Evaluation of Surface Cleaning Procedures in Terms of Gas Sensing Properties of Spray-Deposited CNT Film: Thermal- and O2 Plasma Treatments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of the Modified SWNT Electrodes
2.3. Characterization and Gas Sensing Measurement
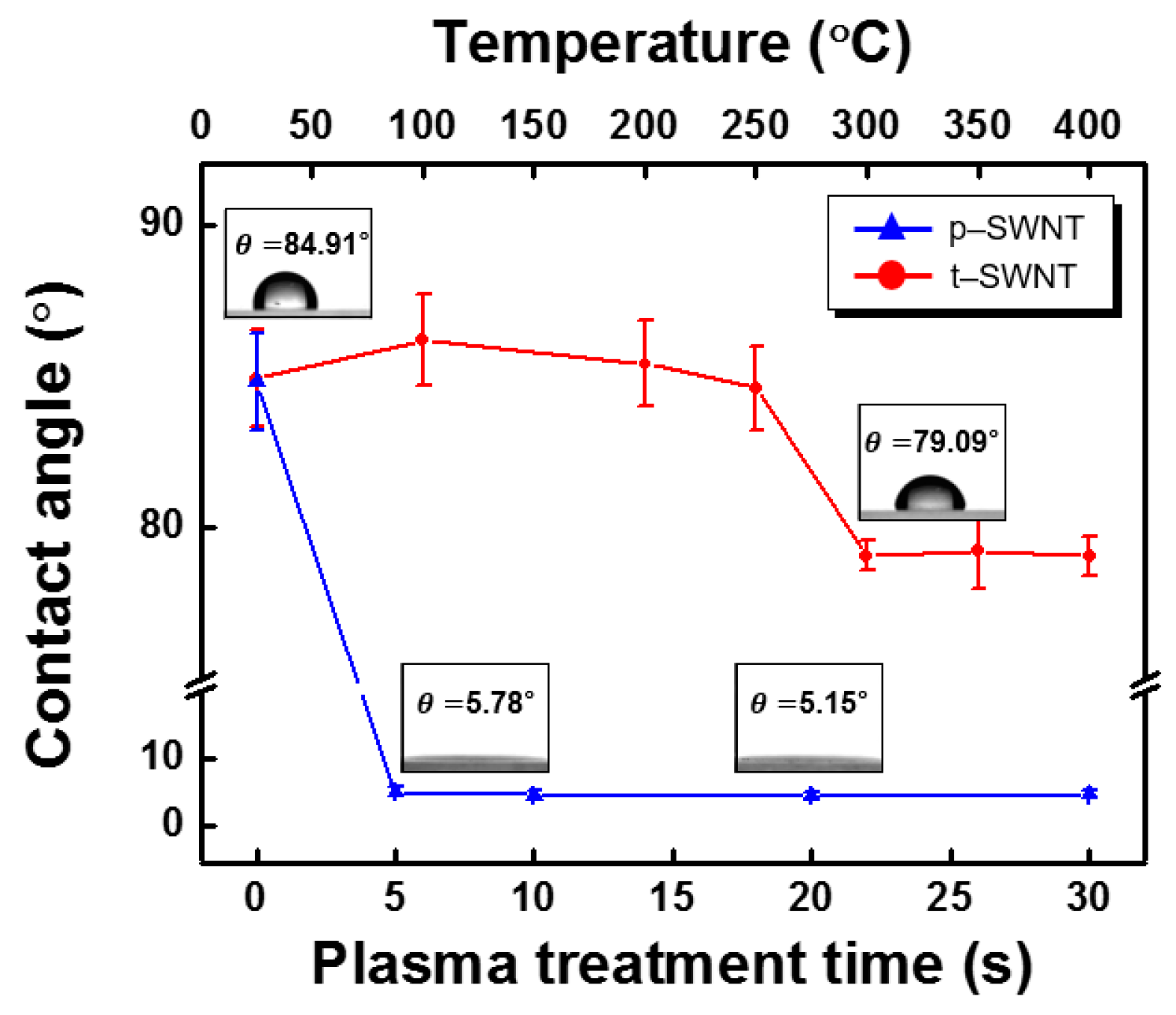
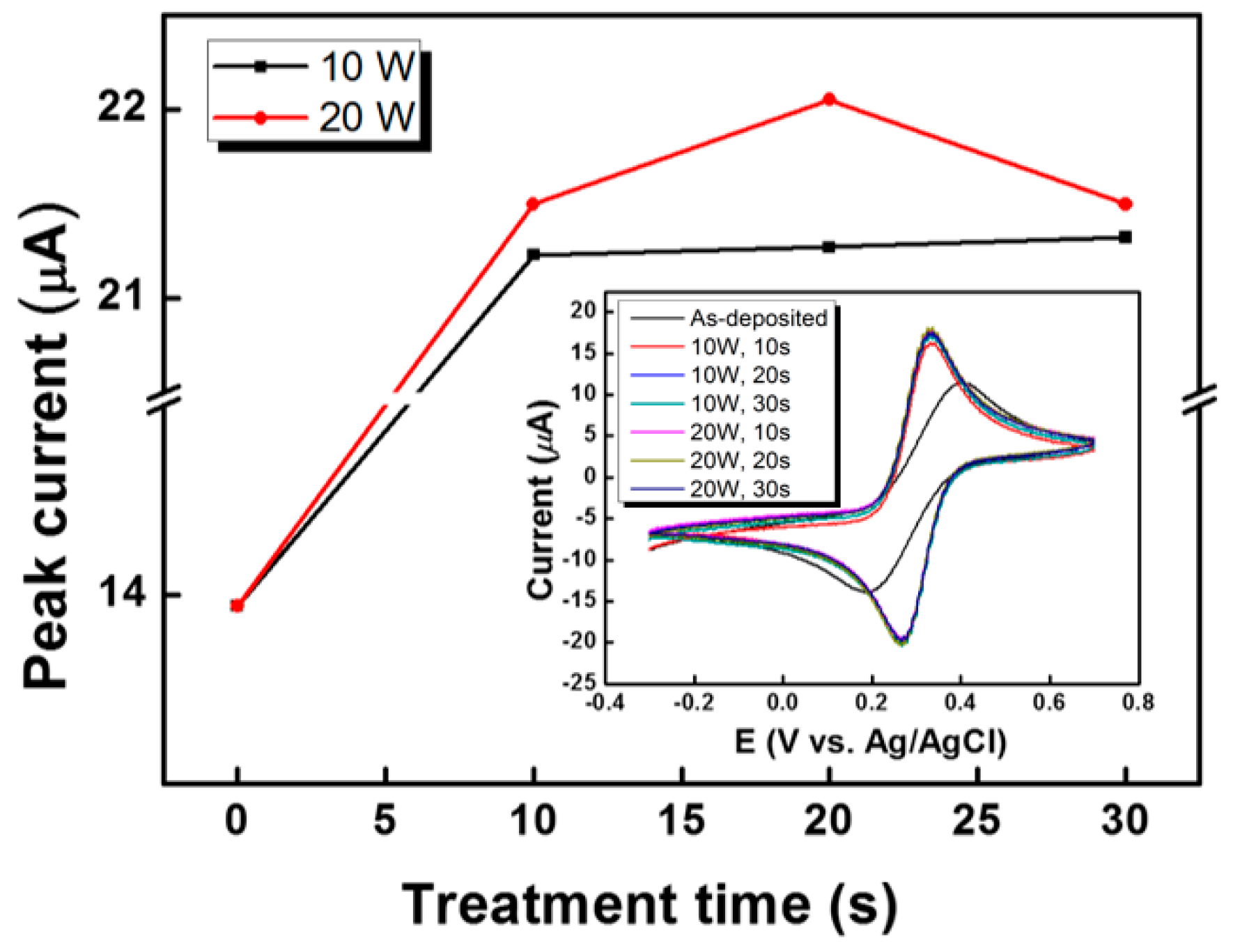
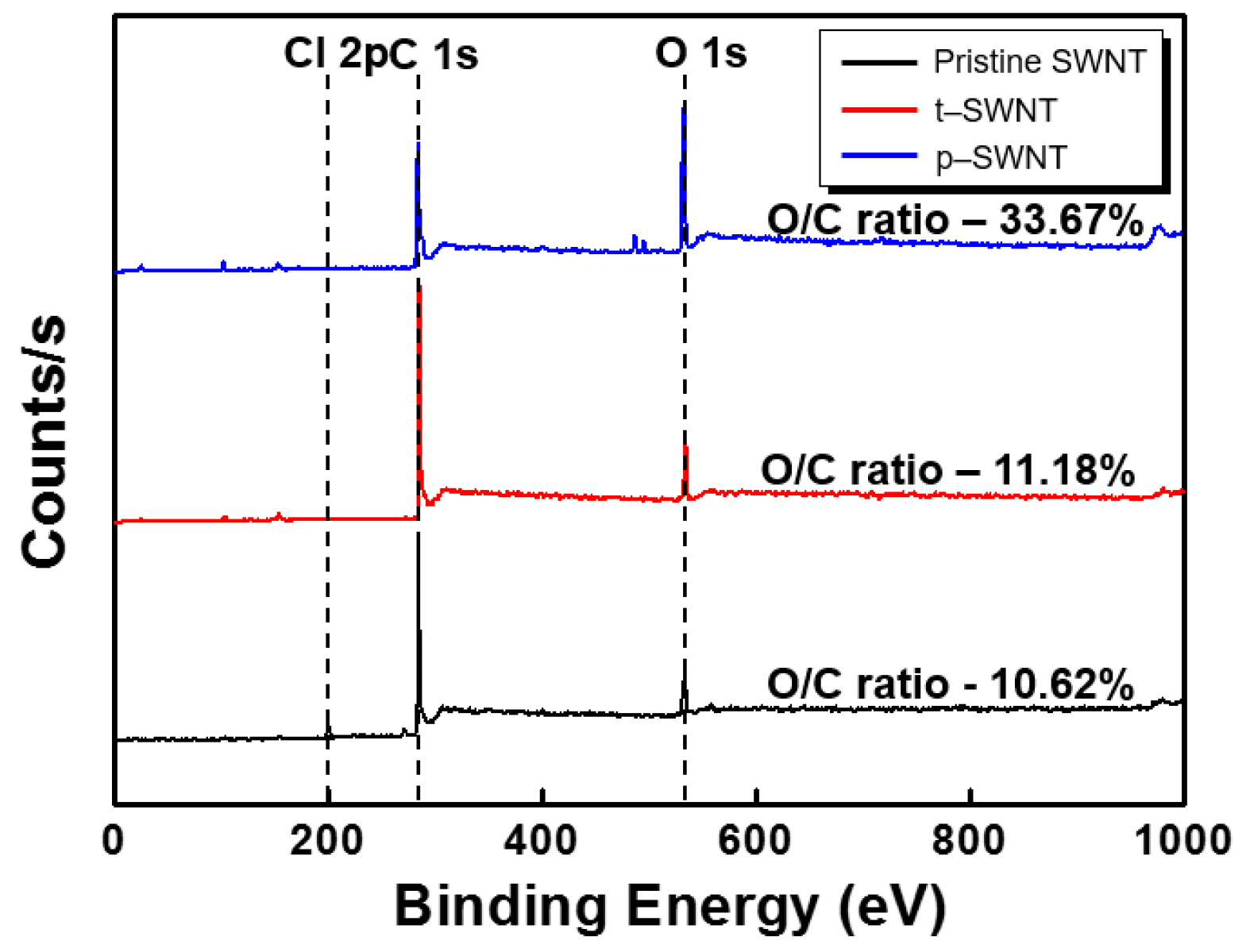
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dube, I.; Jimenez, D.; Fedorov, G.; Boyd, A.; Gayduchenko, I.; Paranjape, M.; Barbara, P. Understanding the electrical response and sensing mechanism of carbon-nanotube-based gas sensors. Carbon 2015, 87, 330–337. [Google Scholar] [CrossRef]
- Salehi-Khojin, A.; Khalili-Araghi, F.; Kuroda, M.A.; Lin, K.Y.; Leburton, J.P.; Masel, R.I. On the sensing mechanism in carbon nanotube chemiresistors. Acs Nano 2011, 5, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Puech, P.; Ondarcuhu, T.; Escoffier, W.; Raquet, B.; Monthioux, M. The effect of adsorbed species and exposure to sulfuric acid on the electrical conductance of individual single-wall carbon nanotube transistors. Carbon 2012, 50, 3953–3956. [Google Scholar] [CrossRef]
- Iqbal, N.; Afzal, A.; Cioffi, N.; Sabbatini, L.; Torsi, L. NOx sensing one- and two-dimensional carbon nanostructures and nanohybrids: Progress and perspectives. Sens. Actuators B Chem. 2013, 181, 9–21. [Google Scholar] [CrossRef]
- Jin, J.H.; Kim, J.H.; Lee, J.Y.; Min, N.K. Voltammetric characterization of a fully integrated, patterned single walled carbon nanotube three-electrode system on a glass substrate. Analyst 2011, 136, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Girifalco, L.A.; Hodak, M.; Lee, R.S. Carbon nanotubes, buckyballs, ropes, and a universal graphitic potential. Phys. Rev. B 2000, 62, 13104–13110. [Google Scholar] [CrossRef]
- Bystrzejewski, M.; Huczko, A.; Lange, H.; Gemming, T.; Buchner, B.; Rummeli, M.H. Dispersion and diameter separation of multi-wall carbon nanotubes in aqueous solutions. J. Colloid Interface Sci. 2010, 345, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.X.; Liu, Z.P.; Chen, G.M. Dispersion of pristine multi-walled carbon nanotubes in common organic solvents. Nano 2010, 5, 103–109. [Google Scholar] [CrossRef]
- Geckeler, K.E.; Premkumar, T. Carbon nanotubes: Are they dispersed or dissolved in liquids? Nanoscale Res. Lett. 2011, 6. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Sharma, S.S.; Agrawal, S.; Kumar, S.; Singh, M.; Vijay, Y.K. Study of chemiresistor type CNT doped polyaniline gas sensor. Synth. Met. 2010, 160, 529–534. [Google Scholar] [CrossRef]
- Bahari, A.; Qhavami, A. Electrical and nanostructural characteristics of R-, Fe-, S-CNT electrodes of microbial field effect transistors. J. Mater. Sci. Mater. Electron. 2016, 27, 5934–5942. [Google Scholar] [CrossRef]
- Cheon, J.; Choi, S.; Heo, Y.J.; Lee, S.H.; Lim, J.; Park, Y.J. Fabrication of n-Type CNT Field-Effect Transistor Using Energy Band Engineering Layer Between CNT and Electrode. IEEE Electron Device Lett. 2013, 34, 1436–1438. [Google Scholar] [CrossRef]
- Pei, T.; Zhang, Z.Y.; Wang, Z.X.; Ding, L.; Wang, S.; Peng, L.M. Temperature Performance of Doping-Free Top-Gate CNT Field-Effect Transistors: Potential for Low-and High-Temperature Electronics. Adv. Funct. Mater. 2011, 21, 1843–1849. [Google Scholar] [CrossRef]
- Ahn, J.; Hong, S.H.; Park, Y. A Double-Side CMOS-CNT Biosensor Array With Padless Structure for Simple Bare-Die Measurements in a Medical Environment. IEEE Trans. Biomed. Circuits Syst. 2015, 9, 815–824. [Google Scholar] [PubMed]
- Zhang, Y.Y.; Arugula, M.A.; Wales, M.; Wild, J.; Simonian, A.L. A novel layer-by-layer assembled multi-enzyme/CNT biosensor for discriminative detection between organophosphorus and non-organophosphrus pesticides. Biosens Bioelectron. 2015, 67, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Pagan, M.; Suazo, D.; del Toro, N.; Griebenow, K. A comparative study of different protein immobilization methods for the construction of an efficient nano-structured lactate oxidase-SWCNT-biosensor. Biosens. Bioelectron. 2015, 64, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.Y.; Prevot, M.S.; Sivula, K. Multiflake thin film electronic devices of solution processed 2D MoS2 enabled by sonopolymer assisted exfoliation and surface modification. Chem. Mater. 2014, 26, 5892–5899. [Google Scholar] [CrossRef]
- Kim, D.S.; Nepal, D.; Geckeler, K.E. Individualization of single-walled carbon nanotubes: Is the solvent important? Small 2005, 1, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Niyogi, S.; Hamon, M.A.; Perea, D.E.; Kang, C.B.; Zhao, B.; Pal, S.K.; Wyant, A.E.; Itkis, M.E.; Haddon, R.C. Ultrasonic dispersions of single-walled carbon nanotubes. J. Phys. Chem. B 2003, 107, 8799–8804. [Google Scholar] [CrossRef]
- Liu, M.H.; Zhu, T.; Li, Z.C.; Liu, Z.F. One-step in situ synthesis of poly(methyl methacrylate)-grafted single-walled carbon nanotube composites. J. Phys. Chem. C 2009, 113, 9670–9675. [Google Scholar] [CrossRef]
- Kim, J.H.; Song, M.J.; Lee, C.J.; Lee, J.H.; Kim, J.H.; Min, N.K. A comparative study of electrochemical and biointerfacial properties of acid- and plasma-treated single-walled carbon-nanotube-film electrode systems for use in biosensors. Carbon 2013, 52, 398–407. [Google Scholar] [CrossRef]
- Okpalugo, T.I.T.; Papakonstantinou, P.; Murphy, H.; McLaughlin, J.; Brown, N.M.D. High resolution XPS characterization of chemical functionalised MWCNTs and SWCNTs. Carbon 2005, 43, 153–161. [Google Scholar] [CrossRef]
- Ham, S.W.; Hong, H.P.; Kim, J.W.; Kim, J.H.; Kim, K.B.; Park, C.W.; Min, N.K. Comparison of Gas Sensors Based on Oxygen Plasma-Treated Carbon Nanotube Network Films with Different Semiconducting Contents. J. Electron. Mater. 2015, 44, 1344–1350. [Google Scholar] [CrossRef]

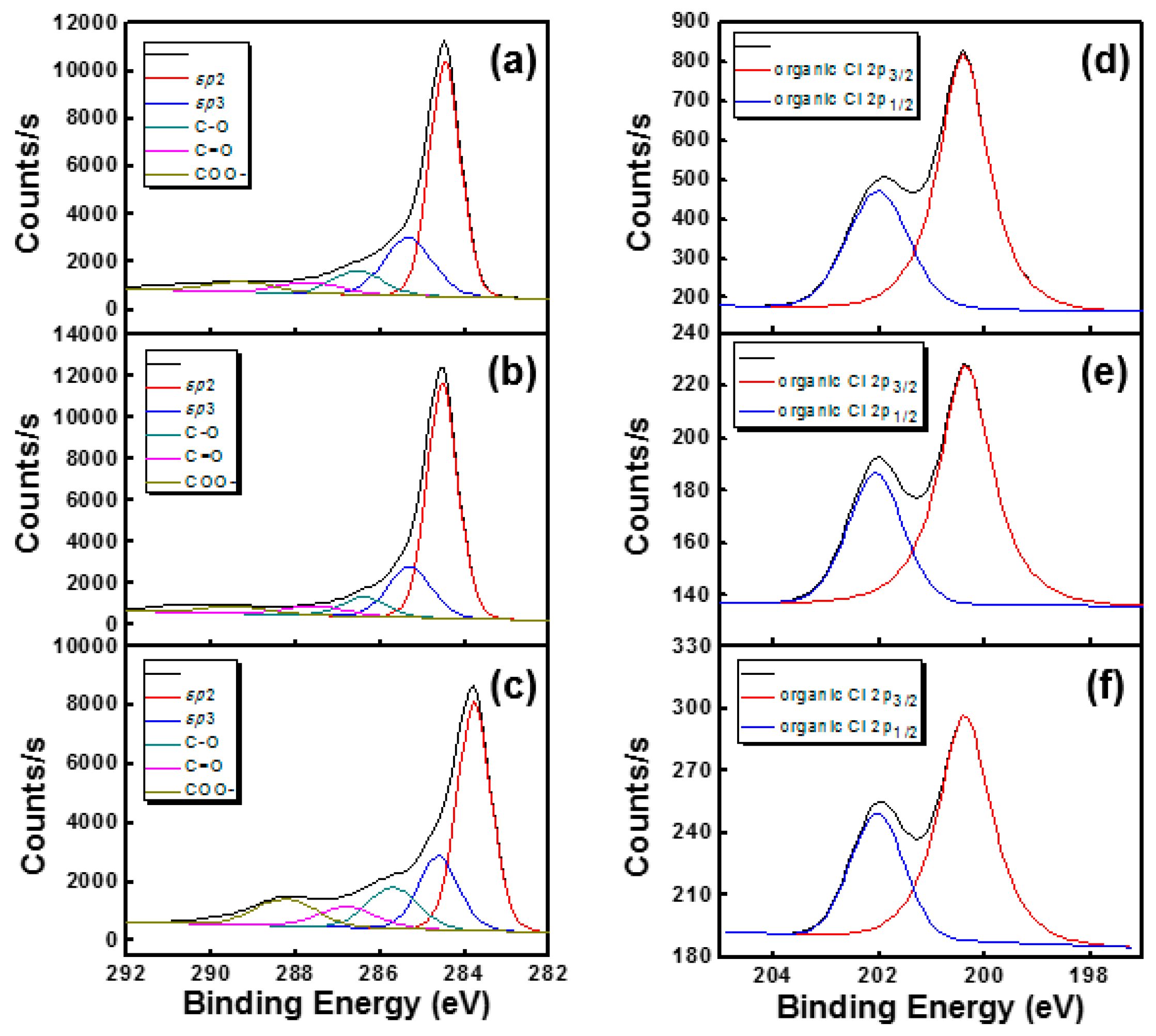
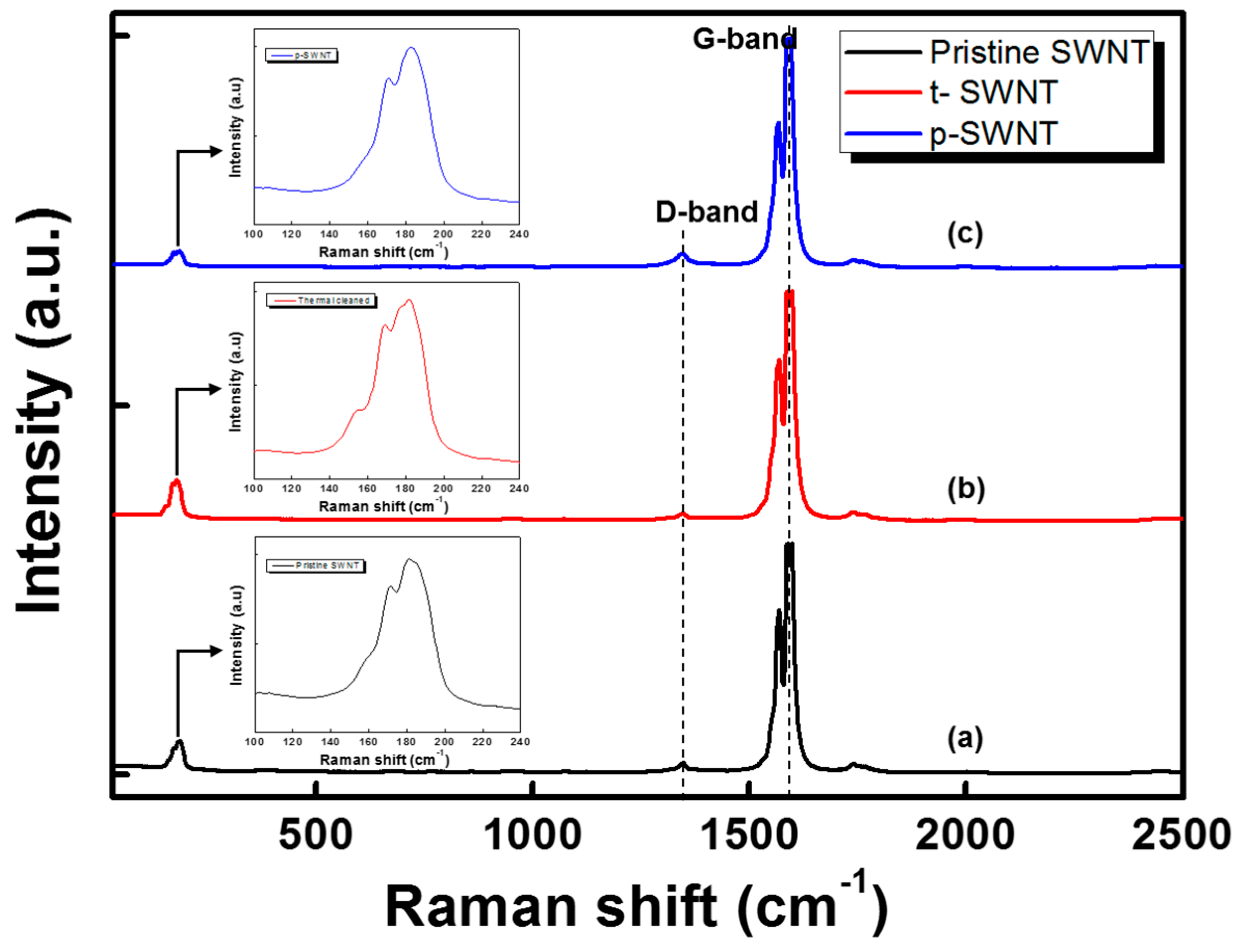
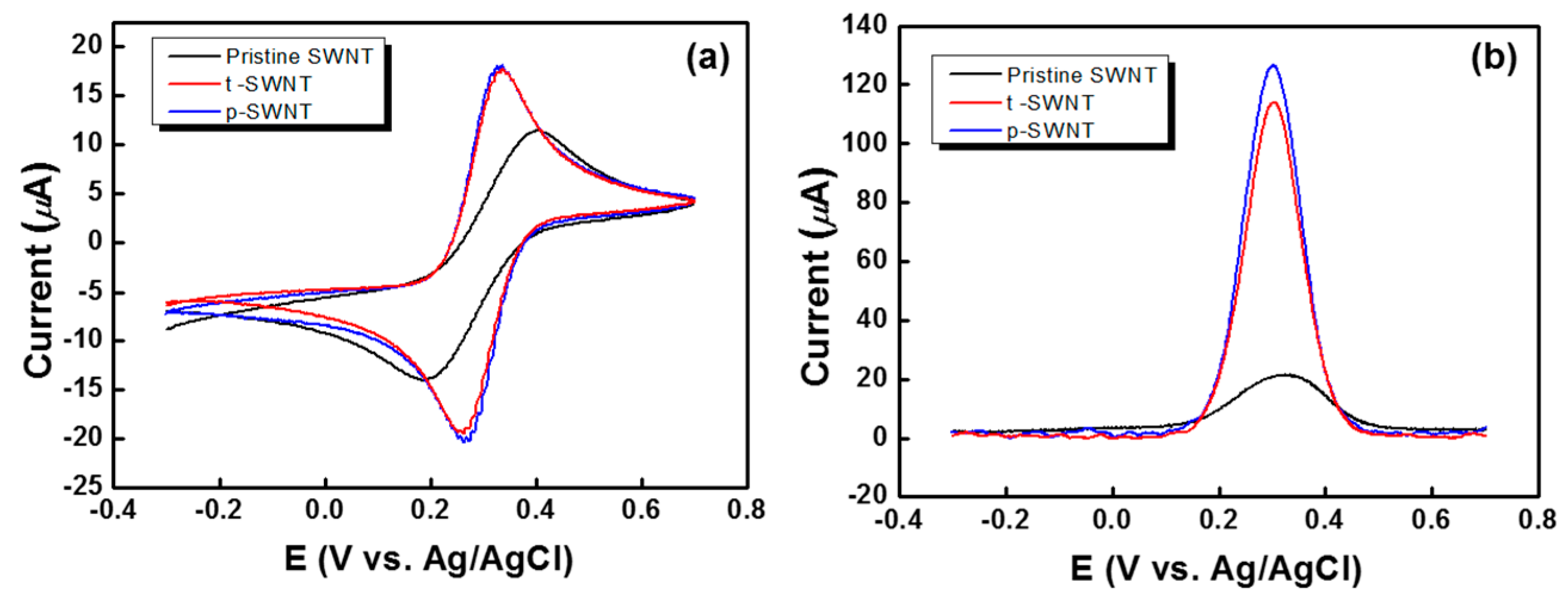
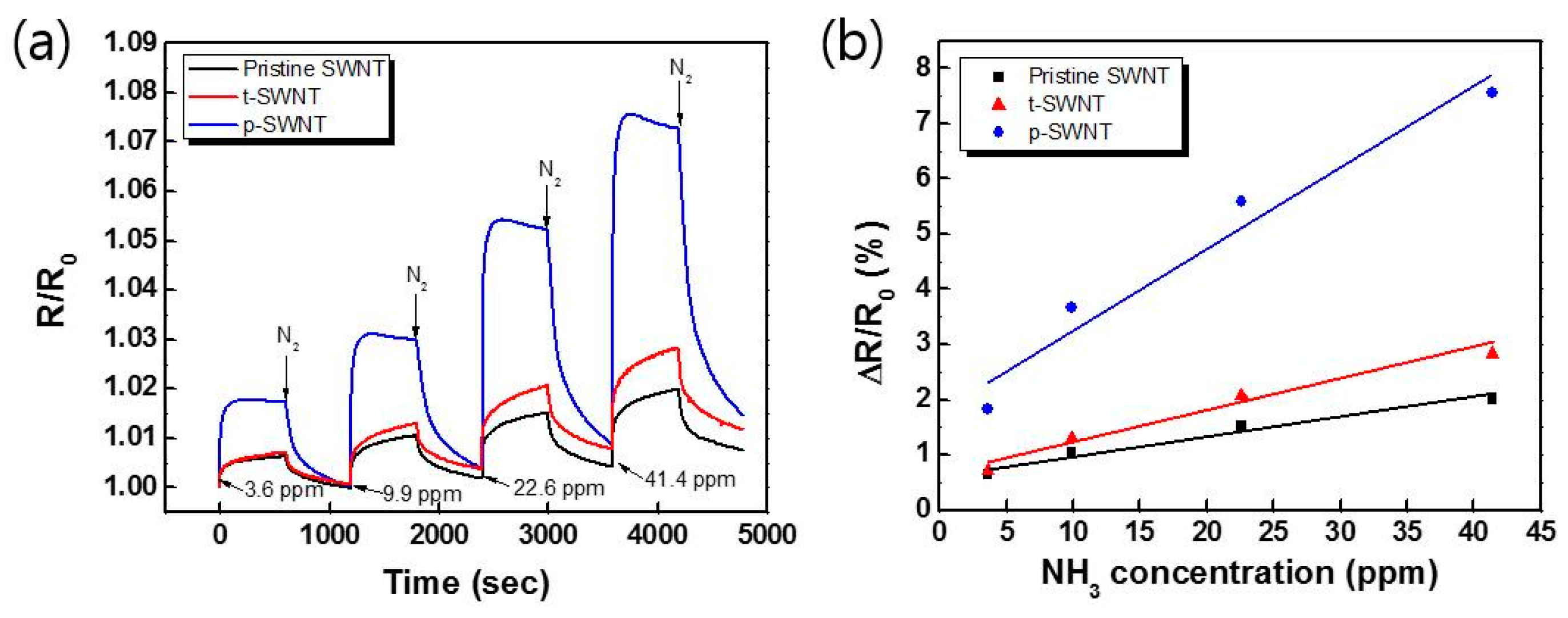
| Sample | sp2 | sp3 | C–O | C=O | –COO | Cl 2p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BE (eV) | % | BE (eV) | % | BE (eV) | % | BE (eV) | % | BE (eV) | % | BE (eV) | % | |
| Pristine SWNT | 284.47 | 69.05 | 285.33 | 16.79 | 286.53 | 6.70 | 287.68 | 3.17 | 289.29 | 2.89 | 200.40 | 3.14 |
| t-SWNT | 284.53 | 71.86 | 285.30 | 15.51 | 286.38 | 5.81 | 287.55 | 2.78 | 289.46 | 2.29 | 200.36 | 0.47 |
| p-SWNT | 284.52 | 57.85 | 285.34 | 18.64 | 286.48 | 10.31 | 287.57 | 5.14 | 288.89 | 6.68 | 200.34 | 0.47 |
| Sample | Peak 1 (cm−1) | d1 (nm) | Peak 2 (cm−1) | d2 (nm) |
|---|---|---|---|---|
| Pristine SWNT | 170.79 | 1.4120 | 180.90 | 1.3272 |
| t-SWNT | 169.10 | 1.4272 | 181.46 | 1.3228 |
| p-SWNT | 170.79 | 1.4120 | 182.58 | 1.3141 |
| Sample | Sensitivity (%/ppm) | Response Time (s) | Recovery Time (s) | Lower Detection Limit (ppm) |
|---|---|---|---|---|
| Pristine SWNT | 0.0348 | 430 | - | ~0.3 |
| t-SWNT | 0.0545 | 424 | - | ~0.3 |
| p-SWNT | 0.145 | 58 | 1066 | ~0.1 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Song, M.-J.; Kim, K.B.; Jin, J.-H.; Min, N.K. Evaluation of Surface Cleaning Procedures in Terms of Gas Sensing Properties of Spray-Deposited CNT Film: Thermal- and O2 Plasma Treatments. Sensors 2017, 17, 73. https://doi.org/10.3390/s17010073
Kim JH, Song M-J, Kim KB, Jin J-H, Min NK. Evaluation of Surface Cleaning Procedures in Terms of Gas Sensing Properties of Spray-Deposited CNT Film: Thermal- and O2 Plasma Treatments. Sensors. 2017; 17(1):73. https://doi.org/10.3390/s17010073
Chicago/Turabian StyleKim, Joon Hyub, Min-Jung Song, Ki Beom Kim, Joon-Hyung Jin, and Nam Ki Min. 2017. "Evaluation of Surface Cleaning Procedures in Terms of Gas Sensing Properties of Spray-Deposited CNT Film: Thermal- and O2 Plasma Treatments" Sensors 17, no. 1: 73. https://doi.org/10.3390/s17010073





