Nonenzymatic Glucose Sensor Based on In Situ Reduction of Ni/NiO-Graphene Nanocomposite
Abstract
:1. Introduction
2. Experimental Methods
2.1. Regents and Apparatus
2.2. Rinse and Activation of SPEs
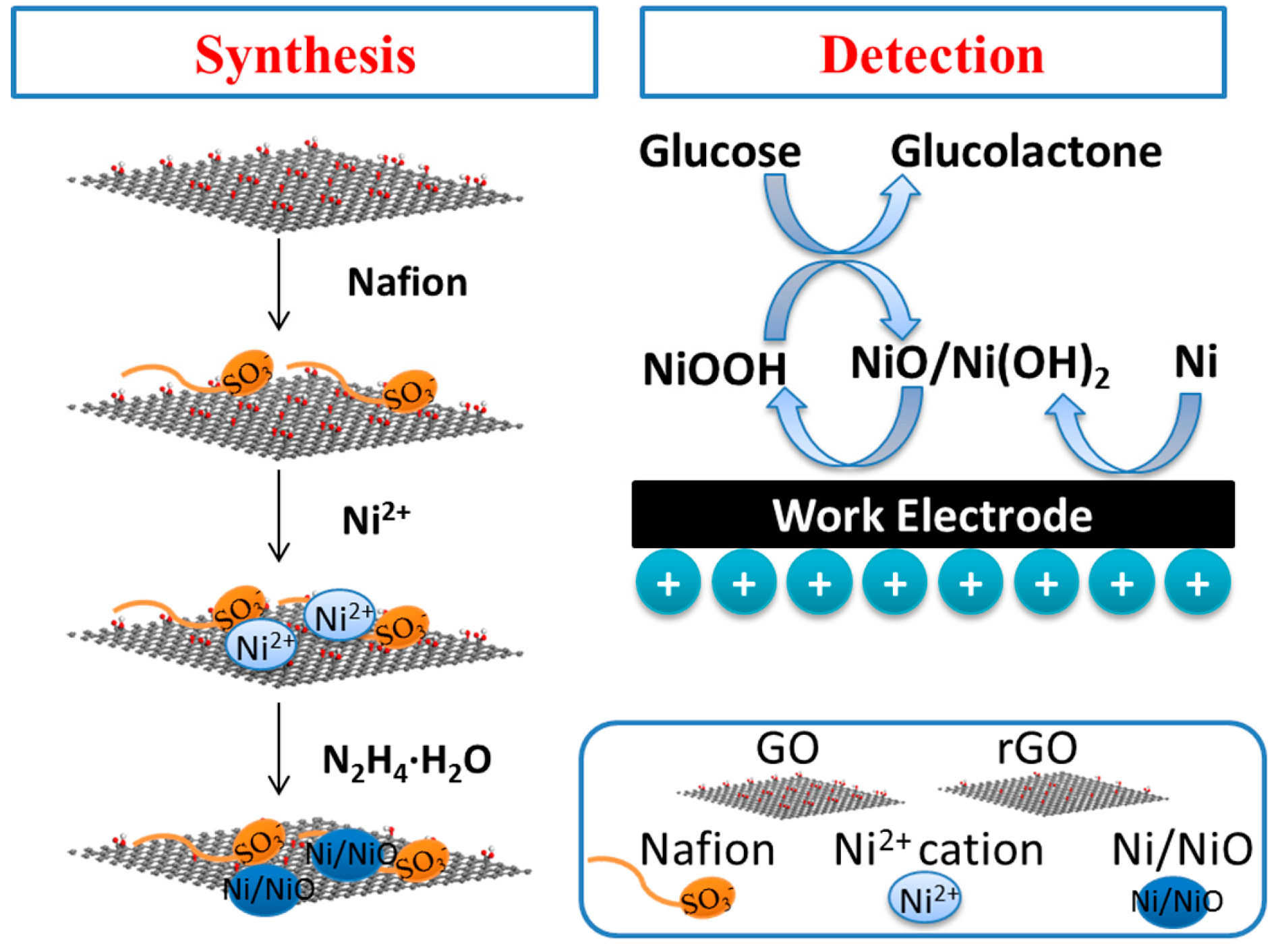
2.3. In Situ Synthesis of Ni/NiO-rGO Modified SPEs
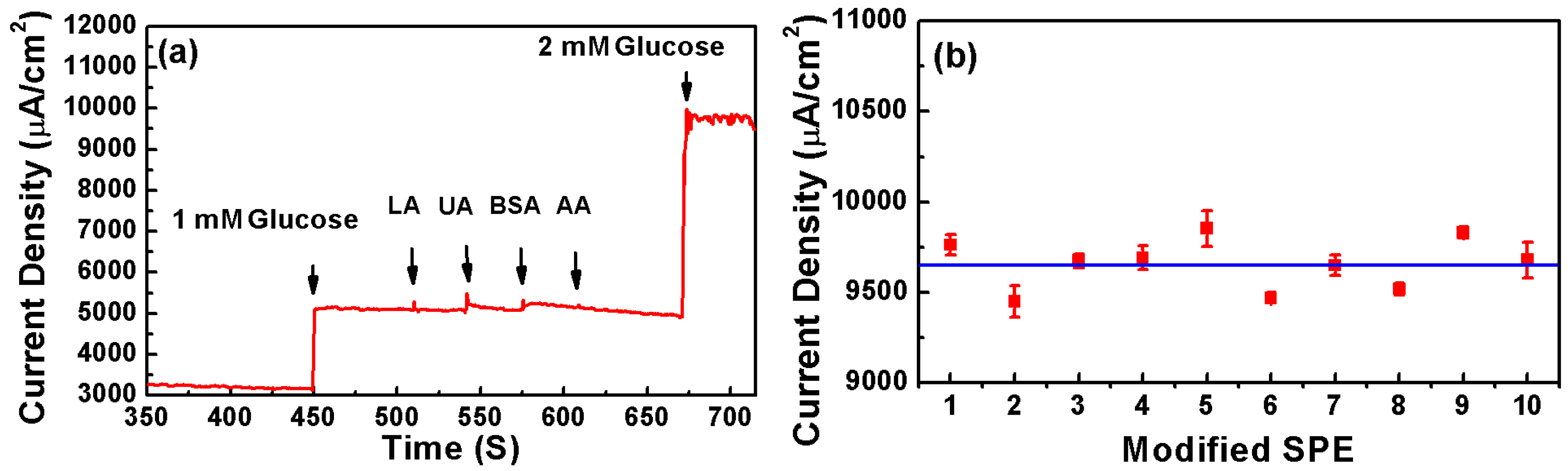
3. Results and Discussion
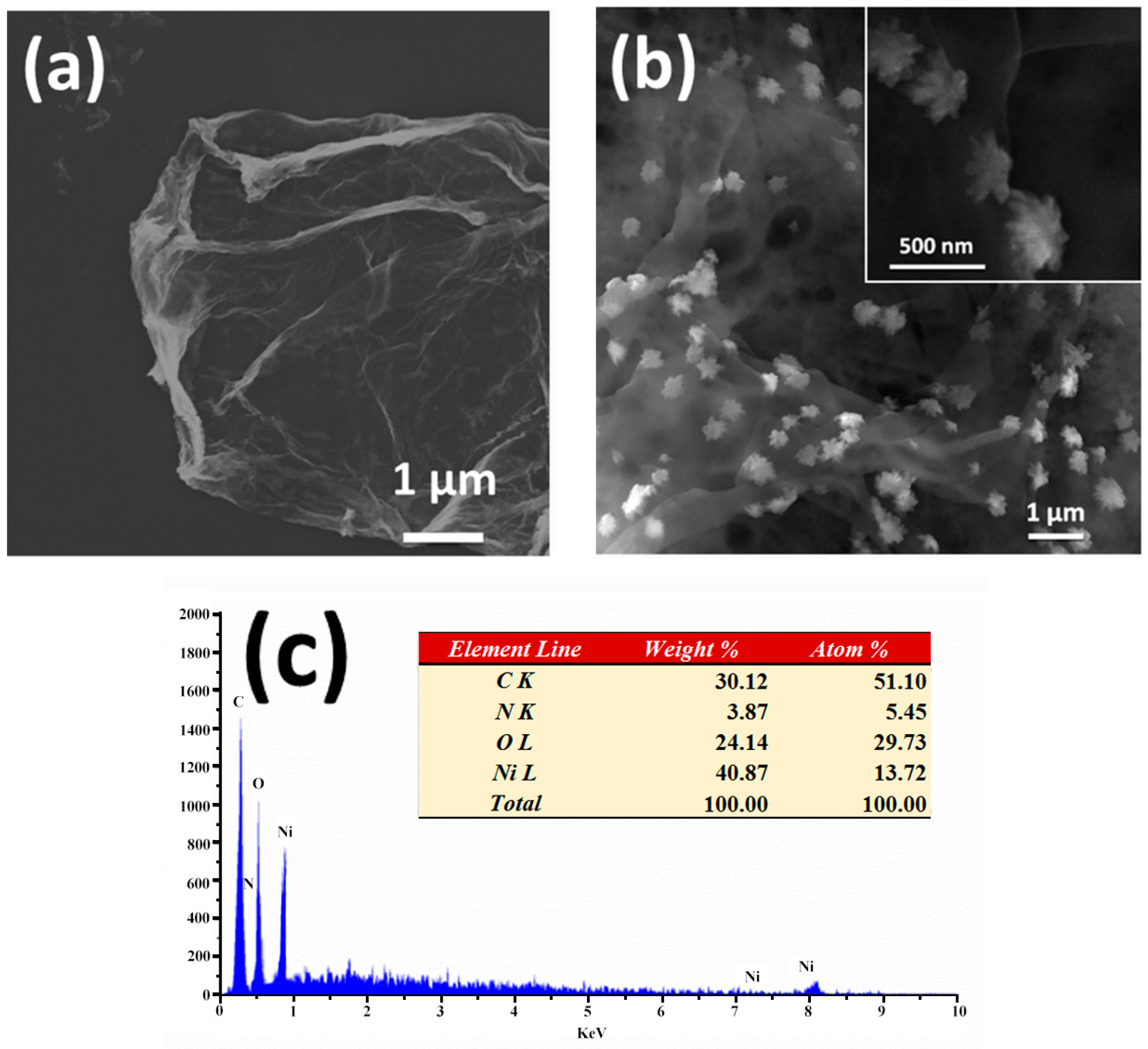
3.1. Morphology and Structure Characterization
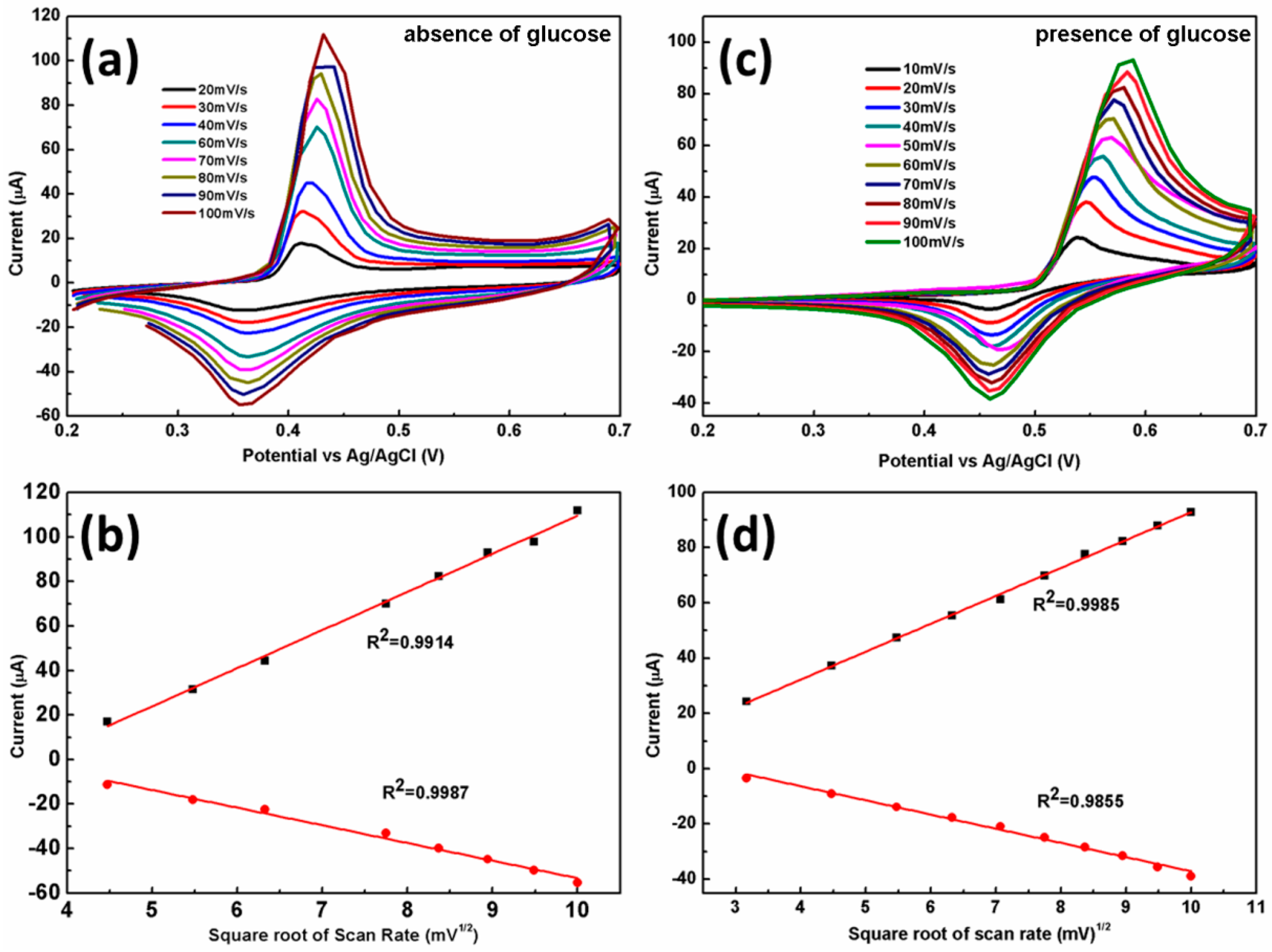
3.2. Electrochemical Characterizations
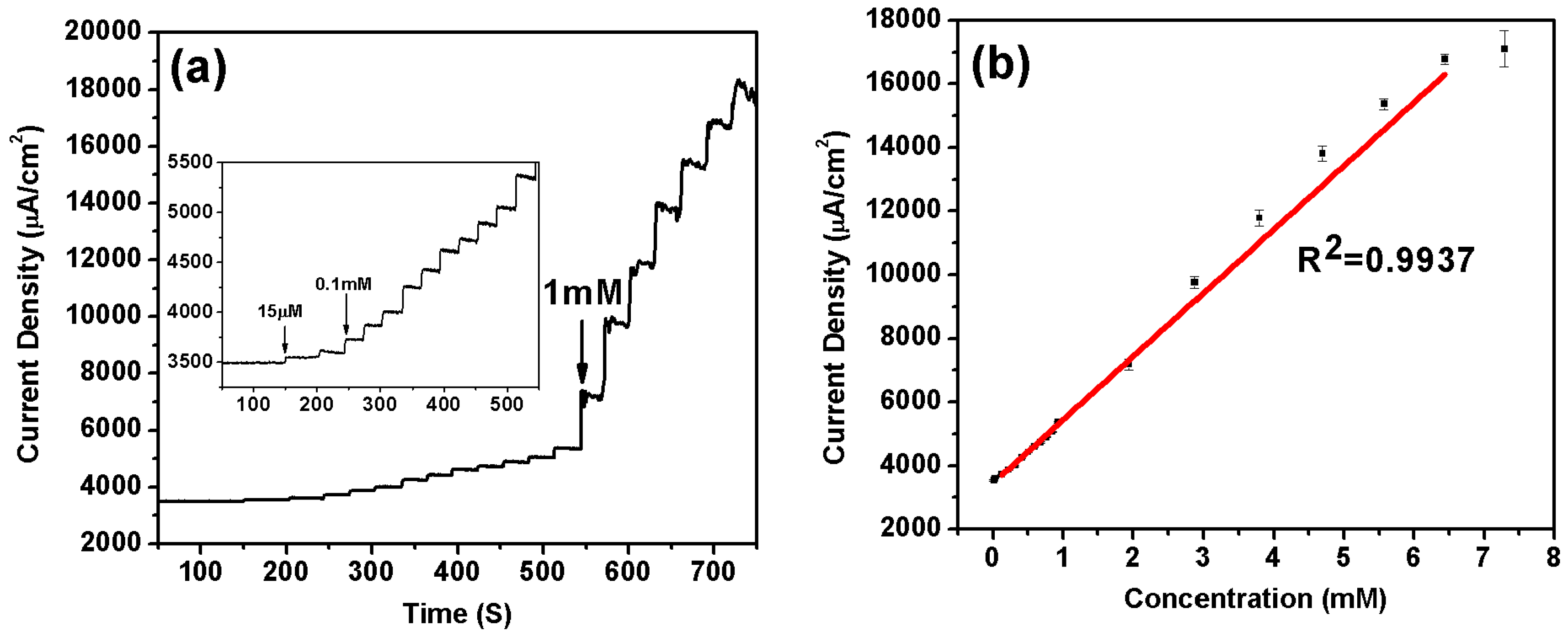
3.3. Performance of Ni/NiO-Nafion-rGO/SPEs Nonenzymatic Glucose Sensors
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Scognamiglio, V. Nanotechnology in glucose monitoring: Advances and challenges in the last 10 years. Biosens. Bioelectron. 2013, 47, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yan, X.; Zhao, J.; Liu, X.; Song, Y.; Luo, N.; Zhang, Y. Improved glucose electrochemical biosensor by appropriate immobilization of nano-ZnO. Colloids Surf. B 2011, 82, 168–72. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Yan, X.Q.; Luo, N.; Song, Y.; Zhang, Y. ZnO nanotetrapod network as the adsorption layer for the improvement of glucose detection via multiterminal electron-exchange. Colloids Surf. A 2010, 361, 169–173. [Google Scholar] [CrossRef]
- Zhang, W.D.; Chen, J.; Jiang, L.C.; Yu, Y.X.; Zhang, J.Q. A highly sensitive nonenzymatic glucose sensor based on NiO-modified multi-walled carbon nanotubes. Microchim. Acta 2010, 168, 259–265. [Google Scholar] [CrossRef]
- Xiao, F.; Zhao, F.Q.; Mei, D.P.; Mo, Z.R.; Zeng, B.Z. Nonenzymatic glucose sensor based on ultrasonic-electrode position of bimetallic PtM (M = Ru, Pd and Au) nanoparticles on carbon nanotubes-ionic liquid composite film. Biosens. Bioelectron. 2009, 24, 3481–3486. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Sun, X.W.; Cai, X.P.; Lei, Y.; Song, L.; Xie, S.S. Nonenzymatic glucose sensor using freestanding single-wall carbon nanotube films. Electrochem. Solid State Lett. 2007, 10, J58–J60. [Google Scholar] [CrossRef]
- Gao, H.C.; Wang, Y.X.; Xiao, F.; Ching, C.B.; Duan, H.W. Growth of Copper Nanocubes on Graphene Paper as Free-Standing Electrodes for Direct Hydrazine Fuel Cells. J. Phys. Chem. C 2012, 116, 7719–7725. [Google Scholar] [CrossRef]
- Xu, F.G.; Sun, Y.J.; Zhang, Y.; Shi, Y.; Wen, Z.W.; Li, Z. Graphene-Pt nanocomposite for nonenzymatic detection of hydrogen peroxide with enhanced sensitivity. Electrochem. Commun. 2011, 13, 1131–1134. [Google Scholar] [CrossRef]
- Lu, L.M.; Li, H.B.; Qu, F.; Zhang, X.B.; Shen, G.L.; Yu, R.Q. In situ synthesis of palladium nanoparticle-graphene nanohybrids and their application in nonenzymatic glucose biosensors. Biosens. Bioelectron. 2011, 26, 3500–3504. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Teng, H.; Hou, H.Q.; You, T.Y. Nonenzymatic glucose sensor based on renewable electrospun Ni nanoparticle-loaded carbon nanofiber paste electrode. Biosens. Bioelectron. 2009, 24, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.M.; Zhang, L.; Qu, F.L.; Lu, H.X.; Zhang, X.B.; Wu, Z.S.; Huan, S.Y.; Wang, Q.A.; Shen, G.L.; Yu, R.Q. A nano-Ni based ultrasensitive nonenzymatic electrochemical sensor for glucose: enhancing sensitivity through a nanowire array strategy. Biosens. Bioelectron. 2009, 25, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Ci, S.; Huang, T.; Wen, Z.; Cui, S.; Mao, S.; Steeber, D.A.; Chen, J. Nickel oxide hollow microsphere for non-enzyme glucose detection. Biosens. Bioelectron. 2014, 54, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, F.G.; Sun, Y.J.; Shi, Y.; Wen, Z.W.; Li, Z. Assembly of Ni(OH)2 nanoplates on reduced graphene oxide: A two dimensional nanocomposite for enzyme-free glucose sensing. J. Mater. Chem. 2011, 21, 16949–16954. [Google Scholar] [CrossRef]
- Wang, G.F.; He, X.P.; Wang, L.L.; Gu, A.X.; Huang, Y.; Fang, B.; Geng, B.Y.; Zhang, X.J. Non-enzymatic electrochemical sensing of glucose. Microchim. Acta 2013, 180, 161–186. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Yang, W.; Zhou, M.; Hu, X. Facile one-step microwave-assisted route towards Ni nanospheres/reduced graphene oxide hybrids for non-enzymatic glucose sensing. Sensors 2012, 12, 4860–4869. [Google Scholar] [CrossRef] [PubMed]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Geim, A.K. Graphene: status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Liao, Q.; Song, Y.; Ma, S. Reduced graphene oxide-functionalized high electron mobility transistors for novel recognition pattern label-free DNA sensors. Small 2013, 9, 4045–4050. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, Q.; Chu, M.; Liu, S.; Zhang, Y. Structure effect on graphene-modified enzyme electrode glucose sensors. Biosens. Bioelectron. 2014, 52, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Du, D.; Gunaratne, D.; Colby, R.; Lin, Y.; Laskin, J. Polyoxometalate-Graphene Nanocomposite Modified Electrode for Electrocatalytic Detection of Ascorbic Acid. Electroanalysis 2014, 26, 178–183. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, X.P.; Sun, Y.J.; Shi, Y.; Dai, H.C.; Ni, P.J.; Hu, J.T.; Li, Z.; Song, Y.H.; Wang, L. Electrochemical Deposition of Nickel Nanoparticles on Reduced Graphene Oxide Film for Nonenzymatic Glucose Sensing. Electroanalysis 2013, 25, 959–966. [Google Scholar] [CrossRef]
- Yang, J.; Yu, J.H.; Rudi Strickler, J.; Chang, W.J.; Gunasekaran, S. Nickel nanoparticle-chitosan-reduced graphene oxide-modified screen-printed electrodes for enzyme-free glucose sensing in portable microfluidic devices. Biosens. Bioelectron. 2013, 47, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, Q.; Liu, S.; Xu, W.; Liu, Y.; Zhang, Y. CuNiO nanoparticles assembled on graphene as an effective platform for enzyme-free glucose sensing. Anal. Chim. Acta 2015, 858, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pedrero, M.; Sakslund, H.; Hammerich, O.; Pingarron, J. Electrochemical activation of screen-printed carbon strips. Analyst 1996, 121, 345–350. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Z.; Zheng, L.; Pan, Y.; Sun, G.; Li, L. Self-powered, visible-light photodetector based on thermally reduced graphene oxide-ZnO (rGO-ZnO) hybrid nanostructure. J. Mater. Chem. 2012, 22, 2589–2595. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large area, few-layer graphene films on arbitrary substrates by chemical vapor deposition. Nano lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.; Marques, P.A.A.P.; Granadeiro, C.M.; Nogueira, H.I.S.; Singh, M.K.; Gracio, J. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 2009, 21, 4796–4802. [Google Scholar] [CrossRef]
- Kim, J.H.; Kwon, S.Y.; Bhattacharjya, D.; Chai, G.S.; Yu, J.S. High-performance quaternary PtRuIrNi electrocatalysts with hierarchical nanostructured carbon support. J. Catal. 2013, 306, 133–145. [Google Scholar] [CrossRef]
- Grim, S.O.; Matienzo, L.J.; Swartz, W.E. X-ray photoelectron spectroscopy of some nickel dithiolate complexes. J. Am. Chem. Soc. 1972, 94, 5116–5117. [Google Scholar] [CrossRef]
- Li-Shing, H.; Williams, R.S. Electronic-structure study of the Ni3Ga and the NiIn intermetallic compounds using X-ray photoemission spectroscopy. J. Phys. Chem. Solids 1994, 55, 305–312. [Google Scholar] [CrossRef]
- Lu, W.; Qin, X.; Asiri, A.M.; Al-Youbi, A.O.; Sun, X. Ni foam: a novel three-dimensional porous sensing platform for sensitive and selective nonenzymatic glucose detection. Analyst 2013, 138, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Prestgard, M.; Tiwari, A. A review of recent advances in nonenzymatic glucose sensors. Mater. Sci. Eng. C 2014, 41, 100–118. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.; Alex, S.; Siegel, G.; Tiwari, A. Enzymatic glucose sensor based on Au nanoparticle and plant-like ZnO film modified electrode. Mater. Sci. Eng. C 2015, 46, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.W.; Wang, C.H.; Wang, Y.C.; Chang, J.K. Ionic-liquid-enhanced glucose sensing ability of non-enzymatic Au/graphene electrodes fabricated using supercritical CO2 fluid. Biosens. Bioelectron. 2013, 46, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.H.; Jiao, Q.F.; Zhang, C.Y.; Zuo, X.X.; Xiao, X.; Liang, Y.; Nan, J.M. Amperometric nonenzymatic determination of glucose based on a glassy carbon electrode modified with nickel(II) oxides and graphene. Microchim. Acta 2013, 180, 477–483. [Google Scholar] [CrossRef]


| Sensor | Detect Limit (μM) | Linear Range (mM) | Sensitivity (μA/mM∙cm−2) | Reference |
|---|---|---|---|---|
| AuNPs-graphene/ILE | 0.062/0.183 | / | 97.8/16.3 | [37] |
| GR-Nafion-PdNPs/GCE | 1 | 0.01–5.0 | / | [11] |
| CS-rGO–NiNPs/SPE | 4.1 | 0.2–9.0 | 318.4 | [24] |
| rGO-Ni(OH)2/GCE | 0.6 | 0.002–3.1 | 11.43 | [15] |
| NiO-GR/GCE | 5 | 0.02–4.5 | / | [38] |
| Ni/NiO-rGO-Nafion/SPE | 1.8 | 0.03–6.44 | 1997 | This work |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Z.; Liao, Q.; Liu, S.; Kang, Z.; Zhang, Y. Nonenzymatic Glucose Sensor Based on In Situ Reduction of Ni/NiO-Graphene Nanocomposite. Sensors 2016, 16, 1791. https://doi.org/10.3390/s16111791
Zhang X, Zhang Z, Liao Q, Liu S, Kang Z, Zhang Y. Nonenzymatic Glucose Sensor Based on In Situ Reduction of Ni/NiO-Graphene Nanocomposite. Sensors. 2016; 16(11):1791. https://doi.org/10.3390/s16111791
Chicago/Turabian StyleZhang, Xiaohui, Zheng Zhang, Qingliang Liao, Shuo Liu, Zhuo Kang, and Yue Zhang. 2016. "Nonenzymatic Glucose Sensor Based on In Situ Reduction of Ni/NiO-Graphene Nanocomposite" Sensors 16, no. 11: 1791. https://doi.org/10.3390/s16111791





