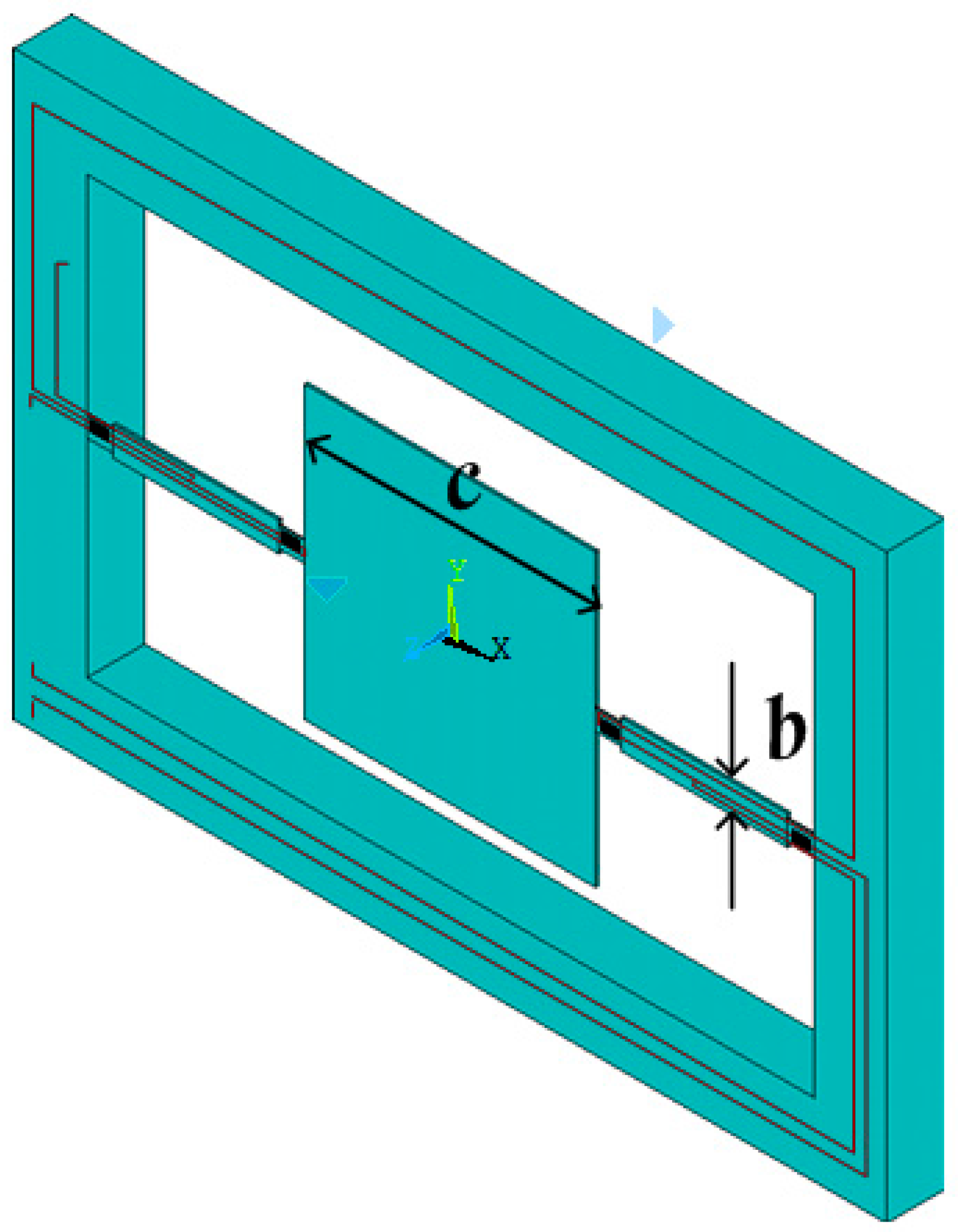
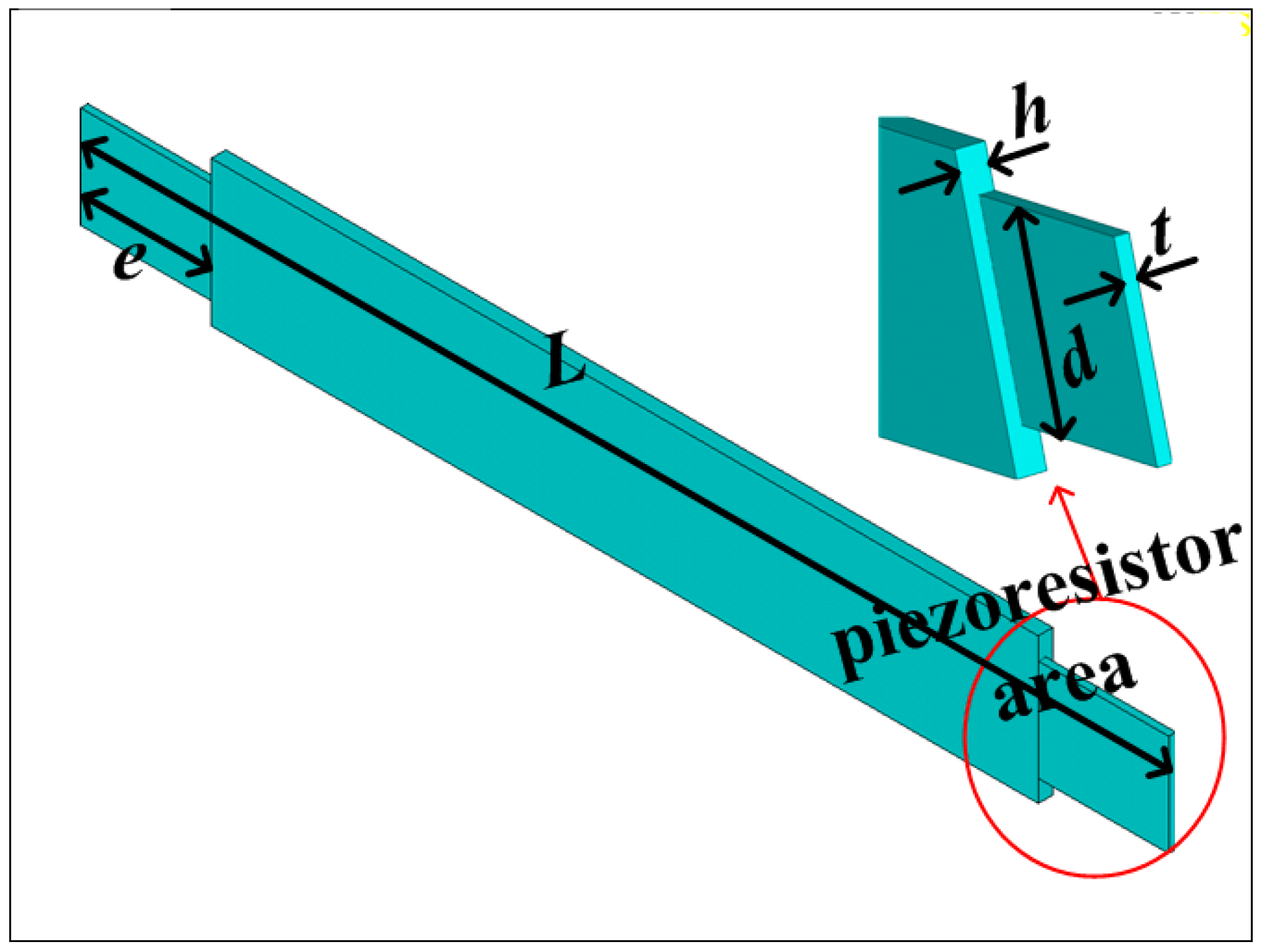
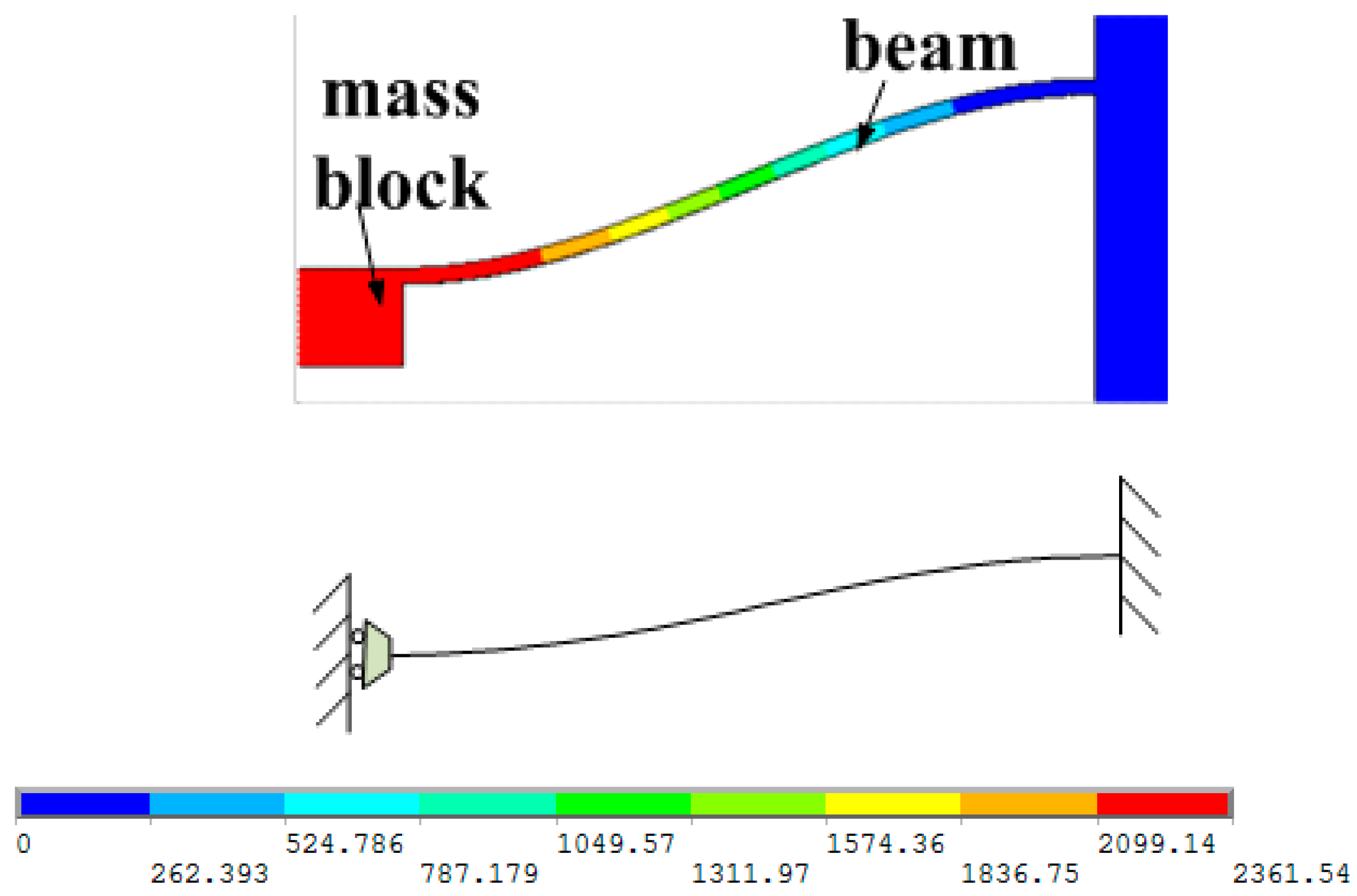
1. Introduction
Auscultation is an important routine examination method in clinical diagnosis. Before the nineteenth century doctors could only put the ear on the patient’s chest for “direct auscultation”. In 1816, French doctor Laennec invented the stethoscope; therefore, “indirect auscultation” becomes possible and the discipline of cardiac auscultation is formed, which has greatly promoted the development of medicine [
1]. However, the traditional stethoscope has poor low-frequency response, and diagnosis highly depends on the subjective judgment of doctors. In addition, the human ear is limited by the inherent limitation whose most sensitive frequency range is 1000~3000 Hz, which is with poor sensitivity for low-frequency sounds [
2]. However the clinically valuable heart sound frequency range is often concentrated in the range of 20~600 Hz, so some important heart sounds with low frequency and small intensity are difficult to capture [
3,
4].
In 1999, the American 3M Littmann Company (São Paulo, MN, USA) developed and produced an electronic stethoscope, which was the first to apply electronic technology to auscultation [
5]. The traditional stethoscope has been replaced by the heart sound electronic auscultation system, which has the advantages of high accuracy, real-time waveform display, ease of use, low cost, and small volume. The common sound sensor used by electronic stethoscopes is the microphone [
6]. The microphone is divided into electret, coil, and capacitance, and electret is the most commonly used. The electronic stethoscope converts the heart sound signals to analog signals, which are amplified, and then the analog signals are converted to digital signals for further processing and transmission. The microphone-type electronic stethoscope receives the heart sound signals via the microphone in the cavity, which is mounted behind the diaphragm. The vibration of the diaphragm causes sound pressure in the cavity, the microphone receives the sound pressure and converts it to an electrical signal. Since the sound propagates through two layers of diaphragm and the internal cavity, namely a three-layer medium, the energy transfer is limited and the sensitivity of the stethoscope is low.
MEMS technology has been applied to the detection, diagnosis, and therapy, which plays an important role in the improvement of medical devices and the prevention, diagnosis and treatment of diseases [
7,
8]. The wireless endoscope technologies of M2A developed by Israeli Given Imaging Company (Tel Aviv-Yafo, Israeli) and NORIKA3 developed by Japanese RF Company (Nagano-ken, Japan) have been applied to clinical practice [
9,
10,
11]. Thousands of biomolecules can be integrated in a centimeter-scale chip by MEMS technology, which could be made into the bio-chip. The bio-chip can show different allergic reactions for the various biochemical reactions to realize the test and analysis of the genes, antigens, ligands and other bioactive substances. MEMS technology can be also used in genetic analysis and genetic diagnosis [
12,
13,
14]. Therefore, MEMS piezoresistive pressure sensors, with the advantages of good performance at low frequencies, comprised of mature and controllable MEMS piezoresistive technology, with low cost, linear output, and simple circuitry, have been widely used in medical diagnostic systems [
15].
This paper proposes an electronic heart sound sensor with a high signal-to-noise ratio and real-time waveform display based on MEMS technology and the piezoresistive principle. Combined with further clinical experience, preliminary diagnosis can be performed by the electronic heart sound sensor and the accuracy of heart attack diagnosis can be improved.
3. Fabrication
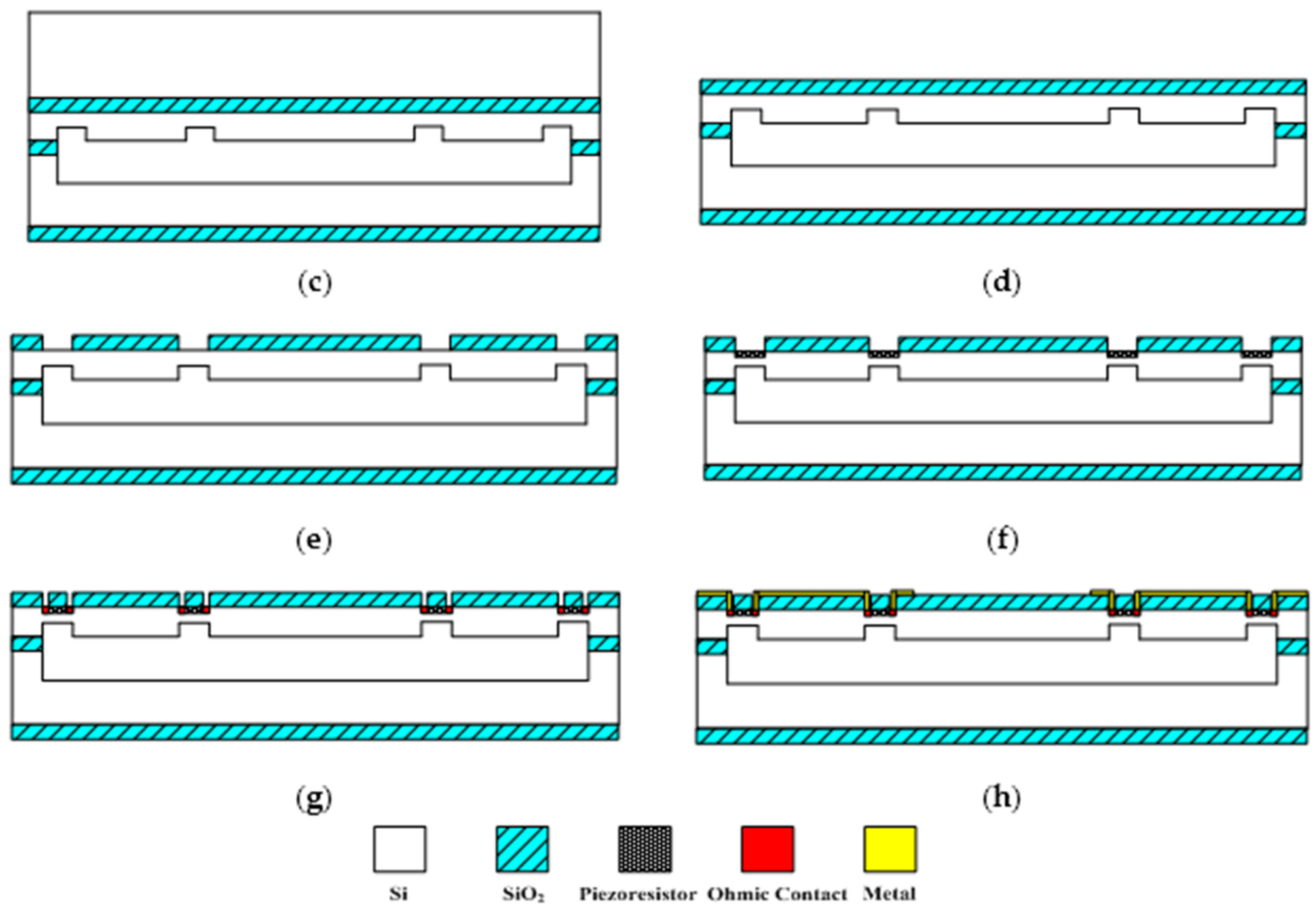
The sensitive microstructure of the MEMS electronic heart sound sensor is processed by MEMS technology based on a Si wafer and SOI (Silicon-On-Insulator) wafer. The fabrication procedures are illustrated in
Figure 9a–h [
18,
19]:
Figure 9a preparing the Si wafer and SOI wafer, a SOI wafer with a 20 µm device layer, 2 µm silicon dioxide layer and 400 µm substrate layer;
Figure 9b RIE etching the device layer of the SOI wafer to form the back structure of the stress-concentrating grooves; oxidizing and RIE etching the Si wafer to form a cavity;
Figure 9c bonding the device layer of the SOI wafer and Si wafer under nitrogen at 900~1100 °C;
Figure 9d removing the substrate layer and silicon dioxide layer of the SOI wafer by TMAH (Tetramethylammonium Hydroxide) solution;
Figure 9e RIE etching to form the stress concentration grooves;
Figure 9f oxidation to form a 1000 Å silicon dioxide layer at 950 °C; etching and implanting boron with 100 KeV energy and 4 × 10
18 cm
−3 density to form the 5000 Ω piezoresistors at the stress concentration grooves;
Figure 9g chemical vapor depositing to form a 1100 Å oxide layer on the top and ICP (Inductively Coupled Plasma) etching, implanting denser boron with 100 KeV energy and 4 × 10
21 cm
−3 density to form P+ area;
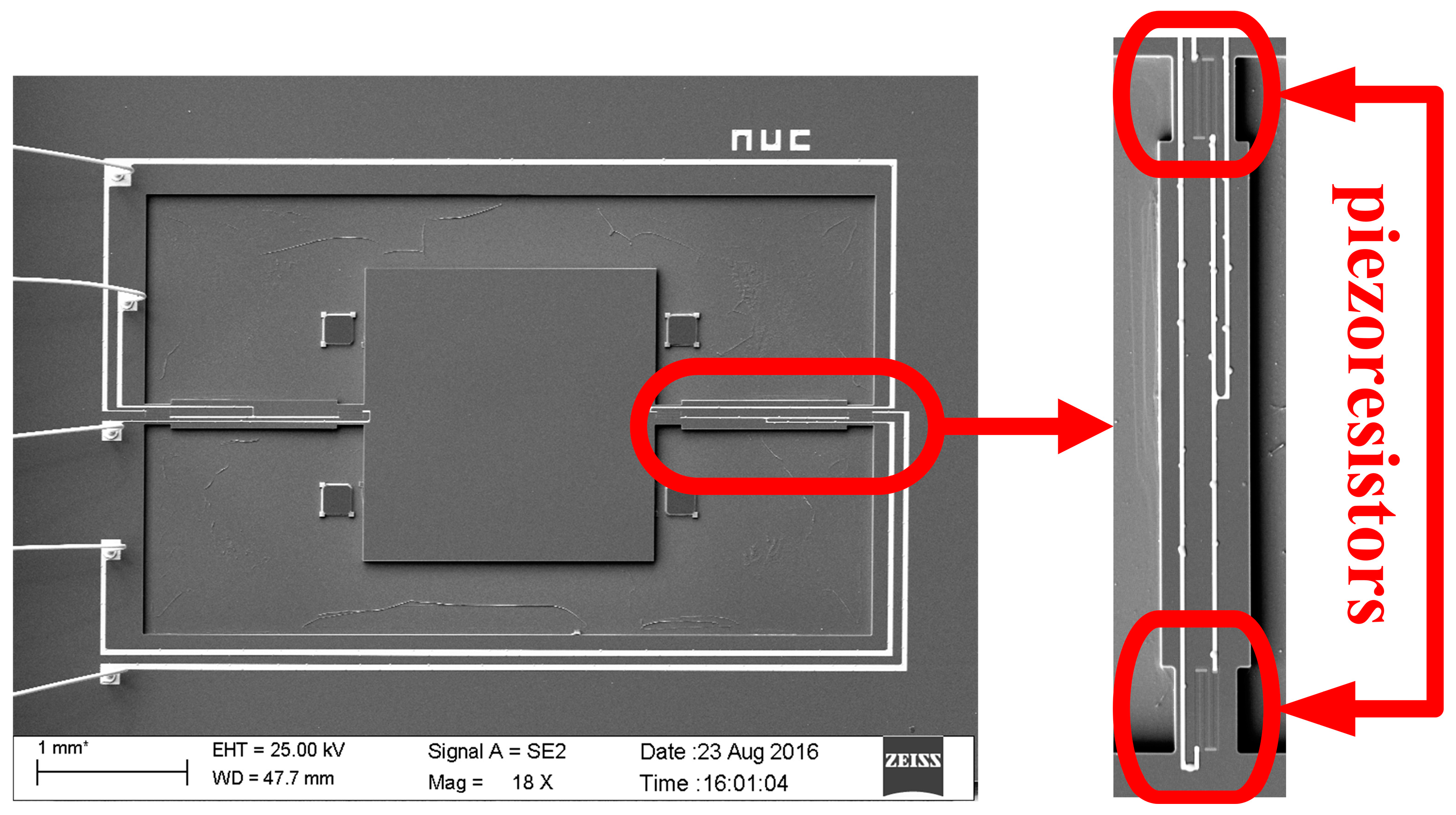
Figure 9h sputtering to form metal interconnects, annealing to form ohmic contact under nitrogen at 1000 °C; ICP etching and releasing the structure. The MEMS microstructure is shown in
Figure 10.
4. Package and Test
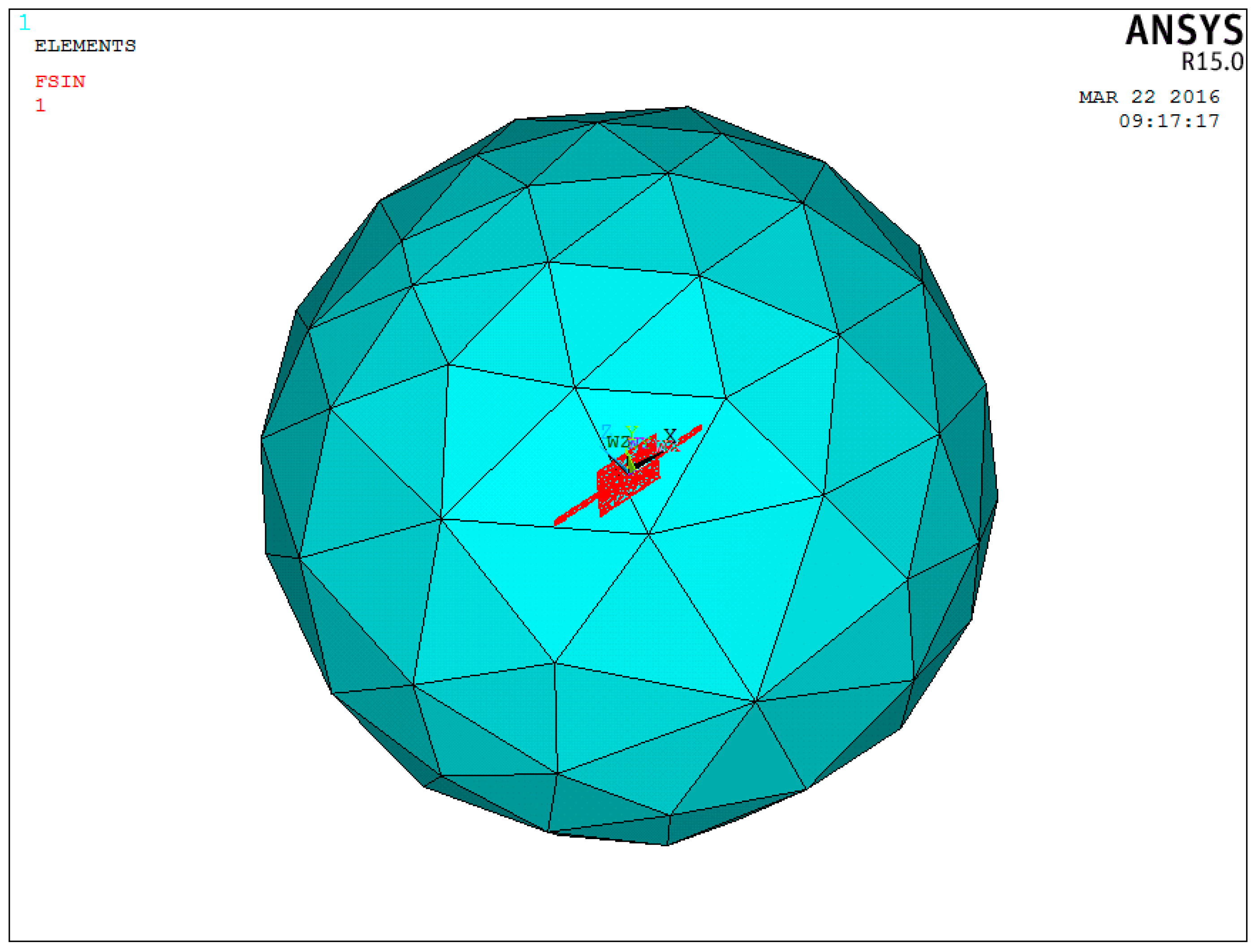
After the microstructure has been fabricated, it needs to be packaged to prevent the microstructure from being destroyed. At the same time, it should be ensured that the external acoustic signal can be maximally transmitted to the microstructure. Thus, acoustic impedance of the packaging structure should match with that of medical coupling agent to reduce the acoustic energy losses and improve the sensitivity of the MEMS electronic heart sound sensor. A theoretical model of the three-layer medium has been established to predict the sound-transparent performance of the packaging structure. Assuming that the acoustic wave is the plane wave when it propagates in the three-layer medium, the model is shown in
Figure 11.
According to the above model, the sound-transparent coefficient can be expressed as Equation (3) [
20]:
where,
Z1 and
Z2, respectively, represent the characteristic impedance of the medical coupling agent and packaging structure,
k2 indicates the wave number, and
L represents the thickness of the package. From Equation (3) it can be seen that when
Z1 ≈
Z2, the sound-transparent coefficient
T ≈ 1. In addition, it can be seen from Equation (3) that if the thickness of the sound-transparent package
L is far smaller than the wavelength, that is
k2L→0,
T ≈ 1.
However, the inherent mechanical properties of the sound-transparent cap acting on the MEMS microstructure would influence the detection of heart sound signal, and the thinner the sound-transparent cap is, the lower its resonant frequency. Considering the sound-transparent performance and the resonant frequency, a polyurethane cap with 2 mm thickness has been selected and the preliminary packaged prototype of the MEMS electronic heart sound sensor is shown in
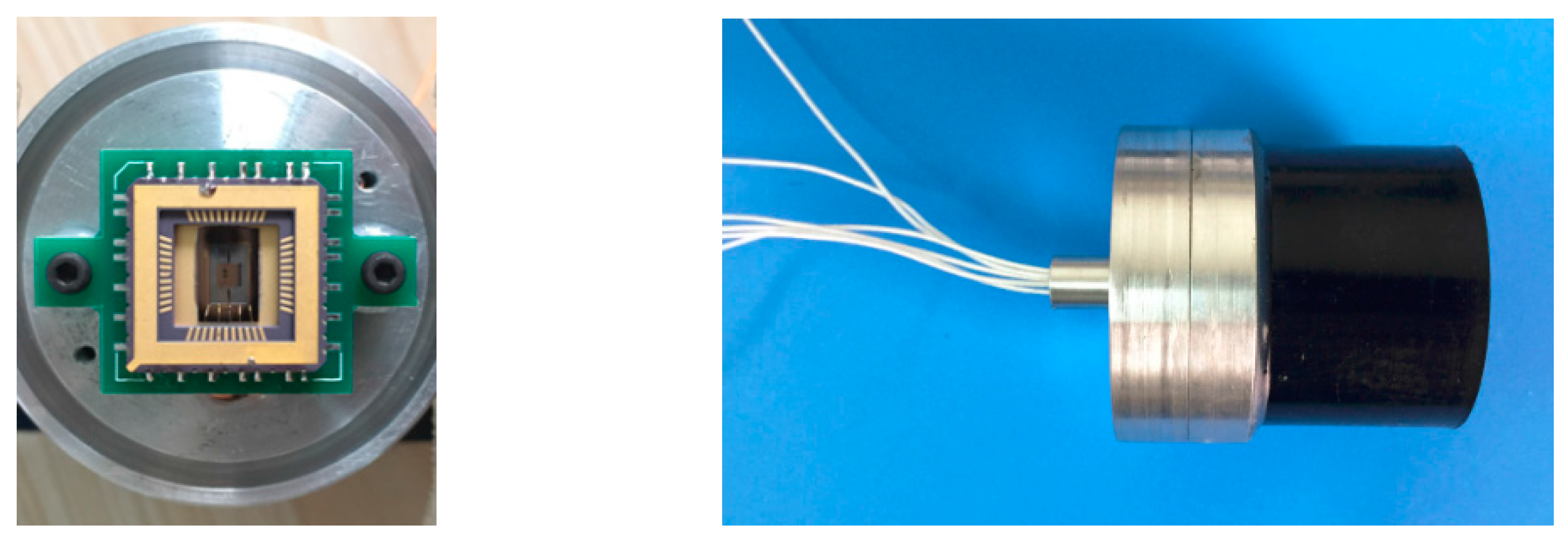
Figure 12.
A random healthy young man has been selected as the auscultation object at room temperature. The MEMS electronic heart sound sensor has been connected to the Agilent 54622A oscilloscope (Palo Alto, CA, USA), and the waveform tested by it is shown in
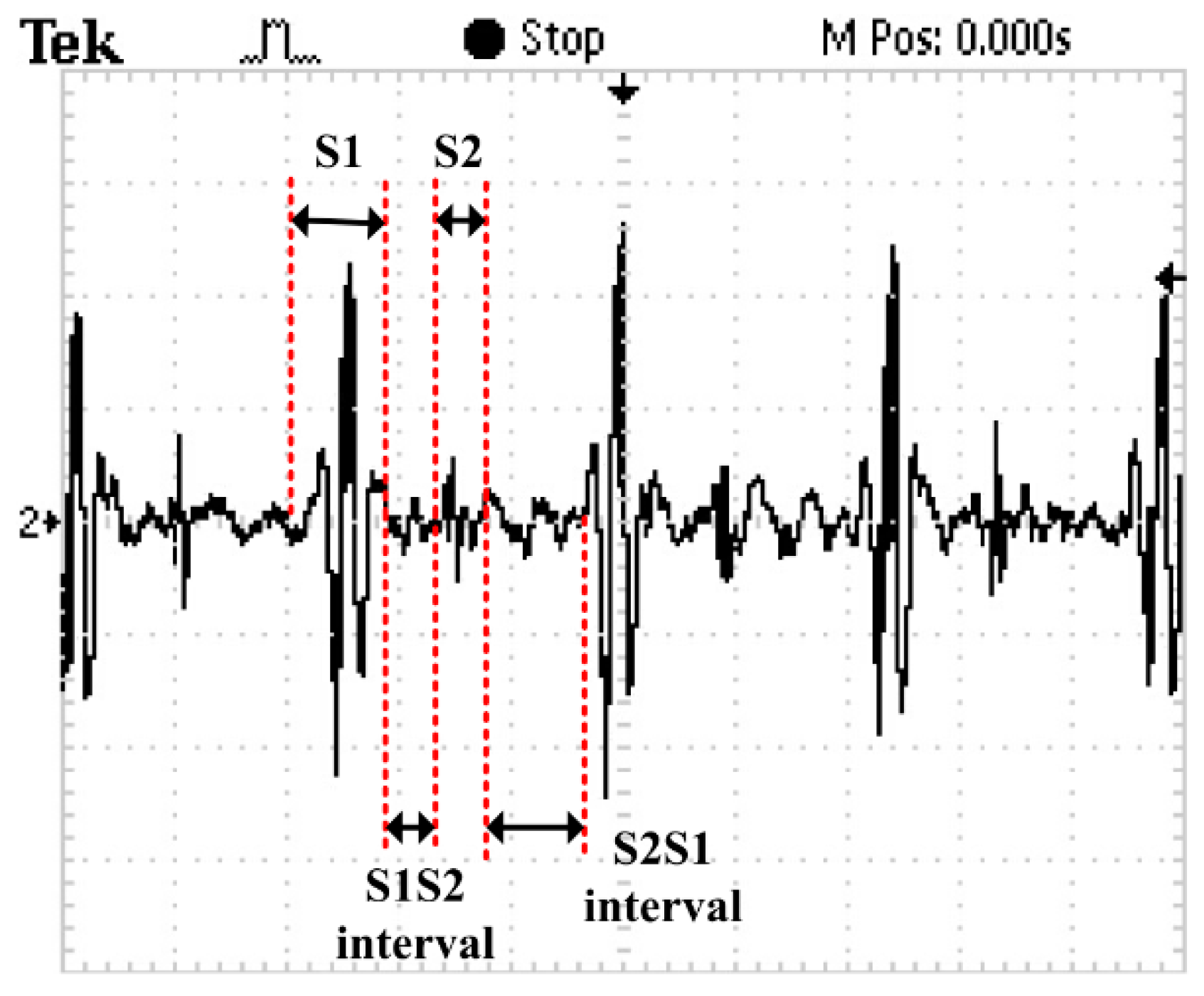
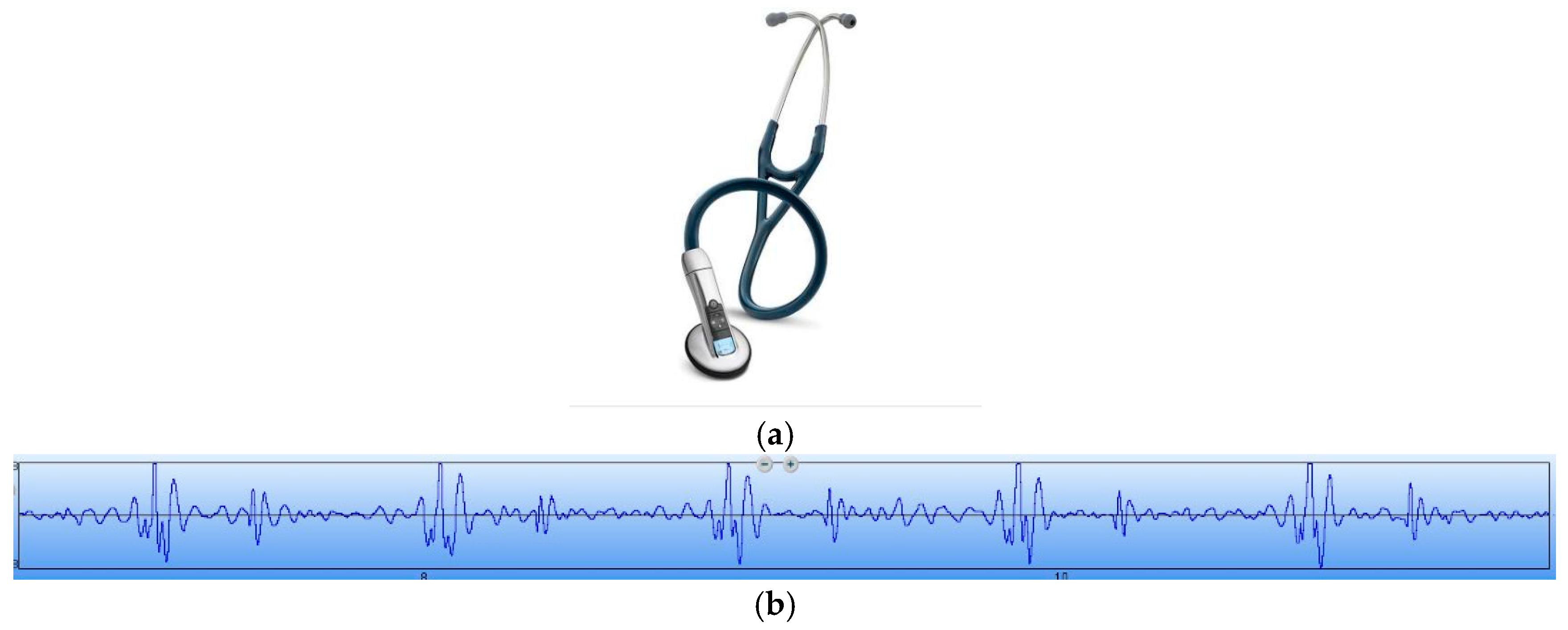
Figure 13. In order to verify the correctness of the test results, the results have been compared with that tested by a 3200-type electronic stethoscope from 3M (
Figure 14). The 3200-type electronic stethoscope is a mature electronic stethoscope on the market, by which heart sound signals can be magnified 24 times and transferred to a computer for subsequent analysis by Bluetooth technology. It can be seen that the trend of the heart sound waveform signal tested by the MEMS electronic heart sound sensor is the same as that tested by the 3200-type electronic stethoscope. From the waveform it can be seen that the first and second heart sounds (S1 and S2) are obvious and his cardiac cycle is about 750 ms, and the heart rate is about 85 beats/min. The ratio of the S1S2 interval and S2S1 interval is approximately 1:2, which is consistent with the normal heart sounds standard.
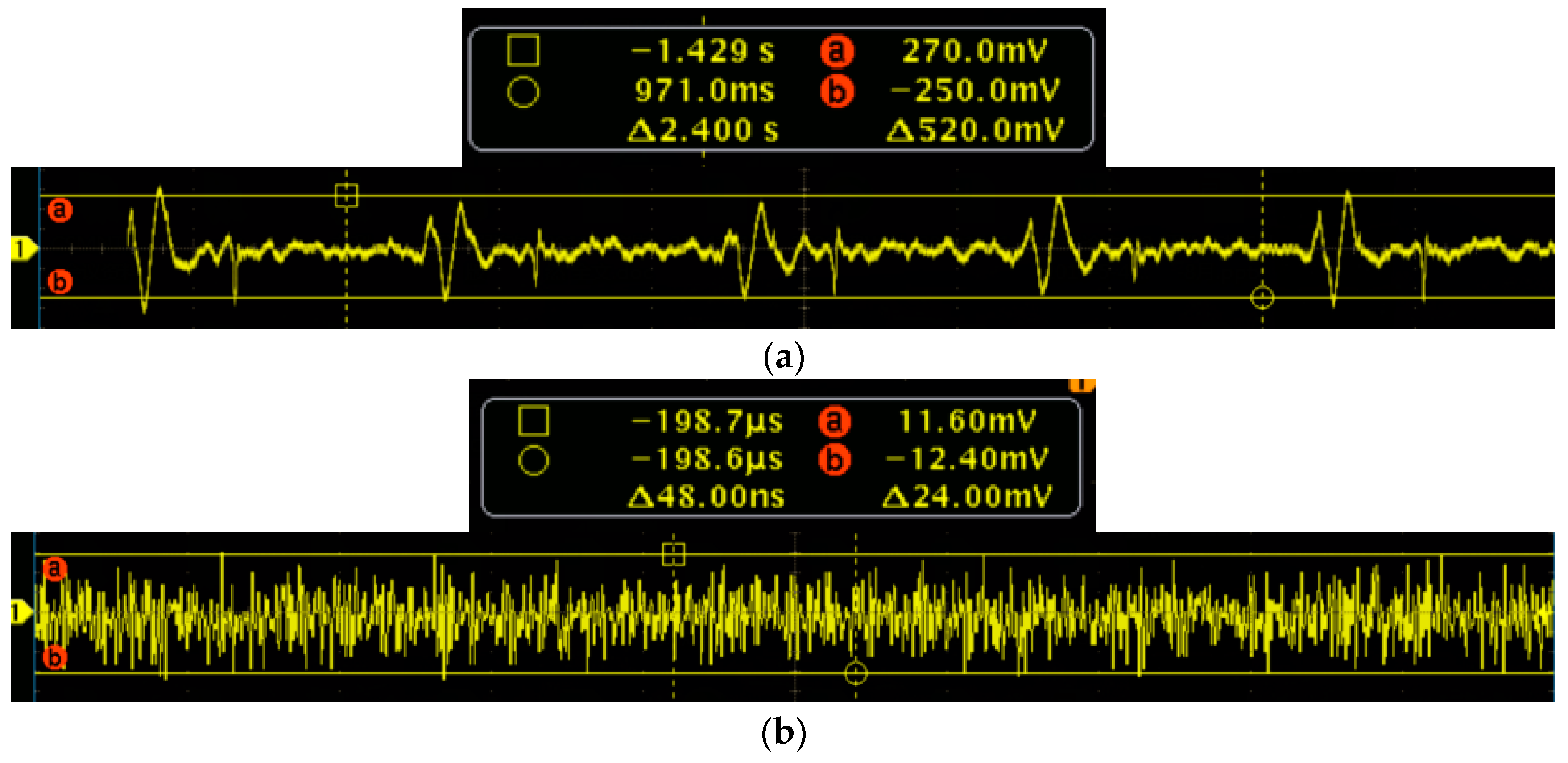
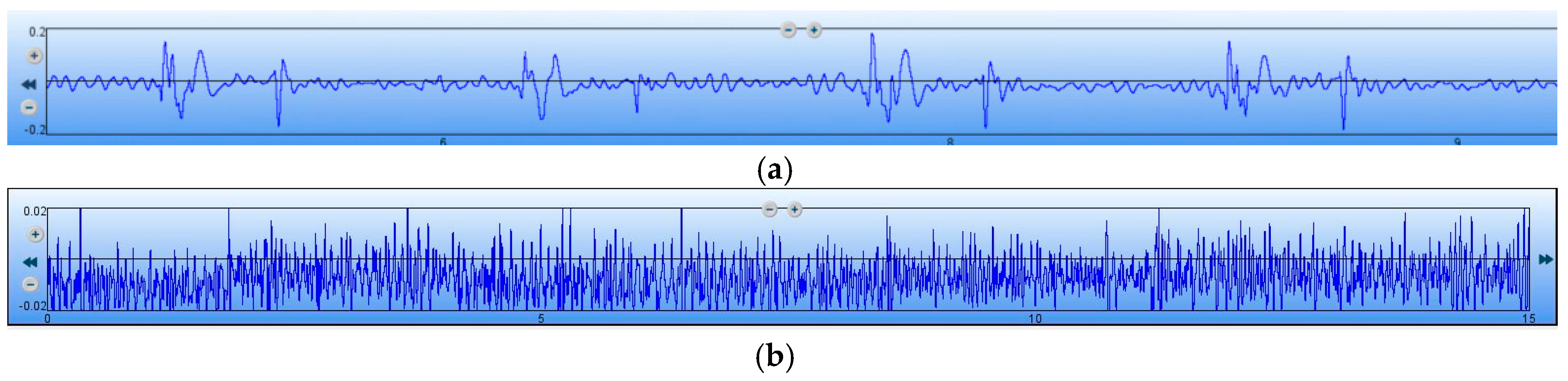
To further verify the performance of the MEMS sensor, the signal-to-noise ratio (SNR) testing experiments of the MEMS sensor and 3200-type electronic stethoscope have been carried out. Firstly, the MEMS sensor has been tested. The test results of the heart sound are shown in
Figure 15a, and it is determined that the maximum of the heart sound signals is 270 mv. The output of the MEMS sensor without the heart sound signals are shown in
Figure 15b, and it is determined that the noise signals is 12.4 mv. Therefore, by calculation, the SNR of the MEMS sensor is 27 dB. Then, the 3200-type electronic stethoscope has been tested. The test results of the heart sound are shown in
Figure 16a, and it is determined that the maximum of the heart sound signals is 0.2. The output of the 3200-type electronic stethoscope without heart sound signals are shown in
Figure 16b, and is determined that the noise signal is 0.02. Therefore, the SNR of the 3200-type electronic stethoscope is 20 dB. All of the above tests show that the SNR of the MEMS sensor is 7 dB higher than the 3200-type electronic stethoscope.
5. Conclusions
This study reveals a new attempt to implement MEMS technology and acoustic sensor technology in the traditional auscultation field of medicine. Heart sound vibration signals are transformed into electrical signals by the MEMS heart sound sensor to be displayed on an oscilloscope, by which the change from the traditional auscultation to heart sound has been realized, and the objectivity and accuracy of diagnosis has been improved. Due to the nature of MEMS technology, the MEMS electronic heart sound sensor has the advantages of small size, low power, visualization, ease of use, low cost, and good prospects for clinical application.