Raman Hyperspectral Imaging for Detection of Watermelon Seeds Infected with Acidovorax citrulli
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria-Infected Watermelon Seeds
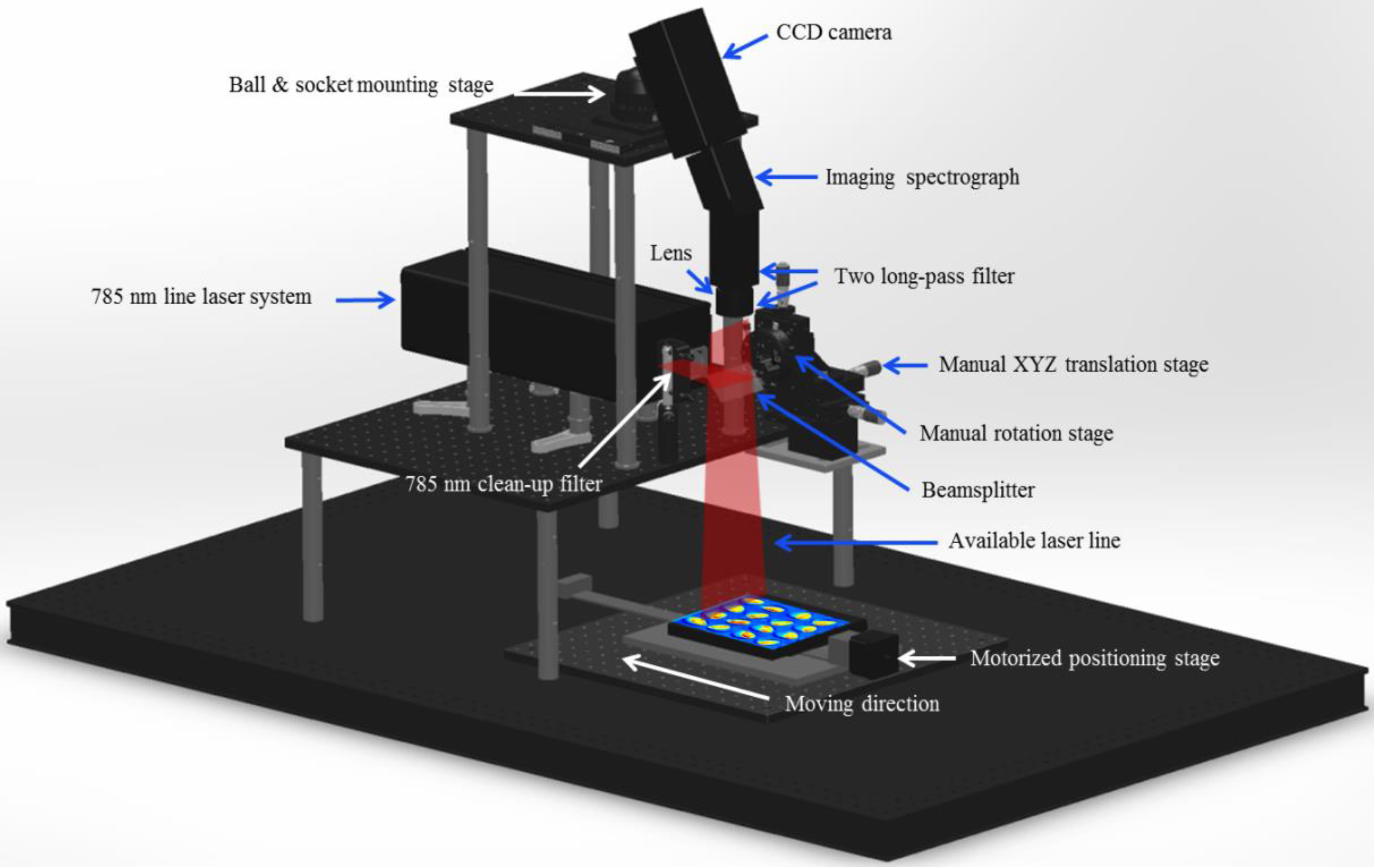
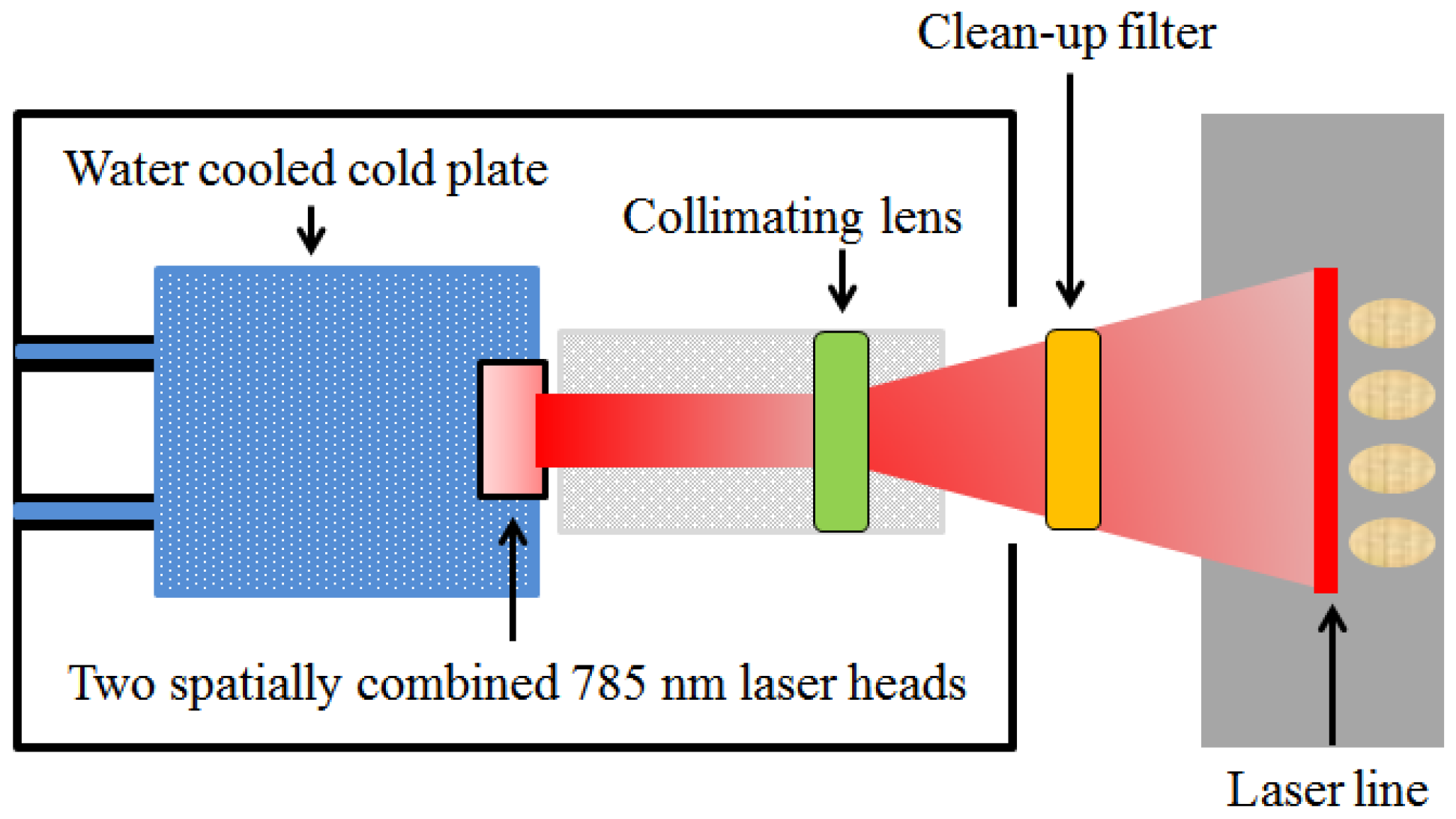
2.2. Raman Hyperspectral Imaging System
2.2.1. System Design, Operation, and Software
2.2.2. System Calibration
2.2.3. Image Acquisition and Spectral Extraction
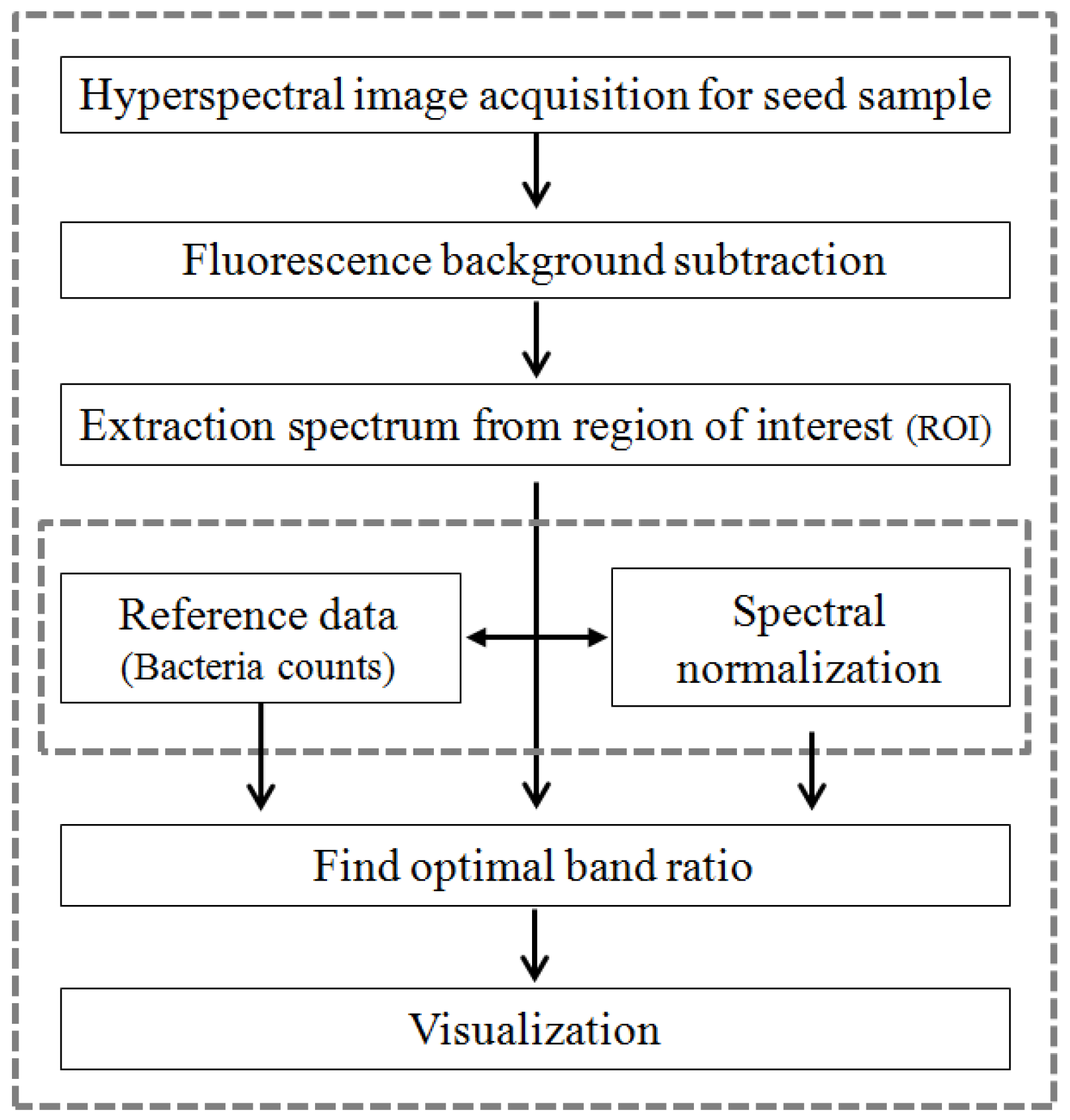
2.3. Baseline Correction and Data Analysis
3. Results and Discussion
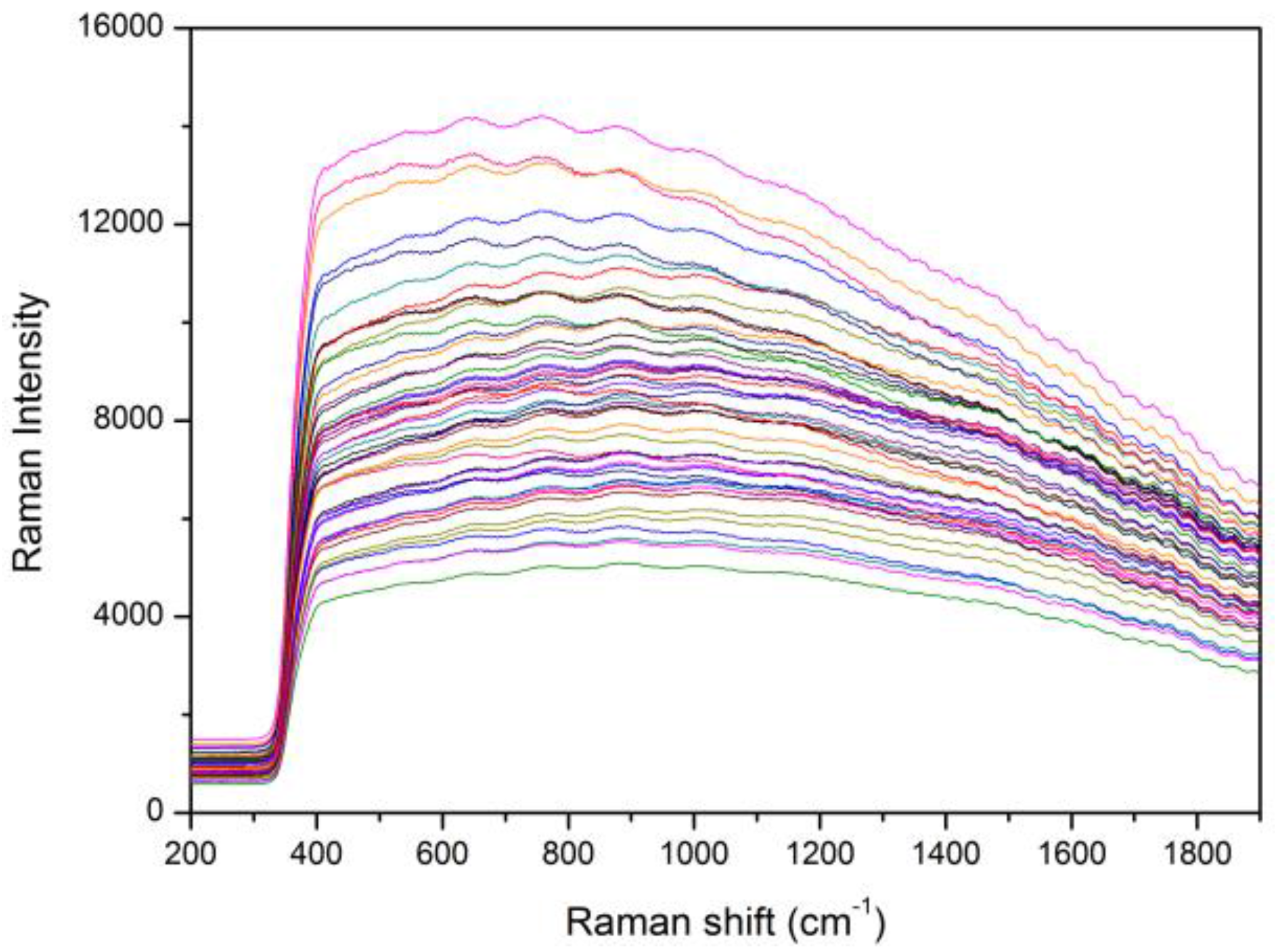
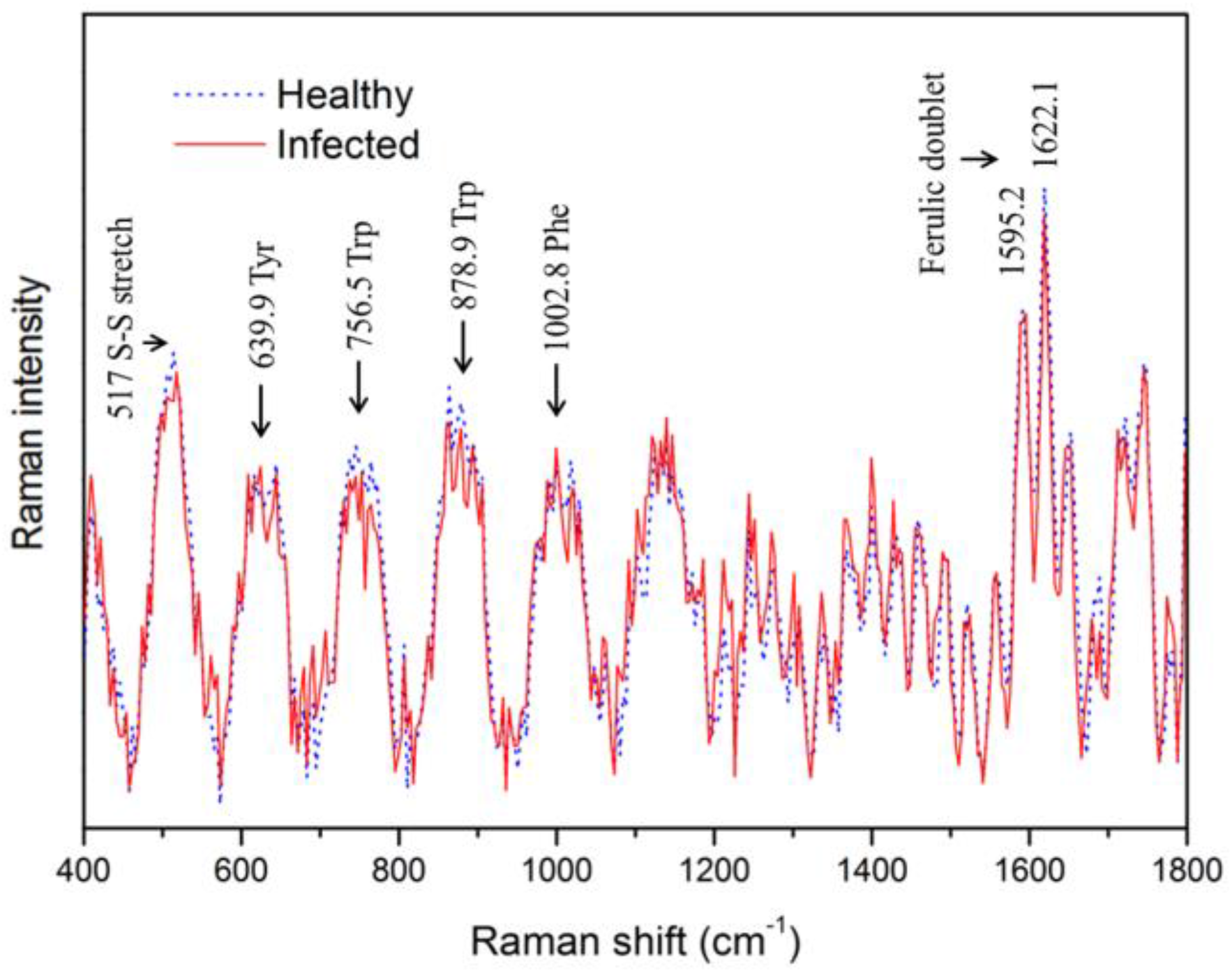
3.1. Spectral Analysis
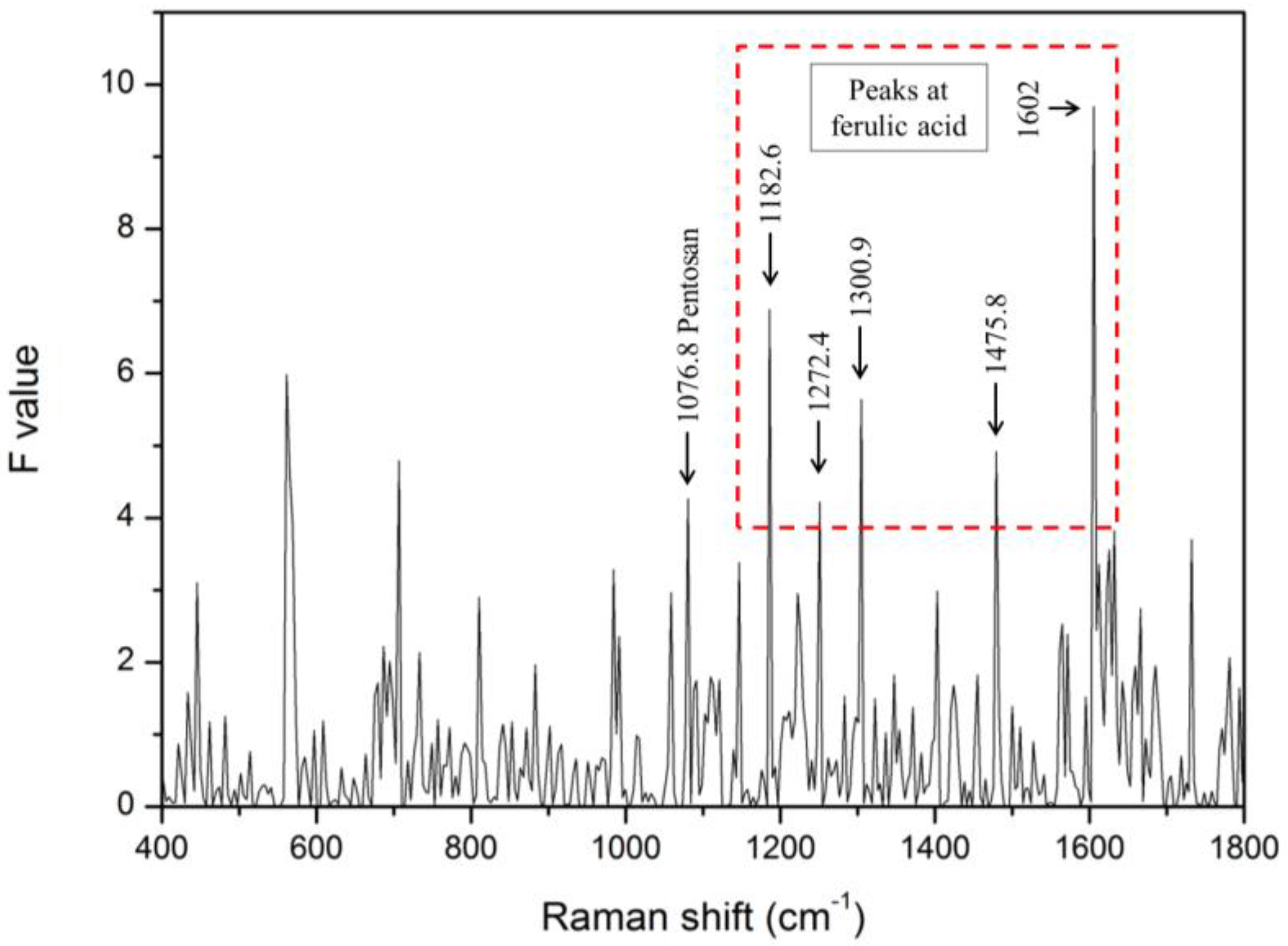
3.2. ANOVA for Classification of Bacteria-Infected and Healthy Seeds
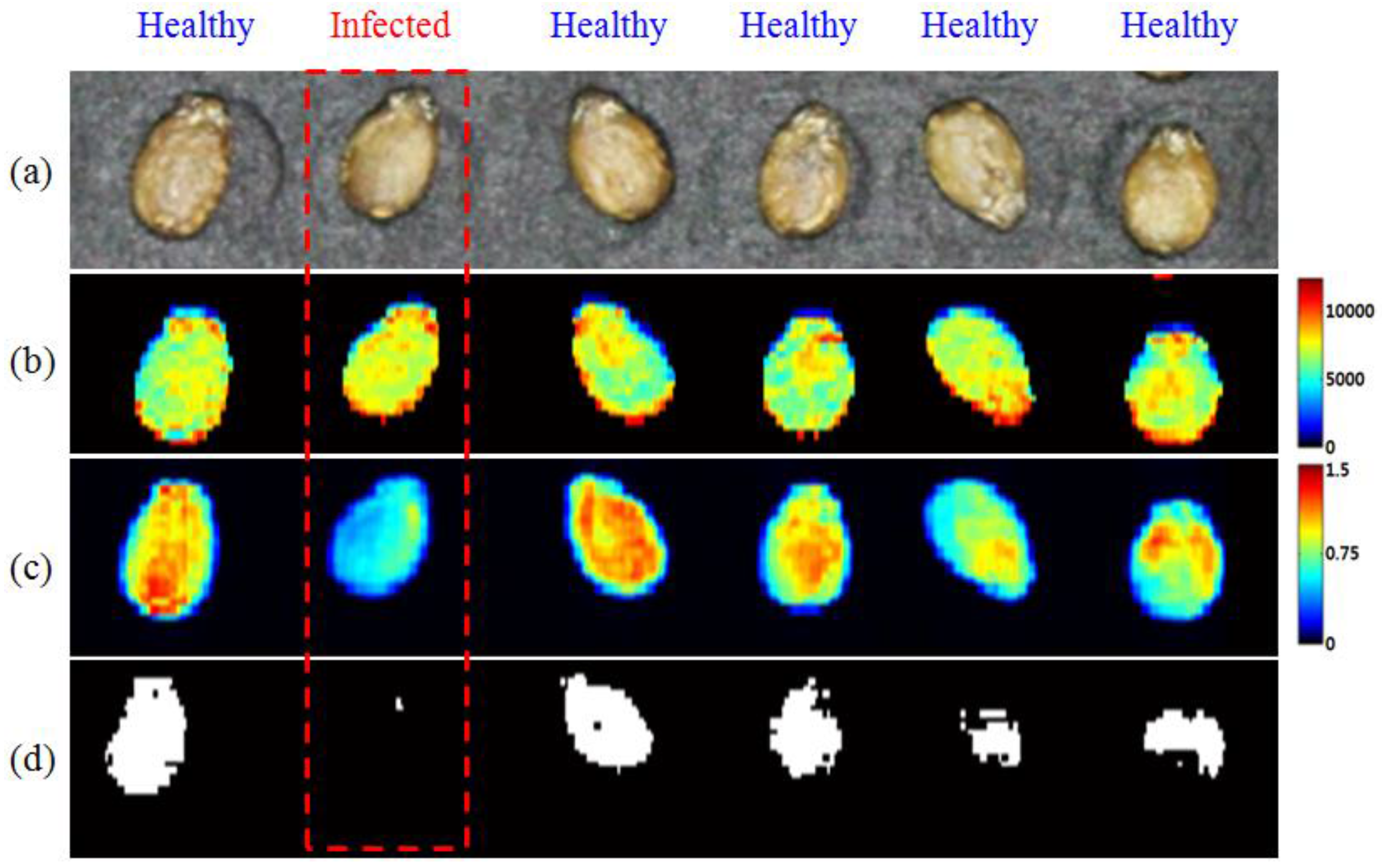
3.3. Visualization of Bacteria-Infected Seeds
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- FAOSTAT. FAOSTAT Statistics Database; Food and Agriculture Organization of the United Nations: FAOSTAT: Roma, Italy, 2014. [Google Scholar]
- Walcott, R.R.; Gitaitis, R.D.; Castro, A.C. Role of blossoms in watermelon seed infestation by Acidovorax avenae subsp citrulli. Phytopathology 2003, 93, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Wall, G.C.; Santos, V.M.; Cruz, F.J.; Nelson, D.A.; Cabrera, I. Outbreak of watermelon fruit blotch in the Mariana Islands. Plant Dis. 1990, 74, 80. [Google Scholar] [CrossRef]
- Sugiyama, M.; Ohara, T.; Sakata, Y. A new source of resistance to Cucumber green mottle mosaic virus in melon. J. Jpn. Soc. Hortic. Sci. 2006, 75, 469–475. [Google Scholar] [CrossRef]
- Pimentel, D.; Hepperly, P.; Hanson, J.; Douds, D.; Seidel, R. Environmental, energetic, and economic comparisons of organic and conventional farming systems. BioScience 2005, 55, 573–582. [Google Scholar] [CrossRef]
- Shang, H.; Xie, Y.; Zhou, X.; Qian, Y.; Wu, J. Monoclonal antibody-based serological methods for detection of Cucumber green mottle mosaic virus. Virol. J. 2011, 8, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Gitaitis, R.; Walcott, R. The epidemiology and management of seedborne bacterial diseases. Annu. Rev. Phytopathol. 2007, 45, 371–397. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Lee, S.Y.; Kim, J.Y.; Jung, H.Y.; Kim, J. Optical sensing method for screening disease in melon seeds by using optical coherence tomography. Sensors 2011, 11, 9467–9477. [Google Scholar] [CrossRef] [PubMed]
- Verboven, P.; Nemeth, A.; Abera, M.K.; Bongaers, E.; Daelemans, D.; Estrade, P.; Herremans, E.; Hertog, M.; Saeys, W.; Vanstreels, E. Optical coherence tomography visualizes microstructure of apple peel. Postharvest Biol. Technol. 2013, 78, 123–132. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, S.Y.; Jung, H.Y.; Kim, J.H. The application of optical coherence tomography in the diagnosis of Marssonina blotch in apple leaves. J. Opt. Soc. Korea 2012, 16, 133–140. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, C.; Kim, J.; Jung, H.Y. Application of optical coherence tomography to detect Cucumber green mottle mosaic virus (CGMMV) infected cucumber seed. Hortic. Environ. Biotechnol. 2012, 53, 428–433. [Google Scholar] [CrossRef]
- Shahin, M.; Tollner, E.; McClendon, R.; Arabnia, H. Apple classification based on surface bruises using image processing and neural networks. Trans. ASAE 2002, 45, 1619–1627. [Google Scholar]
- Jiang, J.A.; Chang, H.Y.; Wu, K.H.; Ouyang, C.S.; Yang, M.M.; Yang, E.C.; Chen, T.W.; Lin, T.T. An adaptive image segmentation algorithm for X-ray quarantine inspection of selected fruits. Comput. Electron. Agric. 2008, 60, 190–200. [Google Scholar] [CrossRef]
- Chuang, C.L.; Ouyang, C.S.; Lin, T.T.; Yang, M.M.; Yang, E.C.; Huang, T.W.; Kuei, C.F.; Luke, A.; Jiang, J.A. Automatic X-ray quarantine scanner and pest infestation detector for agricultural products. Comput. Electron. Agric. 2011, 77, 41–59. [Google Scholar] [CrossRef]
- McKay, R.; Palmer, G.; Ma, X.; Layzell, D.; McKee, B. The use of positron emission tomography for studies of long-distance transport in plants: Uptake and transport of 18F. Plant Cell Environ. 1988, 11, 851–861. [Google Scholar] [CrossRef]
- Alexoff, D.L.; Dewey, S.L.; Vaska, P.; Krishnamoorthy, S.; Ferrieri, R.; Schueller, M.; Schlyer, D.J.; Fowler, J.S. PET imaging of thin objects: Measuring the effects of positron range and partial-volume averaging in the leaf of Nicotiana tabacum. Nucl. Med. Biol. 2011, 38, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Converse, A.; Ahlers, E.; Bryan, T.; Williams, P.; Barnhart, T.; Engle, J.; Nickles, R.; DeJesus, O. Positron emission tomography (PET) of radiotracer uptake and distribution in living plants: Methodological aspects. J. Radioanal. Nucl. Chem. 2013, 297, 241–246. [Google Scholar] [CrossRef]
- Barreiro, P.; Zheng, C.; Sun, D.W.; Hernández-Sánchez, N.; Perez-Sanchez, J.; Ruiz-Cabello, J. Non-destructive seed detection in mandarins: Comparison of automatic threshold methods in FLASH and COMSPIRA MRIs. Postharvest Biol. Technol. 2008, 47, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Kotwaliwale, N.; Curtis, E.; Othman, S.; Naganathan, G.K.; Subbiah, J. Magnetic resonance imaging and relaxometry to visualize internal freeze damage to pickling cucumber. Postharvest Biol. Technol. 2012, 68, 22–31. [Google Scholar] [CrossRef]
- Aristizábal Torres, I.D. The magnetic resonance and its agro-industry applications, a review. Rev. Fac. Nac. Agron. Medellín 2007, 60, 4037–4066. [Google Scholar]
- Sun, D.W.; Li, B. Microstructural change of potato tissues frozen by ultrasound-assisted immersion freezing. J. Food Eng. 2003, 57, 337–345. [Google Scholar] [CrossRef]
- Bhaskaracharya, R.K.; Kentish, S.; Ashokkumar, M. Selected applications of ultrasonics in food processing. Food Eng. Rev. 2009, 1, 31–49. [Google Scholar] [CrossRef]
- Kiani, H.; Zhang, Z.; Delgado, A.; Sun, D.W. Ultrasound assisted nucleation of some liquid and solid model foods during freezing. Food Res. Int. 2011, 44, 2915–2921. [Google Scholar] [CrossRef]
- Mehl, P.M.; Chen, Y.R.; Kim, M.S.; Chan, D.E. Development of hyperspectral imaging technique for the detection of apple surface defects and contaminations. J. Food Eng. 2004, 61, 67–81. [Google Scholar] [CrossRef]
- Kandpal, L.M.; Park, E.; Tewari, J.; Cho, B.K. Spectroscopic Techniques for Nondestructive Quality Inspection of Pharmaceutical Products: A Review. J. Biosyst. Eng. 2015, 40, 394–408. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S.; Lu, R.; Burks, T.F. Hyperspectral and multispectral imaging for evaluating food safety and quality. J. Food Eng. 2013, 118, 157–171. [Google Scholar] [CrossRef]
- Seo, Y.W.; Ahn, C.K.; Lee, H.; Park, E.; Mo, C.; Cho, B.K. Non-destructive sorting techniques for viable pepper (Capsicum annuum L.) seeds using Fourier transform near-infrared and raman spectroscopy. J. Biosyst. Eng. 2016, 41, 51–59. [Google Scholar] [CrossRef]
- Schulmerich, M.V.; Walsh, M.J.; Gelber, M.K.; Kong, R.; Kole, M.R.; Harrison, S.K.; McKinney, J.; Thompson, D.; Kull, L.S.; Bhargava, R. Protein and oil composition predictions of single soybeans by transmission Raman spectroscopy. J. Agric. Food Chem. 2012, 60, 8097–8102. [Google Scholar] [CrossRef] [PubMed]
- Raman, C.V.; Schmid, E.D. Proceedings of the sixth International Conference on Raman Spectroscopy, Bangalore, India, 4–9 September 1978; Heyden: London, UK; Philadelphia, PA, USA; Rheine, Germany, 1978. [Google Scholar]
- Wen, Z.Q. Raman spectroscopy of protein pharmaceuticals. J. Pharm. Sci. 2007, 96, 2861–2878. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.; Nakai, S.; Hirotsuka, M. Raman spectroscopy as a probe of protein structure in food systems. In Protein Structure-Function Relationships in Foods; Springer: Boston, MA, USA, 1994; pp. 163–197. [Google Scholar]
- Herrero, A.M. Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chem. 2008, 107, 1642–1651. [Google Scholar] [CrossRef]
- Lu, X.; Al-Qadiri, H.M.; Lin, M.; Rasco, B.A. Application of mid-infrared and Raman spectroscopy to the study of bacteria. Food Bioprocess Technol. 2011, 4, 919–935. [Google Scholar] [CrossRef]
- Kizil, R.; Irudayaraj, J.; Seetharaman, K. Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem. 2002, 50, 3912–3918. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, B.K.; Kim, M.S.; Lee, W.H.; Tewari, J.; Bae, H.; Sohn, S.I.; Chi, H.Y. Prediction of crude protein and oil content of soybeans using Raman spectroscopy. Sens. Actuators B Chem. 2013, 185, 694–700. [Google Scholar] [CrossRef]
- Baeten, V.; Hourant, P.; Morales, M.T.; Aparicio, R. Oil and fat classification by FT-Raman spectroscopy. J. Agric. Food Chem. 1998, 46, 2638–2646. [Google Scholar] [CrossRef]
- Gowen, A.; O’Donnell, C.; Cullen, P.; Downey, G.; Frias, J. Hyperspectral imaging–an emerging process analytical tool for food quality and safety control. Trends Food Sci. Technol. 2007, 18, 590–598. [Google Scholar] [CrossRef]
- Delwiche, S.R.; Kim, M.S.; Dong, Y. Fusarium damage assessment in wheat kernels by Vis/NIR hyperspectral imaging. Sens. Instrum. Food Qual. Saf. 2011, 5, 63–71. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, Z.; Liu, X.; Ustin, S.L. Detection of stress in tomatoes induced by late blight disease in California, USA, using hyperspectral remote sensing. Int. J. Appl. Earth Obs. Geoinform. 2003, 4, 295–310. [Google Scholar] [CrossRef]
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A review of advanced techniques for detecting plant diseases. Comput. Electron. Agric. 2010, 72, 1–13. [Google Scholar] [CrossRef]
- Wu, D.; Sun, D.W. Advanced applications of hyperspectral imaging technology for food quality and safety analysis and assessment: A review—Part II: Applications. Innov. Food Sci. Emerg. Technol. 2013, 19, 15–28. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M. Raman chemical imaging system for food safety and quality inspection. Trans. ASABE 2010, 53, 1873–1882. [Google Scholar] [CrossRef]
- Qin, J.; Chao, K.; Kim, M.S. Simultaneous detection of multiple adulterants in dry milk using macro-scale Raman chemical imaging. Food Chem. 2013, 138, 998–1007. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chao, K.; Kim, M.S. A line-scan hyperspectral system for high-throughput Raman chemical imaging. Appl. Spectrosc. 2014, 68, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Chao, K.; Kim, M.S.; Cho, B.K. Line-Scan Macro-scale Raman Chemical Imaging for Authentication of Powdered Foods and Ingredients. Food Bioprocess Technol. 2016, 9, 113–123. [Google Scholar] [CrossRef]
- Qin, J.W.; Chao, K.L.; Kim, M.S. Investigation of Raman chemical imaging for detection of lycopene changes in tomatoes during postharvest ripening. J. Food Eng. 2011, 107, 277–288. [Google Scholar] [CrossRef]
- Lieber, C.A.; Mahadevan-Jansen, A. Automated method for subtraction of fluorescence from biological Raman spectra. Appl. Spectrosc. 2003, 57, 1363–1367. [Google Scholar] [CrossRef] [PubMed]
- Lohumi, S.; Lee, S.; Lee, H.; Kim, M.S.; Lee, W.H.; Cho, B.K. Application of hyperspectral imaging for characterization of intramuscular fat distribution in beef. Infrared Phys. Technol. 2016, 74, 1–10. [Google Scholar] [CrossRef]
- Piot, O.; Autran, J.C.; Manfait, M. Spatial distribution of protein and phenolic constituents in wheat grain as probed by confocal Raman microspectroscopy. J. Cereal Sci. 2000, 32, 57–71. [Google Scholar] [CrossRef]
- Saulnier, L.; Sado, P.E.; Branlard, G.; Charmet, G.; Guillon, F. Wheat arabinoxylans: Exploiting variation in amount and composition to develop enhanced varieties. J. Cereal Sci. 2007, 46, 261–281. [Google Scholar] [CrossRef]
- Van der Watt, E.; Pretorius, J.C. Purification and identification of active antibacterial components in Carpobrotus edulis L. J. Ethnopharmacol. 2001, 76, 87–91. [Google Scholar] [CrossRef]
- Brouns, F.; Hemery, Y.; Price, R.; Anson, N.M. Wheat aleurone: Separation, composition, health aspects, and potential food use. Crit. Rev. food Sci. Nutr. 2012, 52, 553–568. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Kim, M.S.; Qin, J.; Park, E.; Song, Y.-R.; Oh, C.-S.; Cho, B.-K. Raman Hyperspectral Imaging for Detection of Watermelon Seeds Infected with Acidovorax citrulli. Sensors 2017, 17, 2188. https://doi.org/10.3390/s17102188
Lee H, Kim MS, Qin J, Park E, Song Y-R, Oh C-S, Cho B-K. Raman Hyperspectral Imaging for Detection of Watermelon Seeds Infected with Acidovorax citrulli. Sensors. 2017; 17(10):2188. https://doi.org/10.3390/s17102188
Chicago/Turabian StyleLee, Hoonsoo, Moon S. Kim, Jianwei Qin, Eunsoo Park, Yu-Rim Song, Chang-Sik Oh, and Byoung-Kwan Cho. 2017. "Raman Hyperspectral Imaging for Detection of Watermelon Seeds Infected with Acidovorax citrulli" Sensors 17, no. 10: 2188. https://doi.org/10.3390/s17102188





