Design and Development for Capacitive Humidity Sensor Applications of Lead-Free Ca,Mg,Fe,Ti-Oxides-Based Electro-Ceramics with Improved Sensing Properties via Physisorption
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of the Sensing Nanomaterial
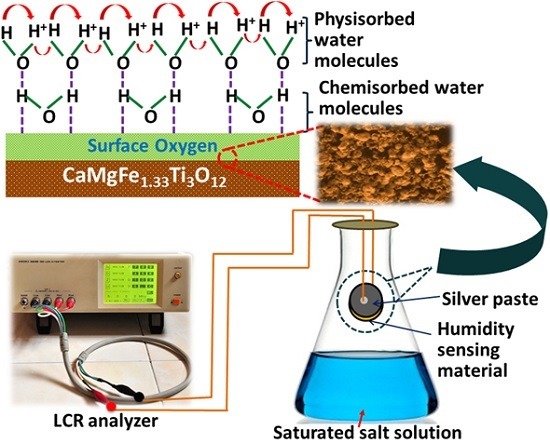
2.2. Fabrication of Humidity Sensor
2.3. Physical Characterizations
2.4. Humidity Sensor Measurements
3. Results and Discussion
3.1. Structural and Morphological Characterization
3.2. Humidity Sensing Measurements
4. Conclusions
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, T.-H.; Chou, J.-C.; Sun, T.-P.; Hsiung, S.-K. A device for skin moisture and environment humidity detection. Sens. Actuators B Chem. 2008, 134, 206–212. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, C. Humidity sensors: A review of materials and mechanisms. Sens. Lett. 2005, 3, 274–295. [Google Scholar] [CrossRef]
- Kim, S.-J.; Park, J.-Y.; Lee, S.-H.; Yi, S.-H. Humidity sensors using porous silicon layer with mesa structure. J. Phys. D Appl. Phys. 2000, 33, 1781. [Google Scholar] [CrossRef]
- Muto, S.; Suzuki, O.; Amano, T.; Morisawa, M. A plastic optical fibre sensor for real-time humidity monitoring. Meas. Sci. Technol. 2003, 14, 746. [Google Scholar] [CrossRef]
- Shuk, P.; Greenblatt, M. Solid electrolyte film humidity sensor. Solid State Ionics 1998, 113, 229–233. [Google Scholar] [CrossRef]
- Kulkarni, M.V.; Viswanath, A.K.; Khanna, P. Synthesis and humidity sensing properties of conducting poly (N-methyl aniline) doped with different acids. Sens. Actuators B Chem. 2006, 115, 140–149. [Google Scholar] [CrossRef]
- Shah, J.; Kotnala, R.; Singh, B.; Kishan, H. Microstructure-dependent humidity sensitivity of porous mgfe 2O4–CeO2 ceramic. Sens. Actuators B Chem. 2007, 128, 306–311. [Google Scholar] [CrossRef]
- Kulwicki, B.M. Humidity sensors. J. Am. Ceramic Soc. 1991, 74, 697–708. [Google Scholar] [CrossRef]
- Traversa, E. Ceramic sensors for humidity detection: The state-of-the-art and future developments. Sens. Actuators B Chem. 1995, 23, 135–156. [Google Scholar] [CrossRef]
- Faia, P.; Furtado, C.; Ferreira, A. Humidity sensing properties of a thick-film titania prepared by a slow spinning process. Sens. Actuators B Chem. 2004, 101, 183–190. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, K.; Ouyang, S.; Luo, L.; Hu, H.; Zhang, Q.; Zhu, Z. Detection of humidity based on quartz crystal microbalance coated with zno nanostructure films. Phys. B Condens. Matter 2005, 368, 94–99. [Google Scholar] [CrossRef]
- Sberveglieri, G.; Rinchetti, G.; Groppelli, S.; Faglia, G. Capacitive humidity sensor with controlled performances, based on porous Al2O3 thin film growm on SiO2-Si substrate. Sens. Actuators B Chem. 1994, 19, 551–553. [Google Scholar] [CrossRef]
- Cheng, B.; Tian, B.; Xie, C.; Xiao, Y.; Lei, S. Highly sensitive humidity sensor based on amorphous Al2O3 nanotubes. J. Mater. Chem. 2011, 21, 1907–1912. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, G. Humidity sensing properties of (Ba,Sr)TiO3 thin films grown by hydrothermal-electrochemical method. Sens. Actuators B Chem. 2002, 85, 205–211. [Google Scholar] [CrossRef]
- Yuk, J.; Troczynski, T. Sol-gel batio 3 thin film for humidity sensors. Sens. Actuat. B Chem. 2003, 94, 290–293. [Google Scholar] [CrossRef]
- Wang, H.Y.; Wang, Y.Q.; Hu, Q.F.; Li, X.J. Capacitive humidity sensing properties of sic nanowires grown on silicon nanoporous pillar array. Sens. Actuators B Chem. 2012, 166, 451–456. [Google Scholar] [CrossRef]
- Chang, D.; Tseng, T. Humidity-sensitivity characteristics of catio 3 porous ceramics. J. Mater. Sci. Lett. 1990, 9, 943–944. [Google Scholar] [CrossRef]
- Matko, V.; Donlagic, D. Sensor for high-air-humidity measurement. IEEE Trans. Instrum. Meas. 1996, 45, 561–563. [Google Scholar] [CrossRef]
- Matko, V. Next generation at-cut quartz crystal sensing devices. Sensors 2011, 11, 4474–4482. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zhou, L.M.; Guo, J.; Hng, H.H.; Oh, J.T.; Hing, P. F spots and domain patterns in rhombohedral PbZr0.90Ti0.10O3. Appl. Phys. Lett. 2003, 83, 3692. [Google Scholar] [CrossRef]
- Ke, S.; Huang, H.; Fan, H. Relaxor behavior in CaCu3Ti4O12 ceramics. Appl. Phys. Lett. 2006, 89, 2904. [Google Scholar] [CrossRef]
- Aria, H.; Ezeki, S.; Shimizu, Y.; Shippo, O.; Seiyama, T. Semiconductive humidity sensor of perovskite-type oxides. Anal. Chem. Symp. Ser. Chem. Sens. 1983, 17, 393–398. [Google Scholar]
- Wang, J.; Xu, B.; Liu, G.; Liu, Y.; Wu, F.; Li, X.; Zhao, M. Influence of doping on humidity sensing properties of nanocrystalline batio3. J. Mater. Sci. Lett. 1998, 17, 857–859. [Google Scholar] [CrossRef]
- Ito, W.; Nagai, T.; Sakon, T. Oxygen separation from compressed air using a mixed conducting perovskite-type oxide membrane. Solid State Ionics 2007, 178, 809–816. [Google Scholar] [CrossRef]
- Alifanti, M.; Kirchnerova, J.; Delmon, B. Effect of substitution by cerium on the activity of LaMnO3 perovskite in methane combustion. Appl. Catal. A Gen. 2003, 245, 231–244. [Google Scholar] [CrossRef]
- Musialik-Piotrowska, A.; Syczewska, K. Combustion of volatile organic compounds in two-component mixtures over monolithic perovskite catalysts. Catal. Today 2000, 59, 269–278. [Google Scholar] [CrossRef]
- Hui, R.; Sun, C.; Yick, S.; Decès-Petit, C.; Zhang, X.; Maric, R.; Ghosh, D. Ba1−xPrxCo1−yFeyO3−δ as cathode materials for low temperature solid oxide fuel cells. Electrochim. Acta 2010, 55, 4772–4775. [Google Scholar] [CrossRef]
- Tian, T.; Wang, W.; Zhan, M.; Chen, C. Catalytic partial oxidation of methane over SrTiO3 with oxygen-permeable membrane reactor. Catal. Commun. 2010, 11, 624–628. [Google Scholar] [CrossRef]
- Park, H.B.; Park, C.Y.; Hong, Y.S.; Kim, K.; Kim, S.J. Structural and dielectric properties of plzt ceramics modified with lanthanide ions. J. Am. Ceram. Soc. 1999, 82, 94–102. [Google Scholar] [CrossRef]
- Zhai, H.F.; Tang, R.L.; Li, A.D.; Guo, H.R.; Xia, Y.D.; Wu, D. Preparation and characterization of relaxor ferroelectric 0.65Pb(Mg1/3Nb2/3)O3-0.35PbTiO3 by a polymerizable complex method. J. Am. Ceram. Soc. 2009, 92, 1256–1261. [Google Scholar] [CrossRef]
- Ranjan, R.; Kumar, R.; Kumar, N.; Behera, B.; Choudhary, R. Impedance and electric modulus analysis of sm-modified Pb(Zr0.55Ti0.45)1−x/4O3 ceramics. J. Alloys Compd. 2011, 509, 6388–6394. [Google Scholar] [CrossRef]
- Liu, J.; Li, W.; Zhang, Y.; Wang, Z. Preparation and characterization of Li+-modified CaxPb1−xTiO3 film for humidity sensor. Sens. Actuators B Chem. 2001, 75, 11–17. [Google Scholar]
- Jeong, Y.-H.; Lee, S.-H.; Yoo, J.-H.; Park, C.Y. Voltage gain characteristics of piezoelectric transformer using PbTiO3 system ceramics. Sens. Actuators A Phys. 1999, 77, 126–130. [Google Scholar] [CrossRef]
- Guo, Y.; Kakimoto, K.-I.; Ohsato, H. Dielectric and piezoelectric properties of lead-free (Na0.5K0.5)NbO3-SrTiO3 ceramics. Solid State Commun. 2004, 129, 279–284. [Google Scholar] [CrossRef]
- Chen, L.; Fan, H.; Zhang, M.; Yang, C.; Chen, X. Phase structure, microstructure and piezoelectric properties of perovskite (K0.5Na0.5)0.95Li0.05NbO3-Bi0.5(K0.15Na0.85)0.5TiO3 lead-free ceramics. J. Alloys Compd. 2010, 492, 313–319. [Google Scholar] [CrossRef]
- Lin, T.F.; Hu, C.T.; Lin, I.N. Influence of cao addition on the electrical properties of BaTiO3 ceramics. J. Appl. Phys. 1990, 67, 1042–1047. [Google Scholar] [CrossRef]
- Tang, X.-G.; Chan, H.L.-W. Effect of grain size on the electrical properties of (Ba,Ca)(Zr,Ti)O3 relaxor ferroelectric ceramics. J. Appl. Phys. 2005, 97, 034109. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, D.; Parkash, O. Valence compensated perovskite oxide system Ca1−xLaxTi1−xCrxO3 part I structure and dielectric behaviour. J. Mater. Sci. 2001, 36, 3641–3648. [Google Scholar] [CrossRef]
- Chung, C.-C.; Chai, Y.-L.; Chang, Y.-S. Dielectric properties of valence compensated Ca1−xBixTi1−xCrxO3 perovskite prepared using the sol-gel process. J. Phys. Chem. Solids 2008, 69, 1877–1882. [Google Scholar] [CrossRef]
- Chung, C.-Y.; Chang, Y.-H.; Chang, Y.-S.; Chen, G.-J. High dielectric permittivity in Ca1−xBixTi1−xCrxO3 ferroelectric perovskite ceramics. J. Alloys Compd. 2004, 385, 298–303. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Manna, A.; Azrin Shah, N.F.; Shasmin, H.N.; Radzi, Z.; Abu Osman, N.A. Synthesis and characterizations of novel Ca-Mg-Ti-Fe-oxides based ceramic nanocrystals and flexible film of polydimethylsiloxane composite with improved mechanical and dielectric properties for sensors. Sensors 2016, 16, 292. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Ataollahi, F.; Pingguan-Murphy, B.; Oshkour, A.A.; Abu Osman, N.A. In vitro study of surface modified poly(ethylene glycol)-impregnated sintered bovine bone scaffolds on human fibroblast cells. Sci. Rep. 2015, 5, 9806. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, S.; Mohd Hanif, A.S.; Pingguan-Murphy, B.; Abu Osman, N.A. Morphological change of heat treated bovine bone: A comparative study. Materials 2013, 6, 65–75. [Google Scholar] [CrossRef]
- Dickey, E.C.; Varghese, O.K.; Ong, K.G.; Gong, D.; Paulose, M.; Grimes, C.A. Room temperature ammonia and humidity sensing using highly ordered nanoporous alumina films. Sensors 2002, 2, 91–110. [Google Scholar] [CrossRef]
- Sun, A.; Huang, H.; Chu, C.; Li, Y. Effect of the pore size of TiO2 porous film on humidity sensitive properties of TiO2/napss composite films. Sens. Actuators B Chem. 2011, 160, 1335–1339. [Google Scholar] [CrossRef]
- Morris, R.V.; Lauer, H.V.; Lawson, C.A.; Gibson, E.K.; Nace, G.A.; Stewart, C. Spectral and other physicochemical properties of submicron powders of hematite (α-Fe2O3), maghemite (γ-Fe2O3), magnetite (Fe3O4), goethite (α-FeOOH), and lepidocrocite (γ-FeOOH). J. Geophys. Res. Solid Earth 1985, 90, 3126–3144. [Google Scholar] [CrossRef]
- Pramanik, S.; Pingguan-Murphy, B.; Cho, J.; Osman, N.A.A. Design and development of potential tissue engineering scaffolds from structurally different longitudinal parts of a bovine-femur. Sci. Rep. 2014, 4, 5843. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Terada, Z.; Hayakawa, S. Humidity-sensitive electrical conduction of MgCr2O4-TiO2 porous ceramics. J. Am. Ceram. Soc. 1980, 63, 295–300. [Google Scholar] [CrossRef]
- Tripathy, A.; Pramanik, S.; Cho, J.; Santhosh, J.; Abu Osman, N.A. Role of morphological structure, doping, and coating of different materials in the sensing characteristics of humidity sensors. Sensors 2014, 14, 16343–16442. [Google Scholar] [CrossRef] [PubMed]
- Ataollahi, F.; Pramanik, S.; Moradi, A.; Dalilottojari, A.; Pingguan-Murphy, B.; Wan Abas, W.A.B.; Abu Osman, N.A. Endothelial cell responses in terms of adhesion, proliferation, and morphology to stiffness of polydimethylsiloxane elastomer substrates. J. Biomed. Mater. Res. Part A 2014, 103, 2203–2213. [Google Scholar] [CrossRef] [PubMed]
- Chuang, S.-H.; Gao, R.-H.; Wang, D.-Y.; Liu, H.-P.; Chen, L.-M.; Chiang, M.Y. Synthesis and characterization of ilmenite-type cobalt titanate powder. J. Chin. Chem. Soc. 2010, 57, 932–937. [Google Scholar] [CrossRef]
- Yazawa, Y.; Yamaguchi, A.; Takeda, H. Lunar minerals and their resource utilization with particular reference to solar power satellites and potential roles for humic substances for lunar agriculture. In Moon; Springer: Berlin, Germany; Heidelberg, Germany, 2012; pp. 105–138. [Google Scholar]
- Wang, J.; Wang, X.-H.; Wang, X.-D. Study on dielectric properties of humidity sensing nanometer materials. Sens. Actuators B Chem. 2005, 108, 445–449. [Google Scholar] [CrossRef]
- Bi, H.; Yin, K.; Xie, X.; Ji, J.; Wan, S.; Sun, L.; Terrones, M.; Dresselhaus, M.S. Ultrahigh humidity sensitivity of graphene oxide. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.C.; Tian, Y.T.; Li, K.; Lu, E.Y.; Gong, D.S.; Li, X.J. Capacitive humidity-sensing properties of Zn2SiO4 film grown on silicon nanoporous pillar array. Appl. Surface Sci. 2013, 273, 372–376. [Google Scholar] [CrossRef]
- Cheng, B.; Ouyang, Z.; Tian, B.; Xiao, Y.; Lei, S. Porous ZnAl2O4 spinel nanorods: High sensitivity humidity sensors. Ceram. Int. 2013, 39, 7379–7386. [Google Scholar] [CrossRef]
- Wang, C.-T.; Wu, C.-L.; Chen, I.-C.; Huang, Y.-H. Humidity sensors based on silica nanoparticle aerogel thin films. Sens. Actuators B Chem. 2005, 107, 402–410. [Google Scholar] [CrossRef]
- Björkqvist, M.; Salonen, J.; Paski, J.; Laine, E. Characterization of thermally carbonized porous silicon humidity sensor. Sens. Actuators A Phys. 2004, 112, 244–247. [Google Scholar] [CrossRef]
- Feng, Z.-S.; Chen, X.-J.; Chen, J.-J.; Hu, J. A novel humidity sensor based on alumina nanowire films. J. Phys. D Appl. Phys. 2012, 45, 225305. [Google Scholar] [CrossRef]
- Ahmad, Z.; Zafar, Q.; Sulaiman, K.; Akram, R.; Karimov, K.S. A humidity sensing organic-inorganic composite for environmental monitoring. Sensors 2013, 13, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Shi, L.; Wu, F.; Yuan, S.; Zhao, Y.; Zhang, M. The sol-gel template synthesis of porous TiO2 for a high performance humidity sensor. Nanotechnology 2011, 22, 275502. [Google Scholar] [CrossRef] [PubMed]
- Agmon, N. The grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Gao, W.; Singh, N.; Song, L.; Liu, Z.; Reddy, A.L.M.; Ci, L.; Vajtai, R.; Zhang, Q.; Wei, B.; Ajayan, P.M. Direct laser writing of micro-supercapacitors on hydrated graphite oxide films. Nat. Nanotechnol. 2011, 6, 496–500. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, E.; Zettlemoyer, A. Adsorption of water vapour on α-Fe2O3. Discuss. Faraday Soc. 1971, 52, 239–254. [Google Scholar] [CrossRef]
- Seiyama, T.; Yamazoe, N.; Arai, H. Ceramic humidity sensors. Sens. Actuators 1983, 4, 85–96. [Google Scholar] [CrossRef]
- Wang, L.L.; Wang, H.Y.; Wang, W.C.; Li, K.; Wang, X.C.; Li, X.J. Capacitive humidity sensing properties of ZnO cauliflowers grown on silicon nanoporous pillar array. Sens. Actuators B Chem. 2013, 177, 740–744. [Google Scholar] [CrossRef]
- Chen, W.-P.; Zhao, Z.-G.; Liu, X.-W.; Zhang, Z.-X.; Suo, C.-G. A capacitive humidity sensor based on multi-wall carbon nanotubes (MWCNTs). Sensors 2009, 9, 7431–7444. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, J.; Wang, Z.; Yan, Q.; Hui, S. Humidity sensing behavior of silicon nanowires with hexamethyldisilazane modification. Sens. Actuators B Chem. 2011, 156, 631–636. [Google Scholar] [CrossRef]
- Wang, Y.; Park, S.; Yeow, J.T.; Langner, A.; Müller, F. A capacitive humidity sensor based on ordered macroporous silicon with thin film surface coating. Sens. Actuators B Chem. 2010, 149, 136–142. [Google Scholar] [CrossRef]
- Kim, Y.; Jung, B.; Lee, H.; Kim, H.; Lee, K.; Park, H. Capacitive humidity sensor design based on anodic aluminum oxide. Sens. Actuators B Chem. 2009, 141, 441–446. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Q.; Zhou, R.; Xu, B. Humidity sensors based on composite material of nano-BaTiO3 and polymer rmx. Sens. Actuators B Chem. 2002, 81, 248–253. [Google Scholar] [CrossRef]
- Song, X.; Qi, Q.; Zhang, T.; Wang, C. A humidity sensor based on KCl-doped SnO2 nanofibers. Sens. Actuators B Chem. 2009, 138, 368–373. [Google Scholar] [CrossRef]
- Su, M.; Wang, J. Preparation and humidity sensitivity of multi-layered zirconia thin films by sol-gel method. Sens. Lett. 2011, 9, 670–674. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Yu, Q.; Wang, R.; Zeng, Y.; Liu, L.; Yang, H. Properties of humidity sensing ZnO nanorods-base sensor fabricated by screen-printing. Sens. Actuators B Chem. 2008, 133, 638–643. [Google Scholar] [CrossRef]













| Steps | Parameters | Sample Details | |||
|---|---|---|---|---|---|
| 450 °C | 650 °C | 850 °C | 1050 °C | ||
| Step-I | Temperature (°C) | 450 | 250 | 350 | 350 |
| Time (h) | 3.5 | 1 | 1 | 1 | |
| Ramp rate (°C/min) | 5 | 5 | 5 | 5 | |
| Step-II | Temperature (°C) | - | 650 | 550 | 550 |
| Time (h) | - | 3.5 | 3.5 | 3.5 | |
| Ramp rate (°C/min) | - | 10 | 10 | 10 | |
| Step-III | Temperature (°C) | - | - | 850 | 1050 |
| Time (h) | - | - | 1.3 | 1.3 | |
| Ramp rate (°C/min) | - | - | 15 | 15 | |
| Step-IV | Temperature (°C) | - | - | 750 | 750 |
| Time (h) | - | - | 3 | 3 | |
| Ramp rate (°C/min) | - | - | 20 | 20 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tripathy, A.; Pramanik, S.; Manna, A.; Bhuyan, S.; Azrin Shah, N.F.; Radzi, Z.; Abu Osman, N.A. Design and Development for Capacitive Humidity Sensor Applications of Lead-Free Ca,Mg,Fe,Ti-Oxides-Based Electro-Ceramics with Improved Sensing Properties via Physisorption. Sensors 2016, 16, 1135. https://doi.org/10.3390/s16071135
Tripathy A, Pramanik S, Manna A, Bhuyan S, Azrin Shah NF, Radzi Z, Abu Osman NA. Design and Development for Capacitive Humidity Sensor Applications of Lead-Free Ca,Mg,Fe,Ti-Oxides-Based Electro-Ceramics with Improved Sensing Properties via Physisorption. Sensors. 2016; 16(7):1135. https://doi.org/10.3390/s16071135
Chicago/Turabian StyleTripathy, Ashis, Sumit Pramanik, Ayan Manna, Satyanarayan Bhuyan, Nabila Farhana Azrin Shah, Zamri Radzi, and Noor Azuan Abu Osman. 2016. "Design and Development for Capacitive Humidity Sensor Applications of Lead-Free Ca,Mg,Fe,Ti-Oxides-Based Electro-Ceramics with Improved Sensing Properties via Physisorption" Sensors 16, no. 7: 1135. https://doi.org/10.3390/s16071135