Development of an FPW Biosensor with Low Insertion Loss and High Fabrication Yield for Detection of Carcinoembryonic Antigen
Abstract
:1. Introduction
2. Theory and Design
2.1. Theoretical Description of the FPW Device
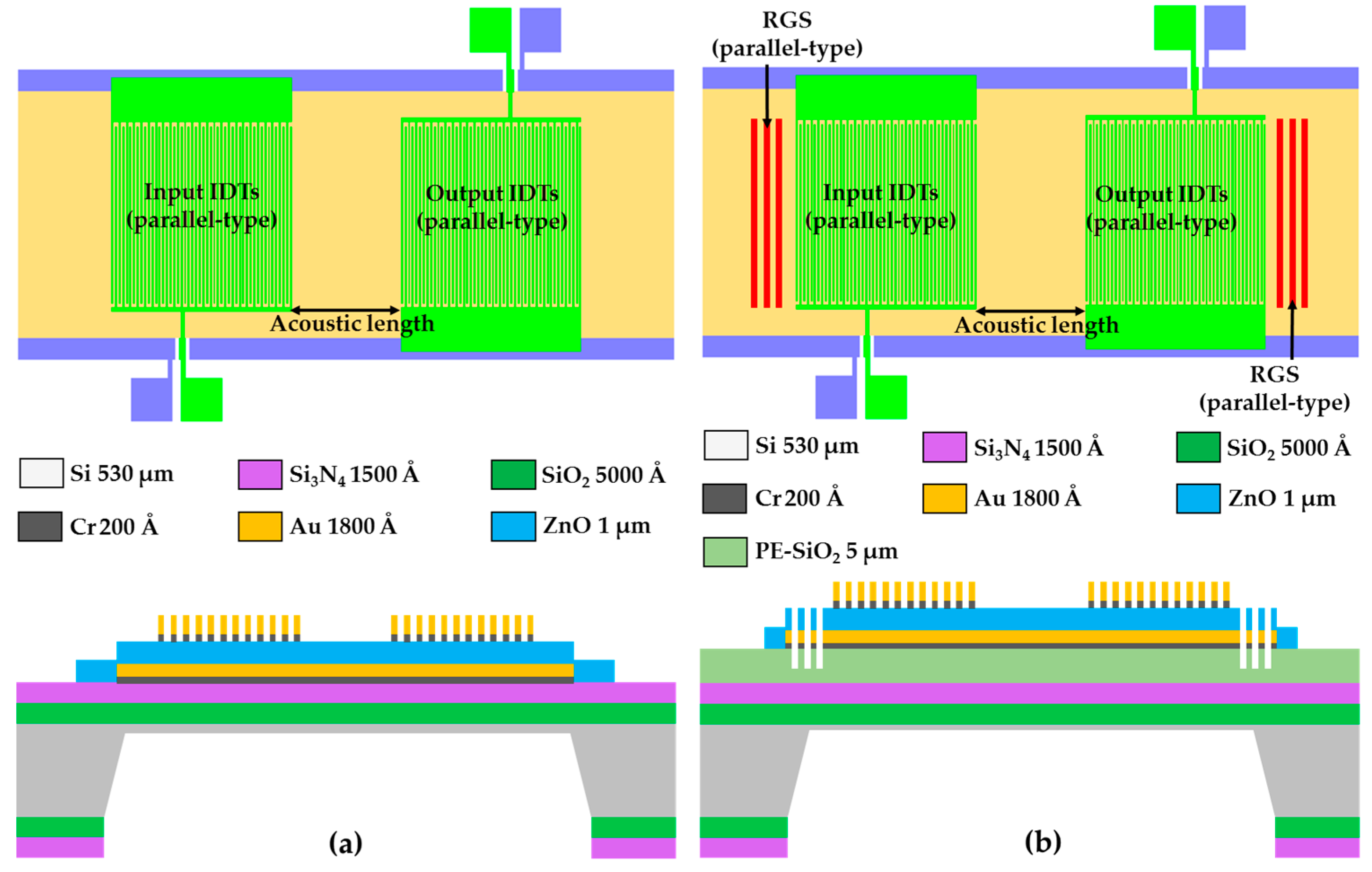
2.2. Layout Specification of the Proposed Focus-Type IDTs/RGS
3. Experimental
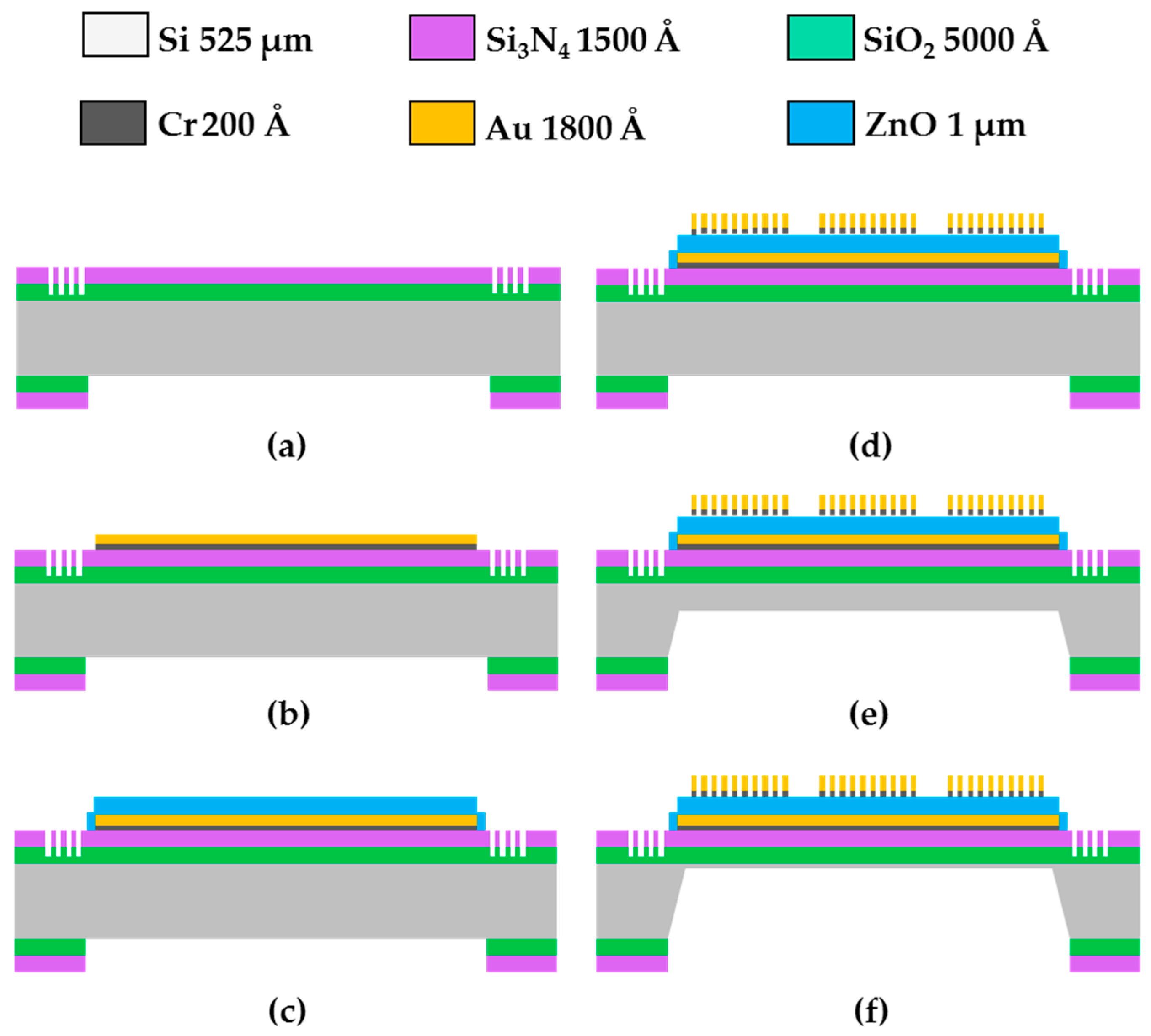
3.1. Fabrication of the FPW Device
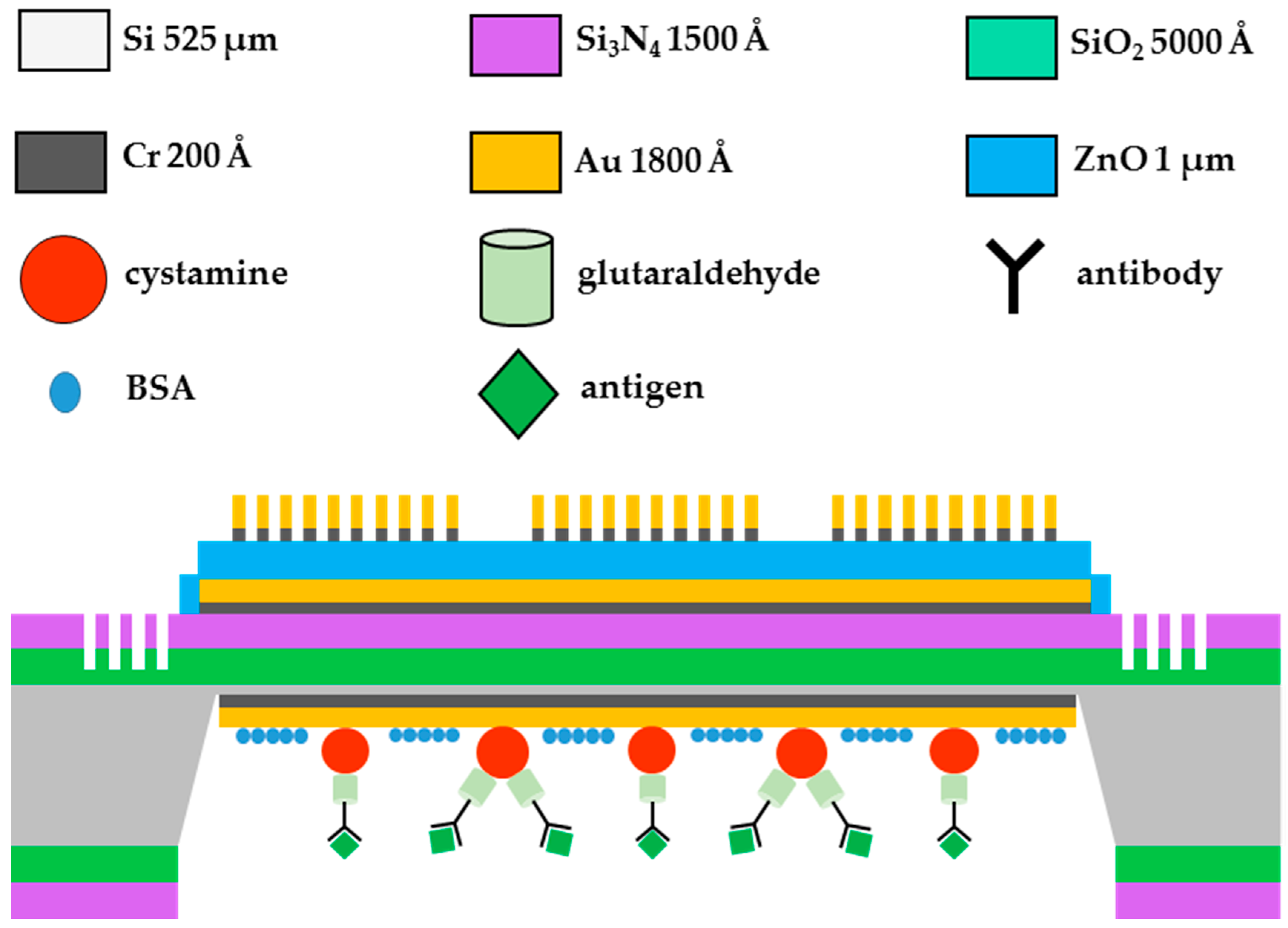
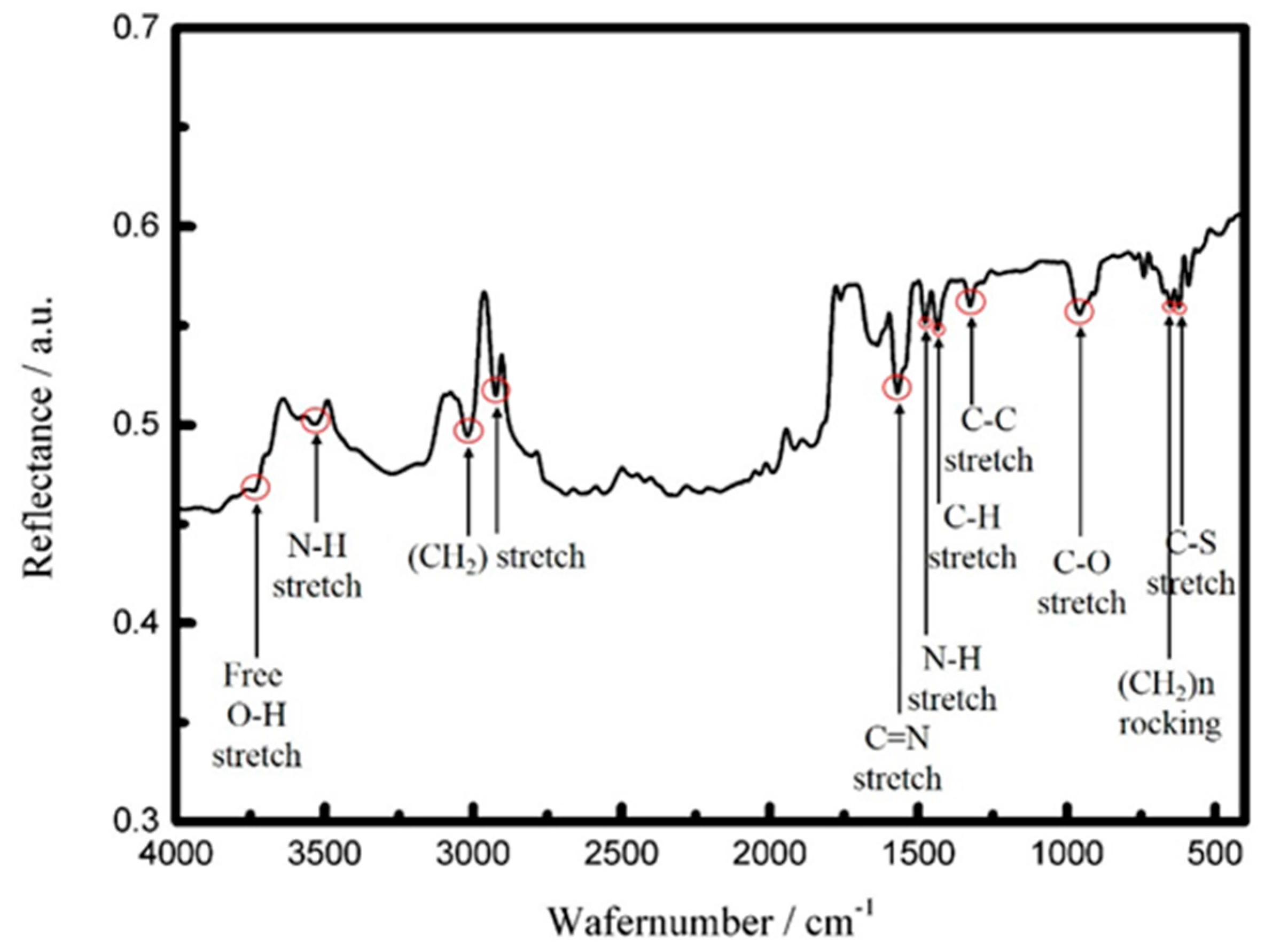
3.2. Fabrication of the FPW-Based CEA Biosensor
- Wash three times with 1 c.c. of phosphate-buffered saline solution;
- Dip 10 μL of diluted rabbit CEA antibody (27 °C, 2 h);
- Inject 200 μL of Tween-20 wash buffer three times;
- Inject 10 μL of 1 wt % BSA solution (27 °C, 0.5 h);
- Inject 200 μL of Tween-20 wash buffer three times; and
- Inject 10 μL of diluted human CEA antigen (27 °C, 2 h).
4. Results and Discussion
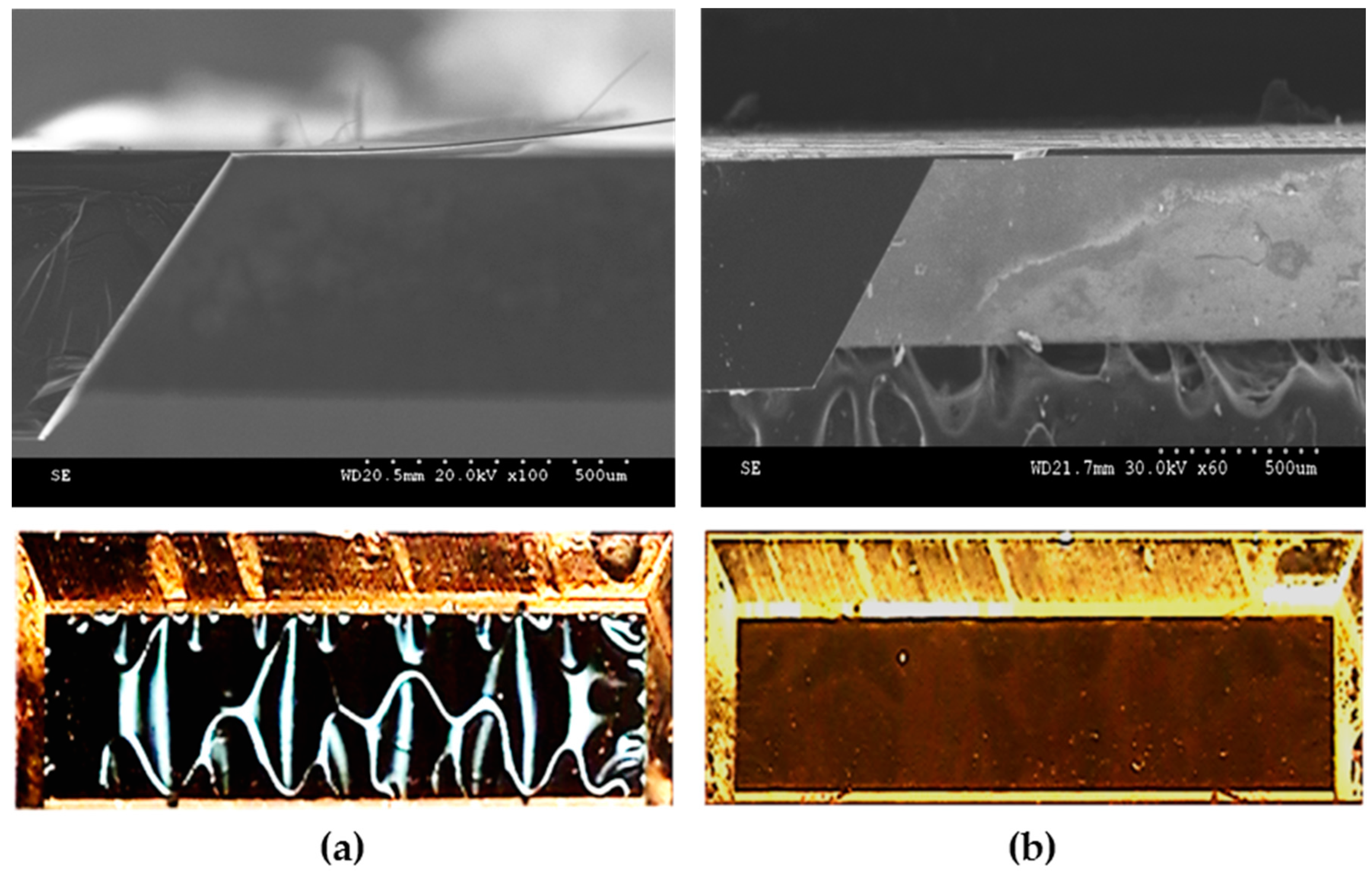
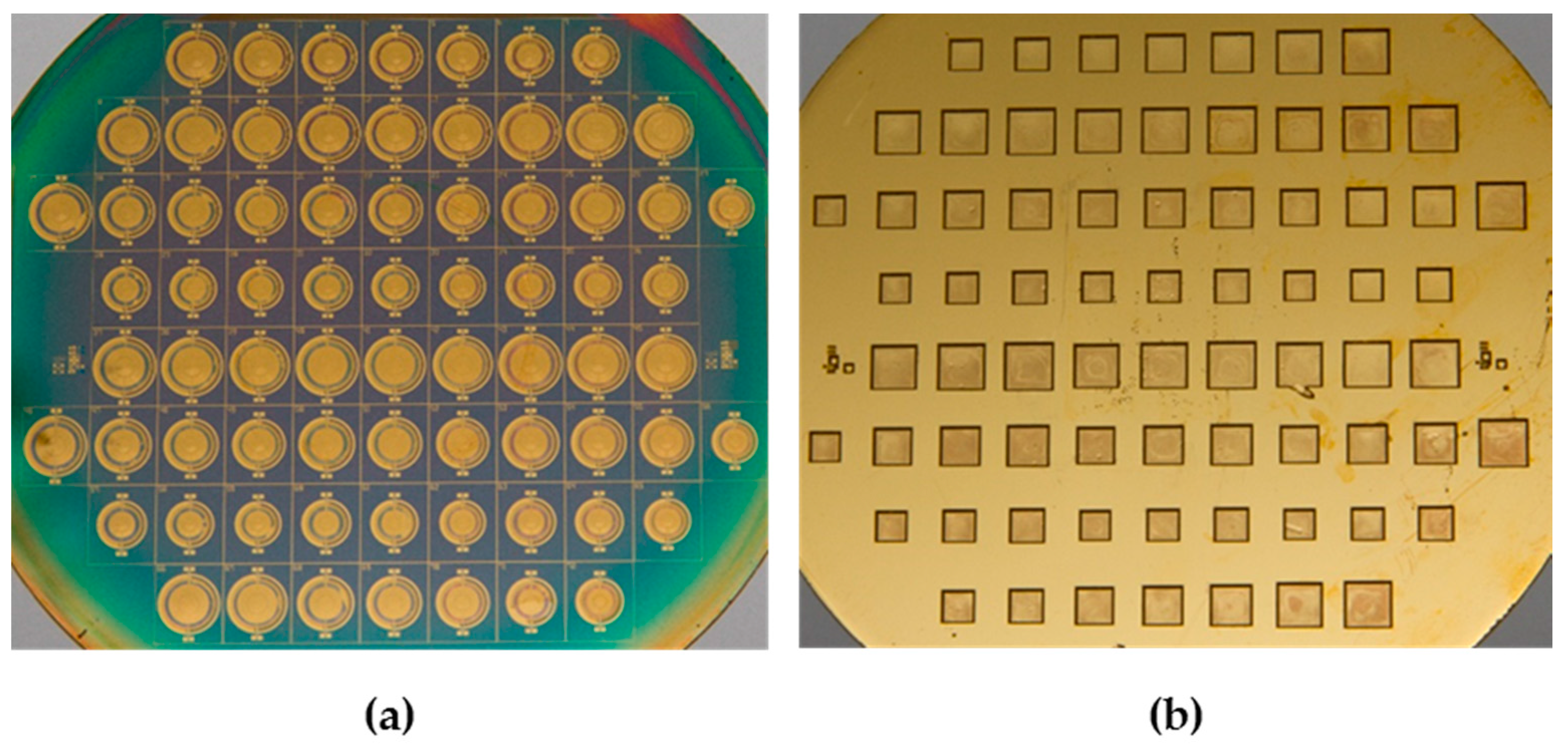
4.1. Structural Inspection and Fabrication Yield Investigation
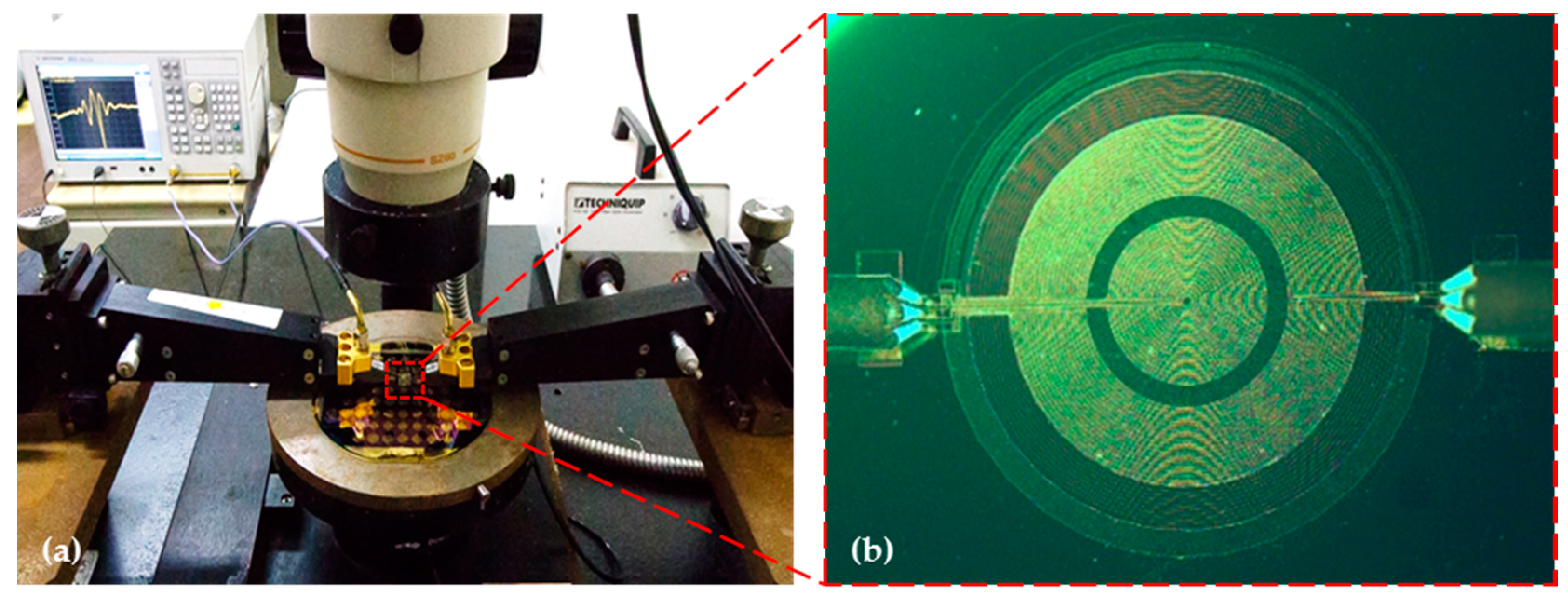
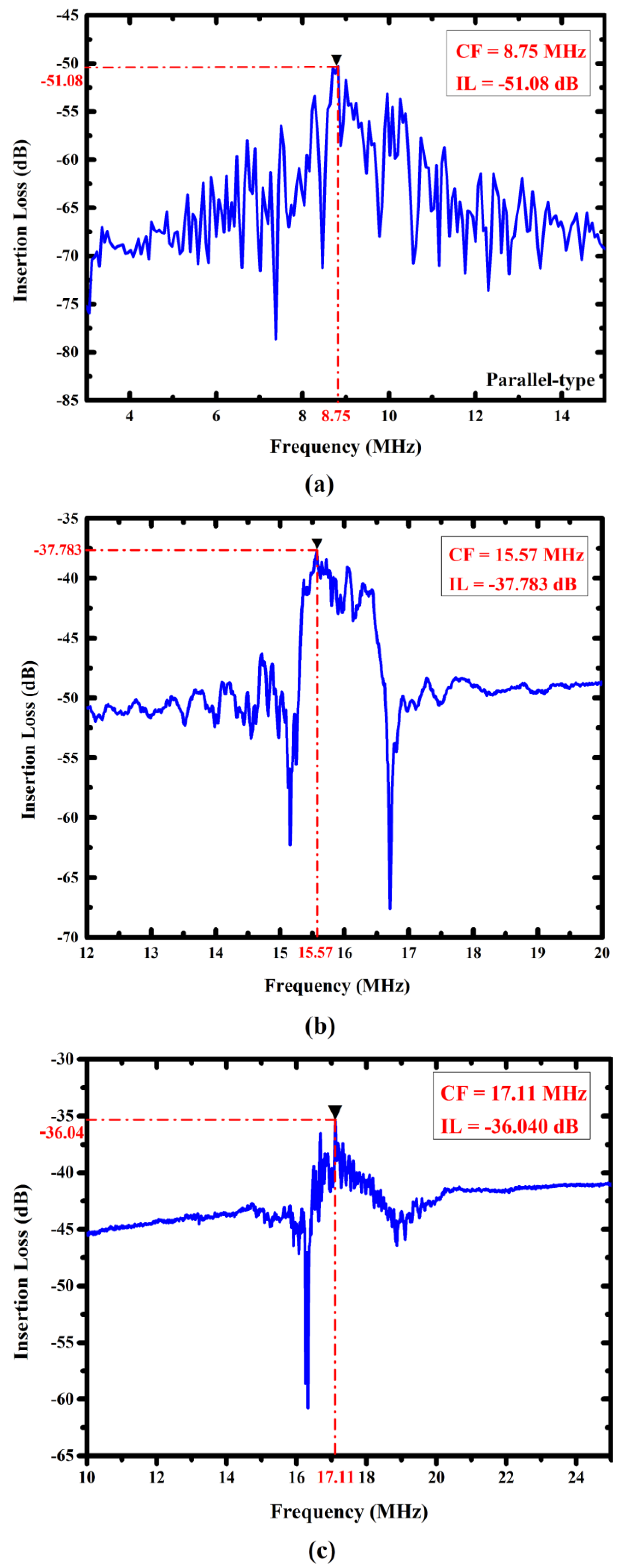
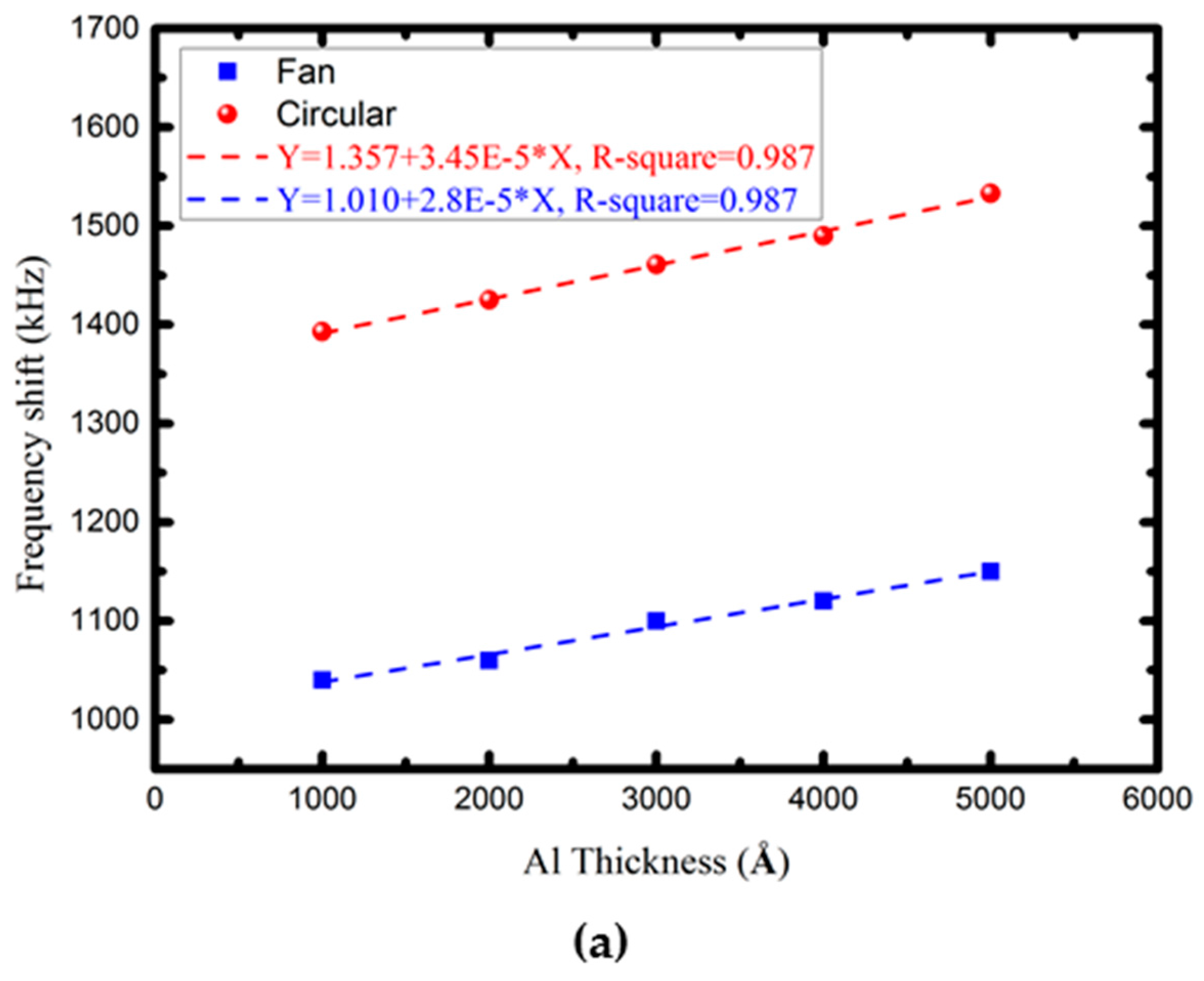
4.2. Characterization of the Proposed FPW Devices
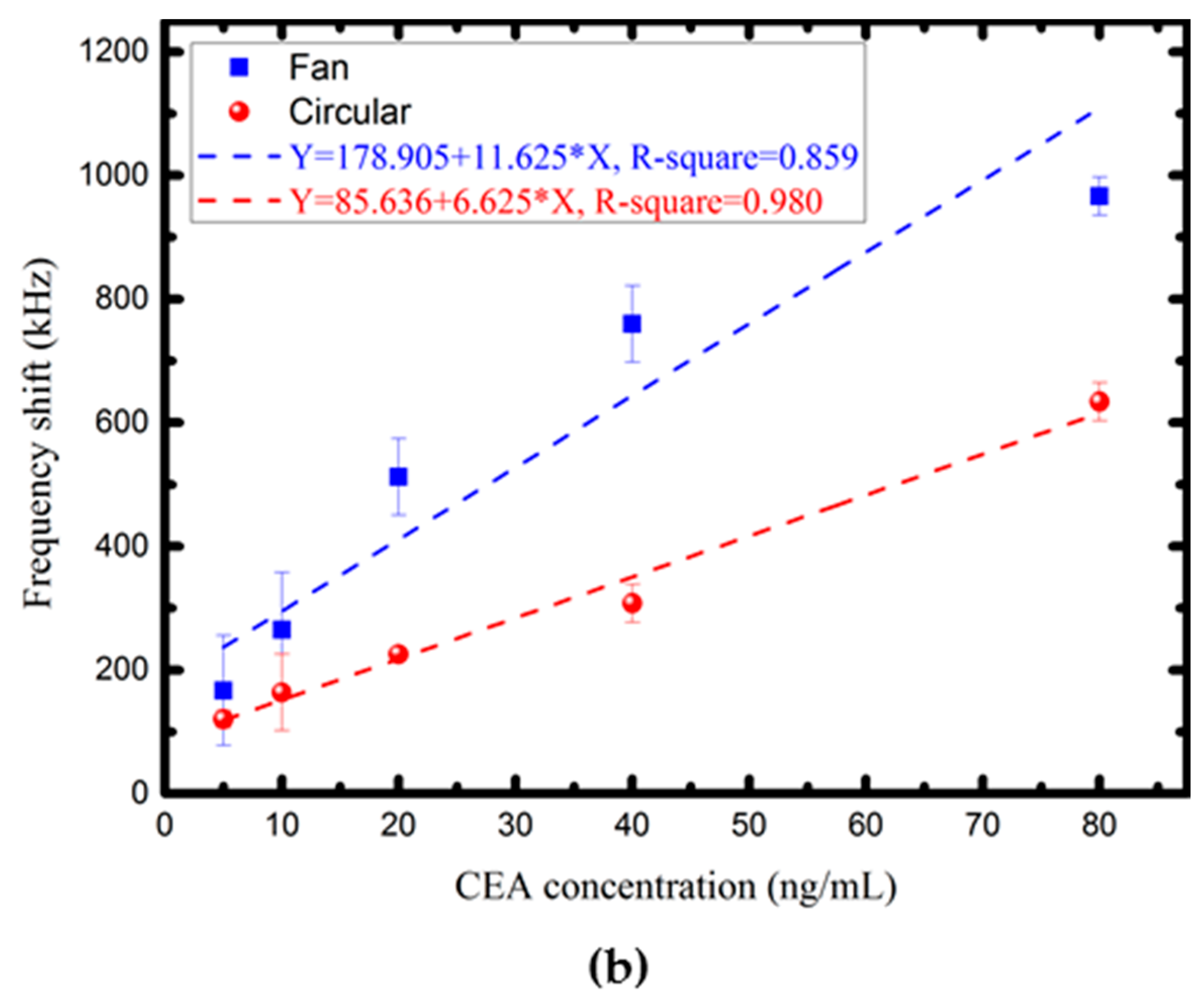
4.3. Characterization of the Proposed FPW-CEA Biosensor
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ballantine, D.S., Jr.; White, R.M.; Martin, S.J.; Ricco, A.J.; Frye, G.C.; Zellars, E.T.; Wohltjen, H. Acoustic Wave Sensors: Theory, Design, and Physicochemical Application; Academic Press: Salt Lake, UT, USA, 1996. [Google Scholar]
- Galipeau, D.W.; Story, P.R.; Vetelino, K.A.; Mileham, R.-D. Surface acoustic wave microsensors and applications. Smart Mater. Struct. 1997, 6, 658–667. [Google Scholar] [CrossRef]
- Gardner, J.W.; Varadan, V.K.; Awadelkarim, O.O. Microsensors MEMS and Smart Devices; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; pp. 309–313. [Google Scholar]
- Velekoop, M.J. Acoustic wave sensors and their technology. Ultrasonics 1998, 36, 7–14. [Google Scholar] [CrossRef]
- Motamedi, M.E.; White, R.M. Acoustic Sensor in Semiconductor Sensor; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1994; pp. 381–383. [Google Scholar]
- Rocha-Gaso, M.I.; Iborra, C.M.; Baides, A.M.; Vives, A.A. Surface Generated Acoustic Wave Biosensors for the Detection of Pathogens. Sensors 2009, 9, 5740–5769. [Google Scholar] [CrossRef] [PubMed]
- Huang, I.Y.; Lee, M.C. Development of a FPW allergy biosensor for human IgE detection by MEMS and cystamine-based SAM technologies. Sens. Actuators B 2008, 132, 340–348. [Google Scholar] [CrossRef]
- Lin, C.Y.; Huang, I.Y.; Lan, J.W. Improvement of insertion loss and quality factor of flexural plate-wave based alpha-fetoprotein biosensor using groove-type reflective grating structures. J. Micro/Nanolithogr. MEMS MOEMS 2013, 12, 013017. [Google Scholar] [CrossRef]
- IARC Launches the Definitive Cancer Statistics Resource. Available online: https://www.iarc.fr/en/media-centre/pr/2010/pdfs/pr201_E.pdf (accessed on 5 October 2016).
- Latest World Cancer Statistics Global Cancer Burden Rises to 14.1 Million New Cases in 2012: Marked Increase in Breast Cancers Must Be Addressed. Available online: https://www.iarc.fr/en/media-centre/pr/2013/pdfs/pr223_E.pdf (accessed on 5 October 2016).
- Wink, T.; Van Zuilen, S.J.; Bult, A.; Van Bennkom, W.P. Self-assembled monolayers for biosensors. Analyst 1997, 122, 43–50. [Google Scholar] [CrossRef]
- Huang, I.Y.; Lee, M.C.; Wang, C.C. Development of a flexural plate-wave (FPW) immunoglobulin-E allergy bio-sensing microsystem. Sens. Actuators B 2012, 162, 184–193. [Google Scholar] [CrossRef]
- Luginbuhl, P.; Collins, S.D.; Racine, G.A.; Gretillat, M.A.; De Rooij, N.F.; Brooks, K.G.; Setter, N. Microfabricated Lamb wave device based on PZT sol–gel thin film for mechanical transport of solid particles and liquids. J. Microelectromech. Syst. 1997, 6, 337–346. [Google Scholar] [CrossRef]
- Sankaranarayanan, K.R.S.; Bhethanabotla, V.R. Design of Efficient Focused Surface Acoustic Wave Devices for Potential Microfluidic Applications. J. Appl. Phys. 2007, 103, 064518. [Google Scholar] [CrossRef]
- Quirk, M.; Serda, J. Semiconductor Manufacturing Technology; Prentice-Hall, Inc.: Upper Saddle River, NJ, USA, 2000; pp. 150–152. [Google Scholar]

| Parameter | Value |
|---|---|
| IDTs finger width/gap | 25 μm/25 μm |
| Wavelength of IDTs | 100 μm |
| Number of IDTs’ finger pairs | 10 |
| RGS pairs | 10 |
| RGS finger width/gap | 25 μm/25 μm |
| Separation between the RGS and IDTs | 37.5 μm |
| References | IDTs/RGS Geometry | Temperature of the Si Etching Process (°C) | Thickness of the Floating Thin-Plate (m) | Insertion Loss (dB) | Fabrication Yield (%) |
|---|---|---|---|---|---|
| I-Yu Huang [7] | Parallel (without RGS) | 60 (one-step) | 5.29 ± 3.5 | −51.08 | <10 |
| Chang-Yu Lin [8] | Parallel (with RGS) | 80 (one-step) | 6.32 ± 5 | −40.85 | 10–15 |
| This Study | Fan-shaped (with RGS) | 60/27 (two-step) | 16.86 ± 2 | −37.78 | 50–86.11 |
| Circular (with RGS) | −36.04 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, J.-W.; Huang, I.-Y.; Lin, Y.-C.; Lin, C.-Y.; Chen, J.-L.; Hsieh, C.-H. Development of an FPW Biosensor with Low Insertion Loss and High Fabrication Yield for Detection of Carcinoembryonic Antigen. Sensors 2016, 16, 1729. https://doi.org/10.3390/s16111729
Lan J-W, Huang I-Y, Lin Y-C, Lin C-Y, Chen J-L, Hsieh C-H. Development of an FPW Biosensor with Low Insertion Loss and High Fabrication Yield for Detection of Carcinoembryonic Antigen. Sensors. 2016; 16(11):1729. https://doi.org/10.3390/s16111729
Chicago/Turabian StyleLan, Je-Wei, I-Yu Huang, Yu-Cheng Lin, Chang-Yu Lin, Jian-Lin Chen, and Chia-Hsu Hsieh. 2016. "Development of an FPW Biosensor with Low Insertion Loss and High Fabrication Yield for Detection of Carcinoembryonic Antigen" Sensors 16, no. 11: 1729. https://doi.org/10.3390/s16111729





