DEP-On-Go for Simultaneous Sensing of Multiple Heavy Metals Pollutants in Environmental Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Preparation of Standard Solutions
2.3. Pre-Treatments of Real Samples
2.4. Procedure for DPV Analysis
3. Results
3.1. Characteristics of the “DEP-On-Go” Sensor
3.2. Standardization of the “DEP-On-Go” Sensor for Multiple Heavy Metal Detection
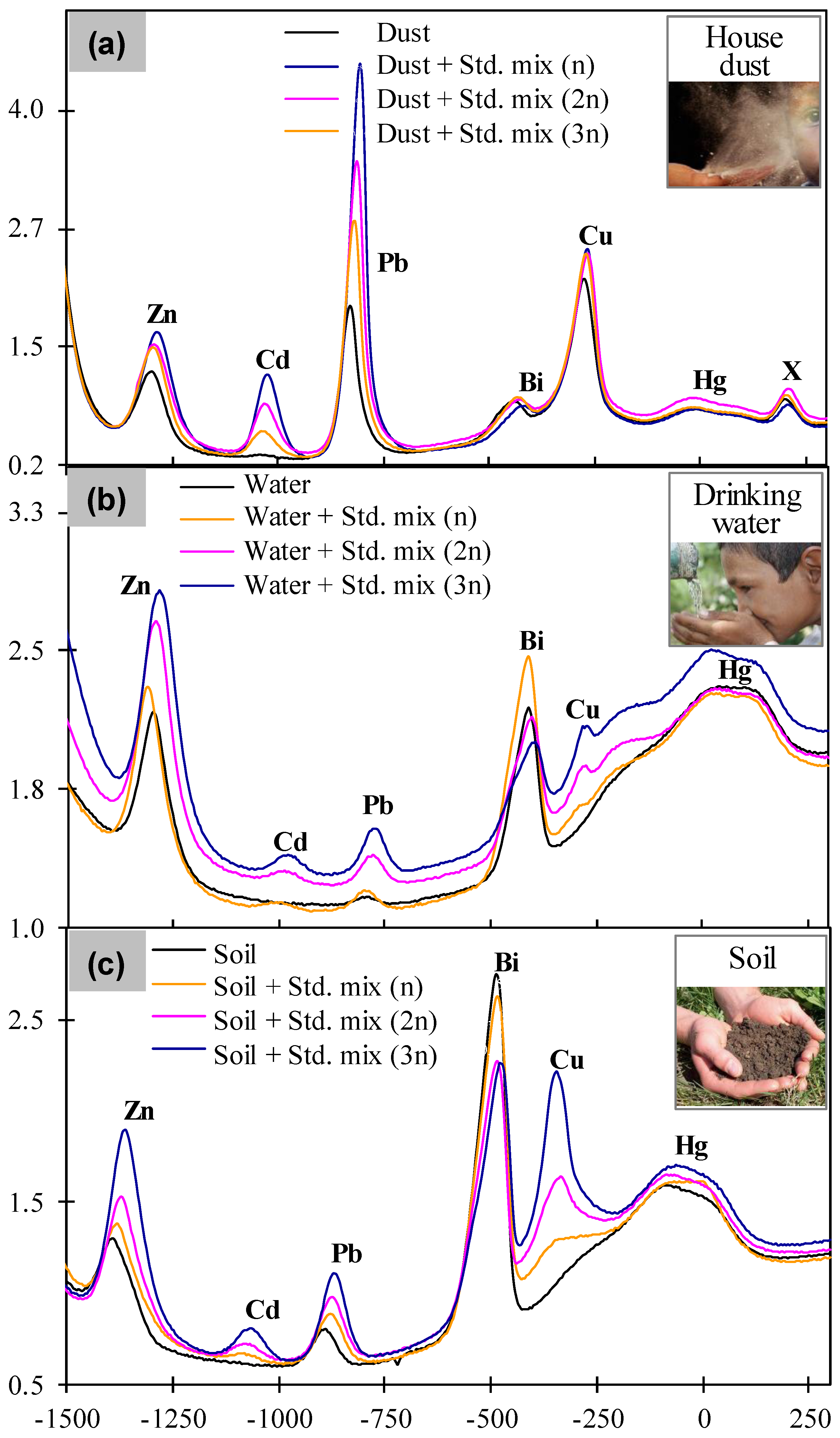
3.3. Simultaneous Detection of Multiple Heavy Metals in Real Environmental Samples
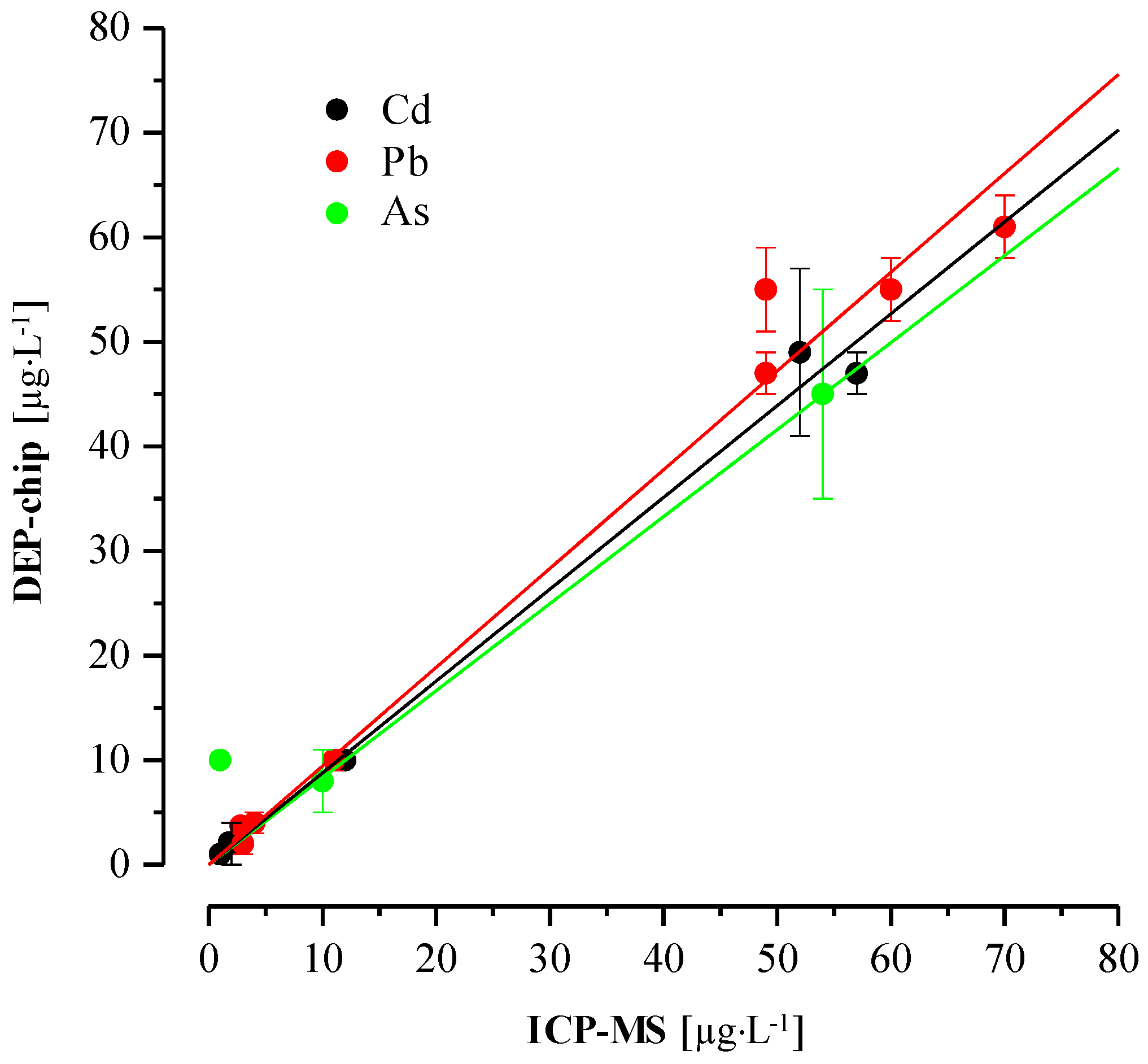
3.4. On-Site Detection and Validation of Heavy Metals in Drinking Groundwater Samples
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Gautam, N.; Mishra, A.; Gupta, R. Heavy metals and living systems: An overview. Indian J. Pharmacol. 2011, 43, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Okoro, K.I.; Igene, J.O.; Ebabhamiegbebho, P.A.; Evivie, S.E. Lead (Pb) and Cadmium (Cd) levels in fresh and smoke-dried grasscutter (Thryonomys swinderianus Temminck) meat. Afr. J. Agric. Res. 2015, 10, 3116–3122. [Google Scholar]
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed]
- Bánfalvi, G. Heavy Metals, Trace Elements and Their Cellular Effects. In Cellular Effects of Heavy Metals; Bánfalvi, G., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 3–28. [Google Scholar]
- Jayasumana, M.A.C.S.; Paranagama, P.A.; Amarasinghe, M.D.; Wijewardane, K.M.R.C.; Dahanayake, K.S.; Fonseka, S.I. Possible link of chronic arsenic toxicity with chronic kidney disease of unknown etiology in Sri Lanka. J. Nat. Sci. Res. 2013, 3, 64–73. [Google Scholar]
- Dusek, P.; Roos, P.M.; Litwin, T.; Schneider, S.A.; Flaten, T.P.; Aaseth, J. The neurotoxicity of iron, copper and manganese in Parkinson’s and Wilson’s diseases. J. Trace Elem. Med. Biol. 2015, 31, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Q.; Young, M.R.; Diwan, B.A.; Coogan, T.P.; Waalkes, M.P. Association of arsenic-induced malignant transformation with DNA hypomethylation and aberrant gene expression. Proc. Natl. Acad. Sci. USA 1997, 94, 10907–10912. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy metal toxicity and the environment. EXS 2012, 101, 133–164. [Google Scholar] [PubMed]
- Chhabra, D.; Oda, K.; Jagannath, P.; Utsunomiya, H.; Takekoshi, S.; Nimura, Y. Chronic heavy metal exposure and gallbladder cancer risk in India, a comparative study with Japan. Asian Pac. J. Cancer Prev. 2012, 13, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gu, Y.; Zhou, Q.; Mao, G.; Zou, B.; Zhao, J. Combined toxicity of heavy metal mixtures in liver cells. J. Appl. Toxicol. 2016, 36, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Soodan, R.K.; Pakade, Y.B.; Nagpal, A.; Katnoria, J.K. Analytical techniques for estimation of heavy metals in soil ecosystem: A tabulated review. Talanta 2014, 125, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Chikae, M.; Kerman, K.; Nagatani, N.; Takamura, Y.; Tamiya, E. An electrochemical on-field sensor system for the detection of compost maturity. Anal. Chim. Acta 2007, 581, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.U.; Hossain, M.M.; Tamiya, E. Electrochemical Biosensors for Medical and Food applications. Electroanalysis 2008, 20, 616–626. [Google Scholar] [CrossRef]
- Biyani, M.; Kawai, K.; Kitamura, K.; Chikae, M.; Biyani, M.; Ushijima, H.; Tamiya, E.; Yoneda, T.; Takamura, Y. PEP-on-DEP: A competitive peptide-based disposable eletrochemical aptasensor for renin diagnostics. Biosens. Bioelectron. 2016, 84, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Metters, J.P.; Kadara, R.O.; Banks, C.E. New directions in screen printed electroanalytical sensors: An overview of recent developments. Analyst 2011, 36, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Marty, J.L. Aptamer based electrochemical sensors for emerging environmental pollutants. Front. Chem. 2014, 2, 41. [Google Scholar] [CrossRef] [PubMed]
- Hayat, A.; Marty, J.L. Disposable screen printed electrochemical sensors: Tools for environmental monitoring. Sensors 2014, 14, 10432–10453. [Google Scholar] [CrossRef] [PubMed]
- Nemiroski, A.; Christodouleas, D.C.; Hennek, J.W.; Kumar, A.A.; Maxwell, E.J.; Fernández-Abedul, M.T.; Whitesides, G.M. Universal mobile electrochemical detector designed for use in resource-limited applications. Proc. Natl. Acad. Sci. USA 2014, 111, 11984–11989. [Google Scholar] [CrossRef] [PubMed]
- Chailapakul, O.; Korsrisakul, S.; Siangproh, W.; Grudpan, K. Fast and simultaneous detection of heavy metals using a simple and reliable microchip-electrochemistry route: An alternative approach to food analysis. Talanta 2008, 74, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Baron-Jaimez, J.; Joya, M.R.; Barba-Ortega, J. Anodic stripping voltammetry—ASV for determination of heavy metals. J. Phys. 2013, 466, 012023. [Google Scholar] [CrossRef]
- Huang, H.; Chen, T.; Liu, X.; Ma, H. Ultrasensitive and simultaneous detection of heavy metal ions based on three-dimensional graphene-carbon nanotubes hybrid electrode materials. Anal. Chim. Acta 2014, 852, 45–54. [Google Scholar] [CrossRef] [PubMed]
- March, G.; Nguyen, T.D.B.; Piro, B. Modified electrodes used for electrochemical detection of metal ions in environmental analysis. Biosensors 2015, 5, 241–275. [Google Scholar] [CrossRef] [PubMed]
- Mafa, P.J.; Idris, A.O.; Mabuba, N.; Arotiba, O.A. Electrochemical co-detection of As(III), Hg(II) and Pb(II) on a bismuth modified exfoliated graphite electrode. Talanta 2016, 153, 99–106. [Google Scholar] [CrossRef] [PubMed]
- World Health Organizations. Guidelines for Drinking—Water Quality, 4th ed.; World Health Organizations: Geneva, Switzerland, 2011. [Google Scholar]
- United States Department of Agriculture. Natural Resources Conservation Service, Soil Quality—Urban; Technical Note No. 3; United States Department of Agriculture: Temple, TX, USA, 2000.
- World Health Organizations. Seventy-Third Report of the Joint FAO/WHO Expert Committee on Food Additives; WHO Technical Report Series 960; World Health Organizations: Geneva, Switzerland, 2011. [Google Scholar]
- Singh, J.; Huerta-Aguilar, C.A.; Singh, H.; Pandiyan, T.; Singh, N. Voltammetric simultaneous determination of Cu2+, Cd2+ and Pb2+ in full aqueous medium using organic nanoparticles of disulfide based receptor. Electroanalysis 2015, 27, 2544–2551. [Google Scholar] [CrossRef]
- Nourifard, F.; Payehghadr, M.; Kalhor, M.; Nejadali, A. An electrochemical sensor for determination of Ultratrace Cd, Cu and Hg in water samples by modified carbon paste electrode base on a new Schiff base ligand. Electroanalysis 2015, 27, 2479–2485. [Google Scholar] [CrossRef]
- Wang, J.; Lu, J.; Hocevar, S.B.; Farias, P.A.M. Bismuth-coated carbon electrodes for anodic stripping voltammetry. Anal. Chem. 2000, 72, 3218–3222. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Parka, S.; Choi, E.; Piao, Y. Voltammetric determination of trace heavy metals using an electrochemically deposited graphene/bismuth nanocomposite film-modified glassy carbon electrode. J. Electroanal. Chem. 2016, 766, 120–127. [Google Scholar] [CrossRef]
- Sardar, R. Maggi stews in lead and MSG pot: Controversy over India’s favourite instant Noodles. Case Stud. J. 2015, 4, 1–6. [Google Scholar]
- Duggal, V.; Rani, A.; Mehra, R. Monitoring of metal contaminations in groundwater in Northern Rajasthan, India. J. Environ. Occup. Sci. 2014, 3, 114–118. [Google Scholar] [CrossRef]
- Dixit, R.C.; Verma, S.R.; Nitnaware, V.; Thacker, N.P. Heavy metals contamination in surface and groundwater supply of an urban city. Indian J. Environ. Health 2003, 45, 107–112. [Google Scholar] [PubMed]

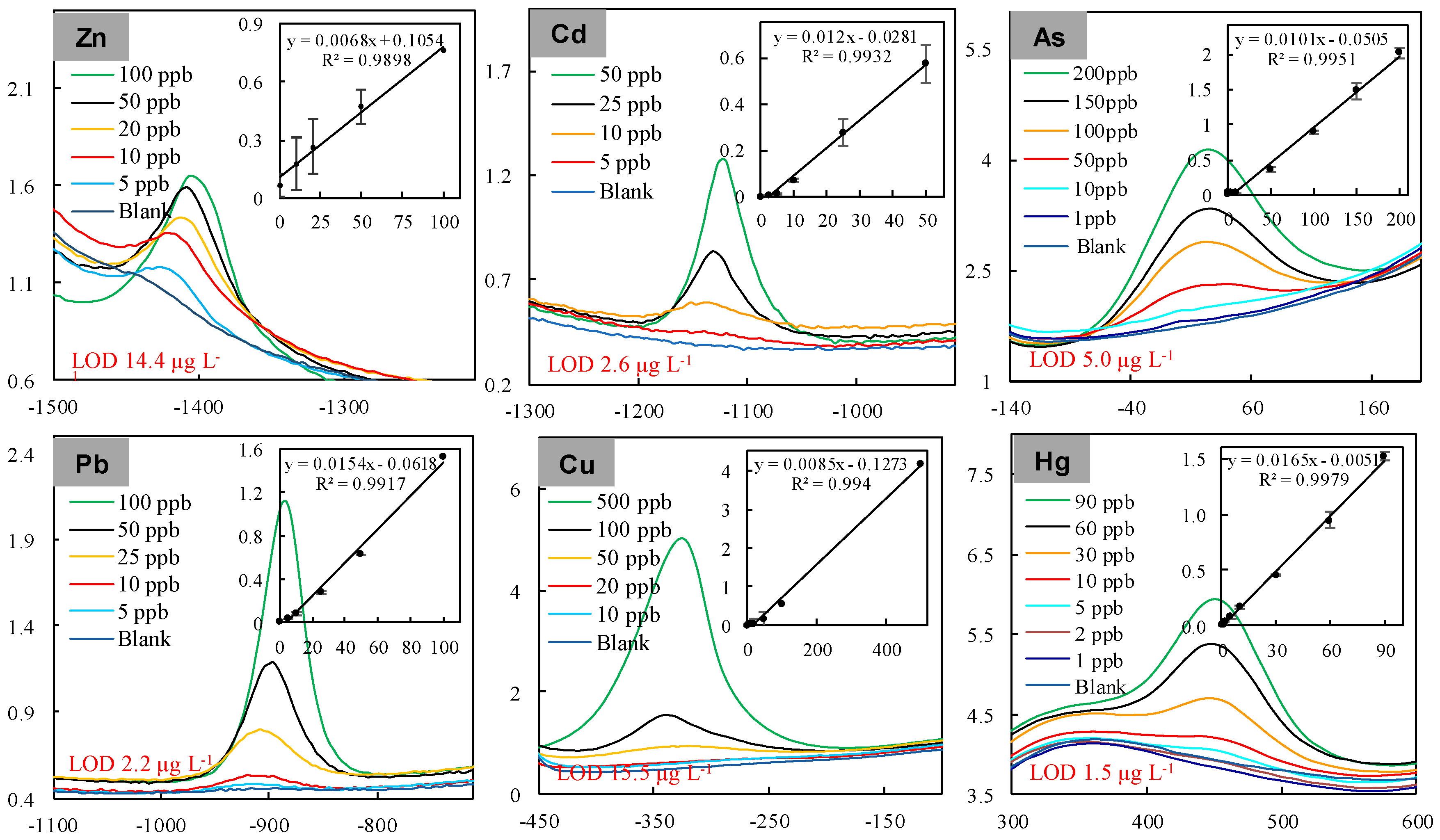

| Limit of Detection | Drinking Water | Air Dust | Soil | ||||
|---|---|---|---|---|---|---|---|
| PEL a | Sample d | PEL b | Sample e | PEL c | Sample f | ||
| (μg·L−1) | (μg·L−1) | (μg∙m−3) | (μg∙g−1) | (μg∙g−1) | |||
| Cd | 2.6 | 3 | 1.73 | 5 | 0.22 | 85 | 0.016 |
| Pb | 4.0 | 10 | 6.5 | 50 | 27.4 | 420 | 0.24 |
| As | 5.0 | 10 | 2.3 | 10 | 0.034 | 75 | 0.025 |
| Hg | 1.5 | 6 | 4.1 * | 25 | 27.2 * | 840 | 0.045 * |
| Cu | 15.5 | 2000 | 14.7 | 1000 | 8.14 | 4300 | 0.175 |
| Zn | 14.4 | 3000 | 1240 | 10,000 | 75.6 | 7500 | 1.4 |
| Samples | ICP-MS | DEP Chip | Recovery (%) | |
|---|---|---|---|---|
| Lead | ||||
| 1 | Noodles (Brand-A, India) 1g/50 mL | 2.8 | 3.7 | 132 |
| 2 | Noodles (Brand-B, Japan) 1g/50 mL | 1 | ND | - |
| 3 | Noodles (Brand-B, Japan + 10 μg·L−1 added) | 11 | 10 ± 1 | 91 |
| 4 | Noodles (Brand-B, Japan + 50 μg·L−1 added) | 49 | 55 ± 4 | 112 |
| 5 | Groundwater (Bikaner, Rajasthan, India) | 70 | 61 ± 3 | 87 |
| 6 | Groundwater (Amarapura, Rajasthan, India) | 60 | 55 ± 3 | 92 |
| 7 | Tap water (Nomi, Ishikawa, Japan) | 3 | 2 ± 1 | 67 |
| 8 | Tap water (Nomi, Ishikawa, Japan + 1 μg·L−1 added) | 4 | 4 ± 1 | 100 |
| 9 | Tap water (Nomi, Ishikawa, Japan + 50 μg·L−1 added) | 49 | 47 ± 2 | 96 |
| 10 | Dust (Jaipur, Rajasthan, India) 1 g/ 50 mL | 910 | 800 ± 63 | 89 |
| 11 | Dust (Nomi, Ishikawa, Japan) 1 g/50 mL | 520 | 510 ± 19 | 98 |
| Cadmium | ||||
| 12 | Rice (Indica brand-A, India) 1g/50 mL | 1.8 | 2.1 | 116 |
| 13 | Rice (Japonica brand-B, Japan) 1g/50 mL | 1 | ND | - |
| 14 | Rice (Japonica brand-B, Japan + 10 μg·L−1 added) | 12 | <10 | - |
| 15 | Rice (Japonica brand-B, Japan + 50 μg·L−1 added) | 52 | 49 ± 8 | 94 |
| 16 | Tap water (Nomi, Ishikawa, Japan) | <1 | <1 | - |
| 17 | Tap water (Nomi, Ishikawa, Japan + 1 μg·L−1 added) | 2 | 2 ± 2 | 100 |
| 18 | Tap water (Nomi, Ishikawa, Japan + 50 μg·L−1 added) | 57 | 47 ± 2 | 83 |
| Arsenic | ||||
| 19 | Tap water (Nomi, Ishikawa, Japan) | <1 | <10 | - |
| 20 | Tap water (Nomi, Ishikawa, Japan + 10 μg·L−1 added) | 10 | 8 ± 3 | 80 |
| 21 | Tap water (Nomi, Ishikawa, Japan + 50 μg·L−1 added) | 54 | 45 ± 10 | 83 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biyani, M.; Biyani, R.; Tsuchihashi, T.; Takamura, Y.; Ushijima, H.; Tamiya, E.; Biyani, M. DEP-On-Go for Simultaneous Sensing of Multiple Heavy Metals Pollutants in Environmental Samples. Sensors 2017, 17, 45. https://doi.org/10.3390/s17010045
Biyani M, Biyani R, Tsuchihashi T, Takamura Y, Ushijima H, Tamiya E, Biyani M. DEP-On-Go for Simultaneous Sensing of Multiple Heavy Metals Pollutants in Environmental Samples. Sensors. 2017; 17(1):45. https://doi.org/10.3390/s17010045
Chicago/Turabian StyleBiyani, Madhu, Radhika Biyani, Tomoko Tsuchihashi, Yuzuru Takamura, Hiromi Ushijima, Eiichi Tamiya, and Manish Biyani. 2017. "DEP-On-Go for Simultaneous Sensing of Multiple Heavy Metals Pollutants in Environmental Samples" Sensors 17, no. 1: 45. https://doi.org/10.3390/s17010045





