Copper Oxide Chitosan Nanocomposite: Characterization and Application in Non-Enzymatic Hydrogen Peroxide Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Chitosan:CuOx Sensor
2.2. Characterization of Chitosan:CuOx Sensor
2.3. Chitosan:CuOx Sensing Tests
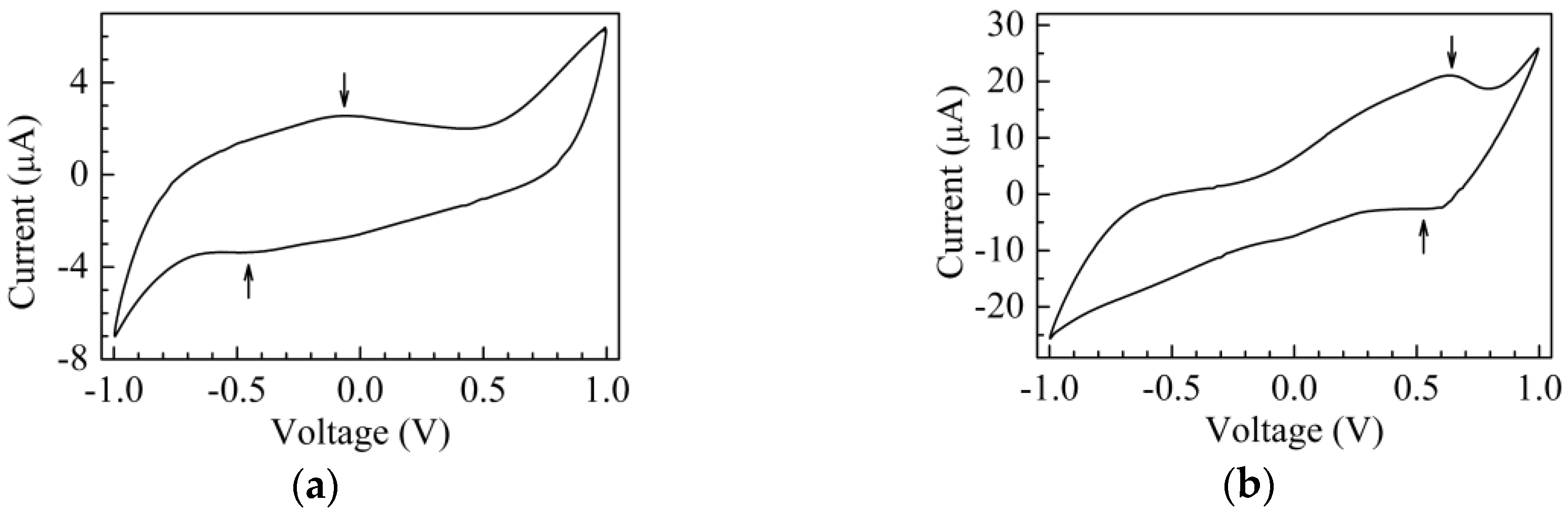
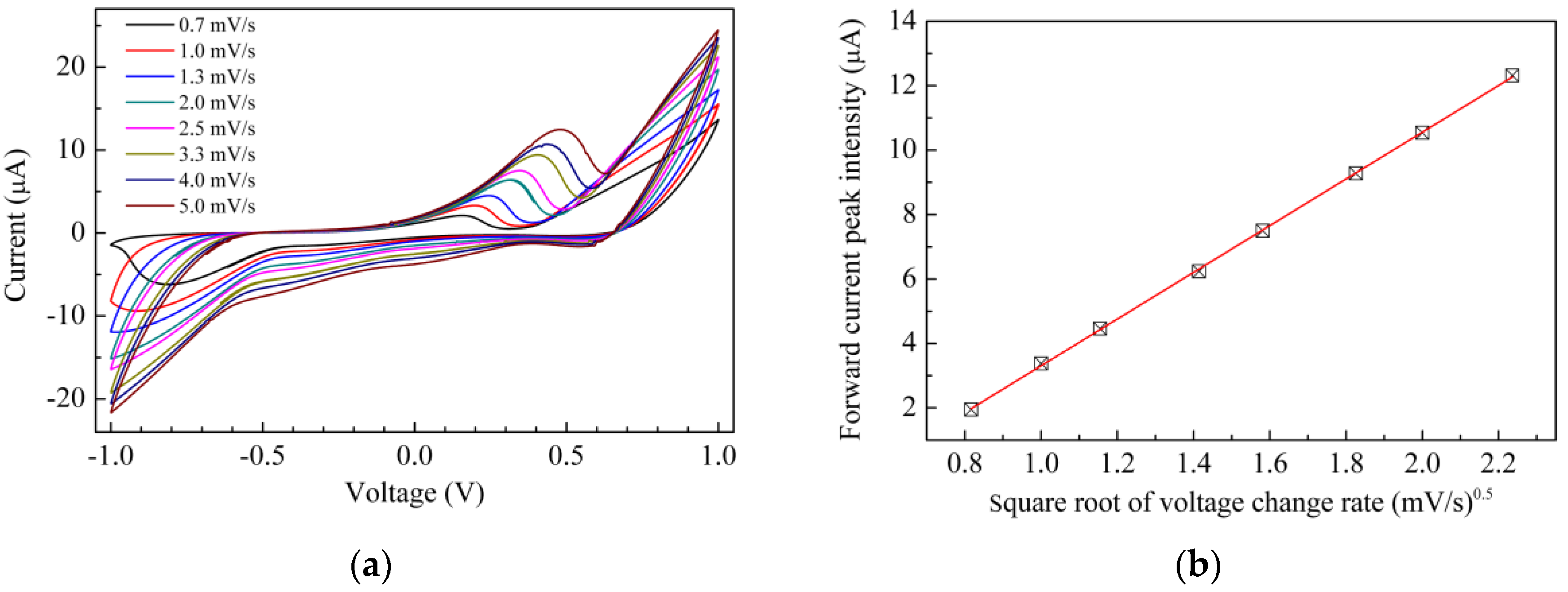
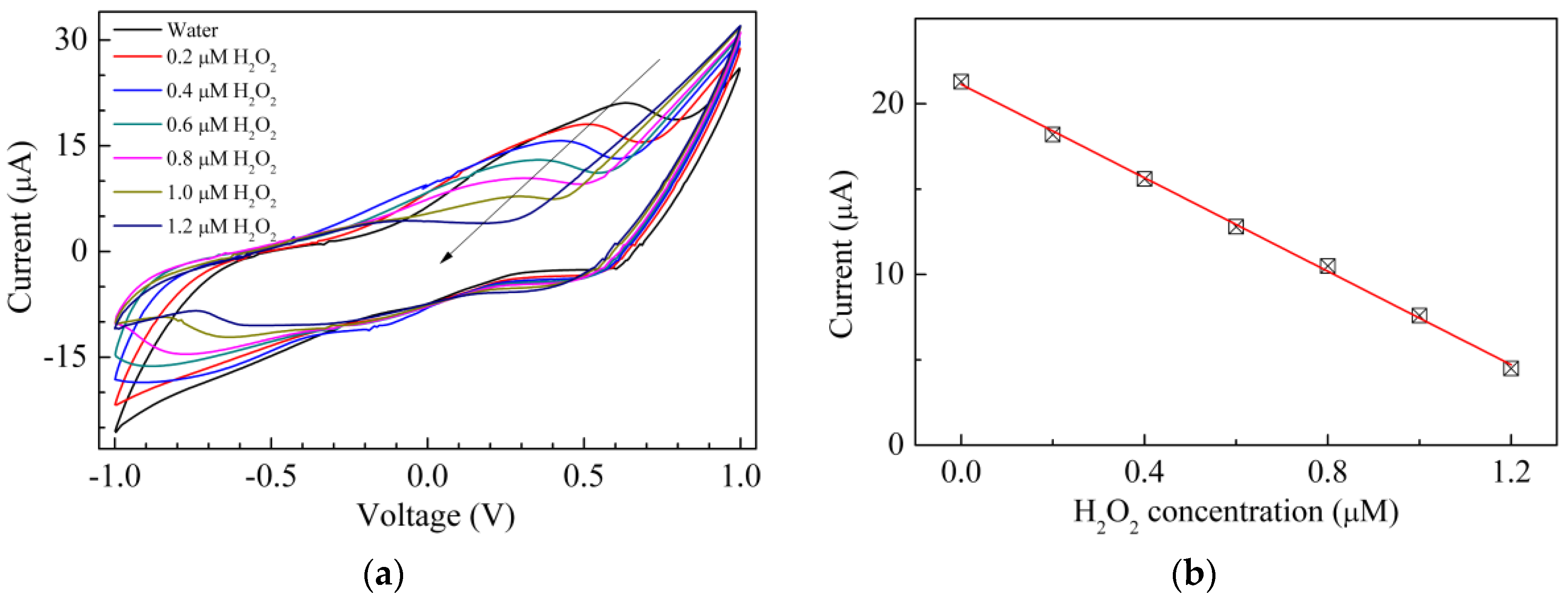
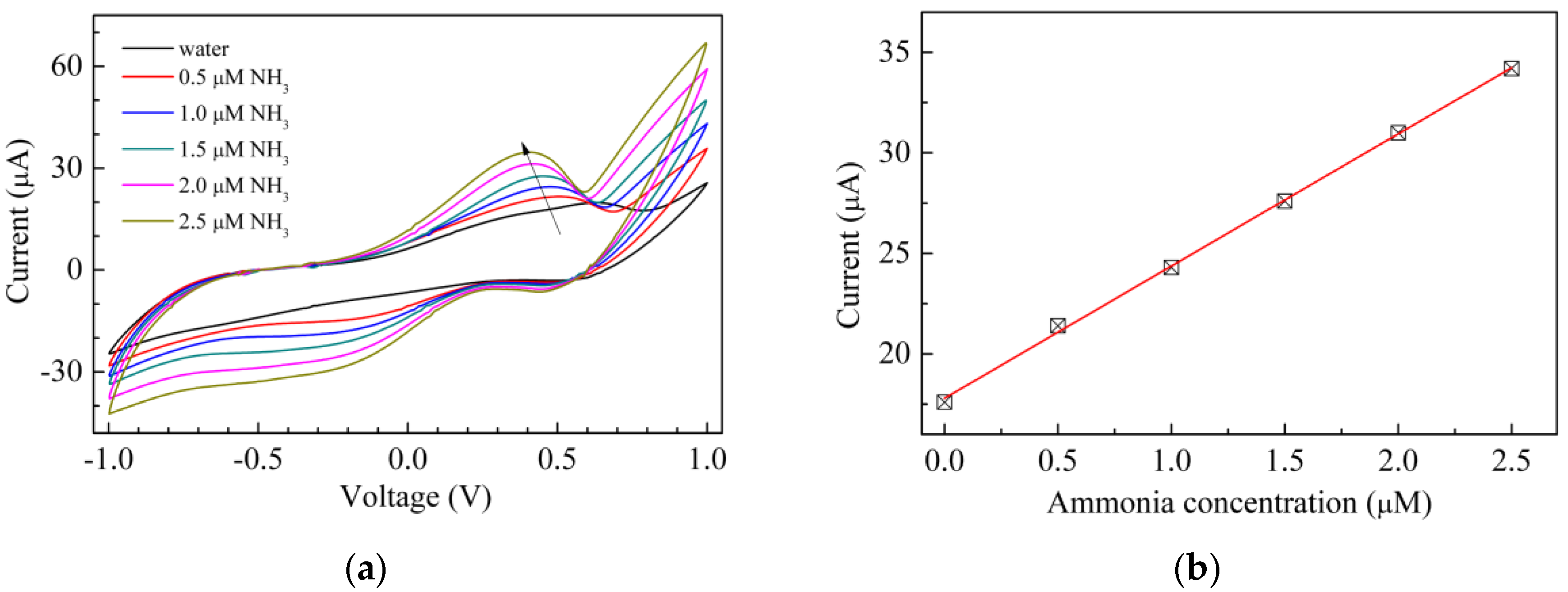
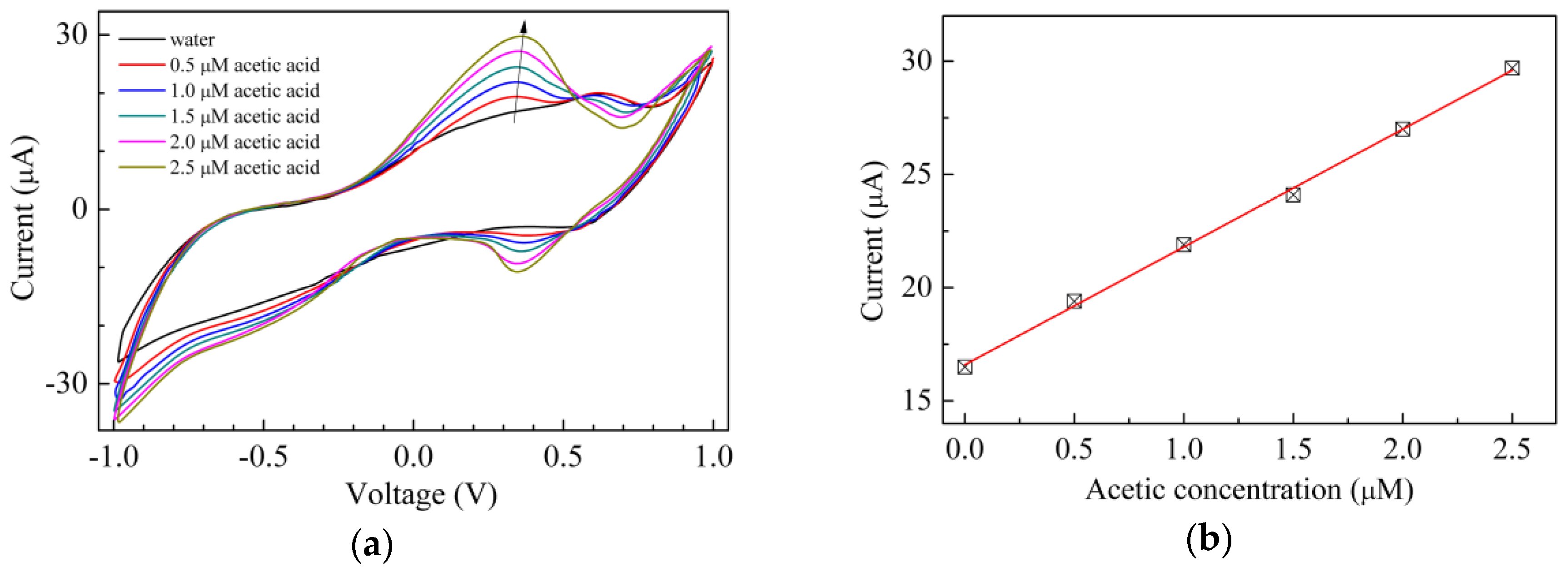
3. Results and Discussion
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Tripathi, B.P.; Shahi, V.K. Organic-inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Romero, P.G.; Chojak, M.; Cuentas-Gallegoa, K.; Asensio, J.A.; Kulesza, P.J.; Casañ-Pastor, N.; Lira-Cantú, M. Hybrid organic-inorganic nanocomposite materials for application in solid state electrochemical supercapacitors. Electrochem. Commun. 2003, 5, 149–153. [Google Scholar] [CrossRef]
- Liu, R. Hybrid Organic/Inorganic Nanocomposites for Photovoltaic Cells. Materials 2014, 7, 2747–2771. [Google Scholar] [CrossRef] [PubMed]
- Bouclé, J.; Ravirajan, P.; Nelson, J. Hybrid polymer–metal oxide thin films for photovoltaic applications. J. Mater. Chem. 2007, 17, 3141–3153. [Google Scholar] [CrossRef]
- Grover, R.; Nanda, O.; Gupta, N.; Saxena, K. Hydrogen peroxide sensing properties of PVA/TiO2/I2 nanocomposite-based free standing membranes. J. Appl. Polym. Sci. 2015, 132, 42257. [Google Scholar] [CrossRef]
- Wang, S.; Kang, Y.; Wang, L.; Zhang, H.; Wang, Y.; Wang, Y. Organic/inorganic hybrid sensors: A review. Sens. Actuators Chem. B 2013, 182, 467–481. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Zhou, P.-J.; Li, J.-L.; Wang, Y. Studies on the photocatalytic performance of cuprous oxide/chitosan nanocomposite activated by visible light. Carbohydr. Polym. 2008, 72, 128–132. [Google Scholar] [CrossRef]
- Sarkar, S.; Guibal, E.; Quignard, F.; SenGupta, A.K. Polymer-supported metals and metal oxide nanoparticles: Synthesis, characterization, and applications. J. Nanopart. Res. 2012, 14, 715. [Google Scholar] [CrossRef]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef]
- Francis Suh, J.-K.; Matthew, H.W.T. Application of chitosan-based polysaccharide biomaterials in cartilage tissue engineering: A review. Biomaterials 2000, 21, 2589–2598. [Google Scholar] [CrossRef]
- Salih, E.; Reicha, F.M.; El-Sherbiny, I.M. Electrochemical Synthesis of New Silver-Chitosan/Polyvinyl Alcohol Hybrid Nanoparticles and Evaluation of Their Antibacterial Activities. J. Nanosci. Nanotechnol. 2016, 2, 94–96. [Google Scholar]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Lalov, I.G.; Guerginov, I.I.; Krysteva1, M.A.; Fartsov, K. Treatment of waste water from distilleries with chitosan. Water Res. 2000, 34, 1503–1506. [Google Scholar] [CrossRef]
- Biró, E.; Németh, Á.S.; Sisak, C.; Feczkó, T.; Gyenis, J. Preparation of chitosan particles suitable for enzyme immobilization. J. Biochem. Biophys. Methods 2008, 70, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Hu, X. An Optical Fiber H2O2-Sensing Probe Using a Titanium(IV) Oxyacetylacetonate Immobilized Nafion Coating on an Bent Optical Fiber Probe. IEEE Sens. J. 2011, 11, 2032–2036. [Google Scholar] [CrossRef]
- Yoon, S.H.; Jeong, W.T.; Kim, K.C.; Kim, K.J.; Oh, M.C.; Lee, S.M. Development of the Biopolymeric Optical Planar Waveguide with Nanopattern. J. Surf. Eng. Mater. Adv. Technol. 2011, 1, 56–61. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Utilization of Chitosan-Based Sensor Thin Films for the Detection of Lead Ion by Surface Plasmon Resonance Optical Sensor. IEEE Sens. J. 2013, 13, 1413–1418. [Google Scholar] [CrossRef] [Green Version]
- Nasution, T.I.; Nainggolan, I.; Hutagalung, S.D.; Ahmad, K.R.; Ahmad, Z.A. The sensing mechanism and detection of low concentration acetone using chitosan-based sensors. Sens. Actuators Chem. B 2013, 177, 522–528. [Google Scholar] [CrossRef]
- Murray, C.A.; Dutcher, J.R. Effect of Changes in Relative Humidity and Temperature on Ultrathin Chitosan Films. Biomacromolecules 2006, 12, 3460–3465. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ibáñez, N.; García-Cruz, L.; Montiel, V.; Foster, C.W.; Banks, C.E.; Iniesta, J. Electrochemical lactate biosensor based upon chitosan/carbon nanotubes modified screen-printed graphite electrodes for the determination of lactate in embryonic cell cultures. Biosens. Bioelectron. 2016, 77, 1168–1174. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Wang, J.; Wu, H.; Aksay, I.A.; Liu, J.; Lin, Y. Glucose-Oxidase-graphene-chitosan modified electrode for direct electrochemistry and glucose sensing. Biosens. Bioelectron. 2009, 25, 901–905. [Google Scholar] [CrossRef]
- Wang, G.; Xu, J.J.; Ye, L.H.; Zhu, J.J.; Chen, H.Y. Highly sensitive sensors based on the immobilization of tyrosinase in chitosan. Bioelectrochemistry 2002, 57, 33–38. [Google Scholar] [CrossRef]
- Mao, A.; Li, H.; Jin, D.; Yu, L.; Hu, X. Fabrication of electrochemical sensor for paracetamol based on multi-walled carbon nanotubes and chitosan–copper complex by self-assembly technique. Talanta. 2015, 144, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Dehdashtian, S.; Gholivand, M.B.; Shamsipur, M.; Kariminia, S. Construction of a sensitive and selective sensor for morphine using chitosan coated Fe3O4 magnetic nanoparticle as a modifier. Mater. Sci. Eng. C 2016, 58, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Rovina, K.; Siddiquee, S. Electrochemical sensor based rapid determination of melamine using ionic liquid/zinc oxide nanoparticles/chitosan/gold electrode. Food Cont. 2016, 59, 801–808. [Google Scholar] [CrossRef]
- Wang, H.S.; Pan, Q.X.; Wang, G.X. A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide. Sensors 2005, 5, 266–276. [Google Scholar] [CrossRef]
- Yalçıner, F.; Çevik, E.; Şenel, M.; Baykal, A. Development of an Amperometric Hydrogen Peroxide Biosensor based on the Immobilization of Horseradish Peroxidase onto Nickel Ferrite Nanoparticle-Chitosan Composite. Nano-Micro Lett. 2011, 3, 91–98. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y.; Li, Y.; Li, S. A sensitive enzyme-free hydrogen peroxide sensor based on a chitosan–graphene quantum dot/silver nanocube nanocomposite modified electrode. Anal. Methods 2016, 8, 2448–2455. [Google Scholar] [CrossRef]
- Jia, N.; Huang, B.; Chen, L.; Tan, L.; Yao, S. A simple non-enzymatic hydrogen peroxide sensor using gold nanoparticles-graphene-chitosan modified electrode. Sens. Actuators Chem. B 2014, 195, 165–170. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, D.; Chen, Q. Non-enzymatic Hydrogen Peroxide Sensors Based on Multi-wall Carbon Nanotube/Pt Nanoparticle Nanohybrids. Materials 2014, 7, 2945–2955. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, G.; Arena, A.; Ciofi, C.; Saitta, G. Electrical Characterization and Hydrogen Peroxide Sensing Properties of Gold/Nafion:Polypyrrole/MWCNTs Electrochemical Devices. Sensors 2013, 13, 3878–3888. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, G.; Arena, A.; Ciofi, C.; Saitta, G.; Lanza, M. Electrochemical Detection of p-Aminophenol by Flexible Devices Based on Multi-Wall Carbon Nanotubes Dispersed in Electrochemically Modified Nafion. Sensors 2014, 14, 8926–8939. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Wang, X.; Guo, X.; Zhang, Z.; Chen, Y.; Wang, Y. Electrodeposition of chitosan based on coordination with metal ions in situ-generated by electrochemical oxidation. J. Mater. Chem. B 2016, 4, 3331–3338. [Google Scholar] [CrossRef]
- Rhazi, M.; Desbrières, J.; Tolaimate, A.; Rinaudo, M.; Vottero, P.; Alagui, A. Contribution to the study of the complexation of copper by chitosan and oligomers. Polymer 2002, 43, 1267–1276. [Google Scholar] [CrossRef]
- Omar, F.A.; El-Tonsy, M.M.; Oraby, A.H.; Reicha, F.M. Copper and Copper Oxides Nanoparticles Synthesized by Electrochemical Technique in Chitosan Solution. Inter. J. Sci. Eng. Appli. 2016, 3, 180–187. [Google Scholar] [CrossRef]
- Basumallick, S.; Santra, S. Chitosan coated copper-oxides nano particles: A novel electro-catalyst for CO2 reduction. RSC Adv. 2014, 4, 63685–63690. [Google Scholar] [CrossRef]
- Yang, G.; Chen, F.; Yang, Z. Electrocatalytic Oxidation of Hydrogen Peroxide Based on the Shuttlelike Nano-CuO-Modified Electrode. Inter. J. Electrochem. 2012, 194183. [Google Scholar] [CrossRef]
- Kamyabi, M.A.; Hajari, N. Low Potential and Non-Enzymatic Hydrogen Peroxide Sensor Based on Copper Oxide Nanoparticle on Activated Pencil Graphite Electrode. J. Braz. Chem. Soc. 2016, 28, 808–818. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, F.; Zhang, X.; Yang, L. Facile synthesis of flower like copper oxide and their application to hydrogen peroxide and nitrite sensing. Chem. Cent. J. 2011, 5, 75. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, S.K.; Palani, B.; Pillai, K.C. Highly stable and redox active nano copper species stabilized functionalized-multiwalled carbon nanotube/chitosan modified electrode for efficient hydrogen peroxide detection. Colloids Surf. A Physicochem. Eng. Asp. 2012, 395, 207–216. [Google Scholar] [CrossRef]
- Zaman, S.; Asif, M.H.; Zainelabdin, A.; Willander, M. CuO nanoflowers as an electrochemical pH sensor and the effect of pH on the growth. J. Electroanal. Chem. 2011, 662, 421–425. [Google Scholar] [CrossRef]
- Devasenathipathy, R.; Liu, Y.-X.; Yang, C.; Kohilarany, K.; Wang, S.-F. Simple electrochemical growth of copper nanoparticles decorated silver nanoleaves for the sensitive determination of hydrogen peroxide in clinical lens cleaning solutions. Sens. Actuators Chem. B 2017, 252, 862–869. [Google Scholar] [CrossRef]
- Jin, J.; Wu, W.; Min, H.; Wu, H.; Wang, S.; Ding, Y.; Yang, S. A glassy carbon electrode modified with FeS nanosheets as a highly sensitive amperometric sensor for hydrogen peroxide. Microchem. Acta. 2017, 184, 1389–1396. [Google Scholar] [CrossRef]
- Habibi, B.; Azhar, F.F.; Fakkar, J.; Rezvani, Z. Ni–Al/layered double hydroxide/Ag nanoparticle composite modified carbon-paste electrode as a renewable electrode and novel electrochemical sensor for hydrogen peroxide. Anal. Methods 2017, 9, 1956–1964. [Google Scholar] [CrossRef]
- Sheng, Q.; Shen, Y.; Zhang, J.; Zheng, J. Ni doped Ag@C core–shell nanomaterials and their application in electrochemical H2O2 sensing. Anal. Methods 2017, 9, 163–169. [Google Scholar] [CrossRef]
- Li, S.J.; Xing, Y.; Yang, H.Y.; Huang, J.Y.; Wang, W.T.; Liu, R.T. Electrochemical Synthesis of a Binary Mn-Co Oxides Decorated Graphene Nanocomposites for Application in Nonenzymatic H2O2 Sensing. Int. J. Electrochem. Sci. 2017, 12, 6566–6576. [Google Scholar] [CrossRef]
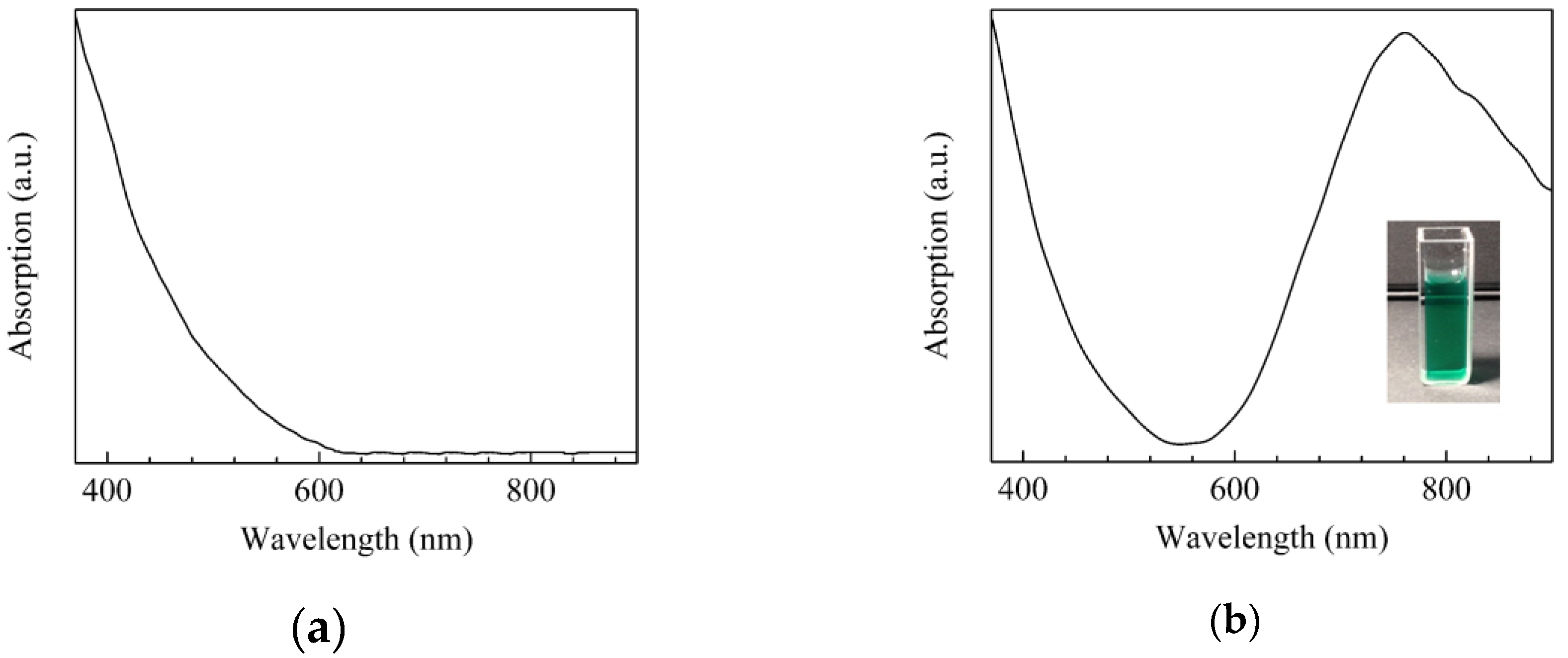
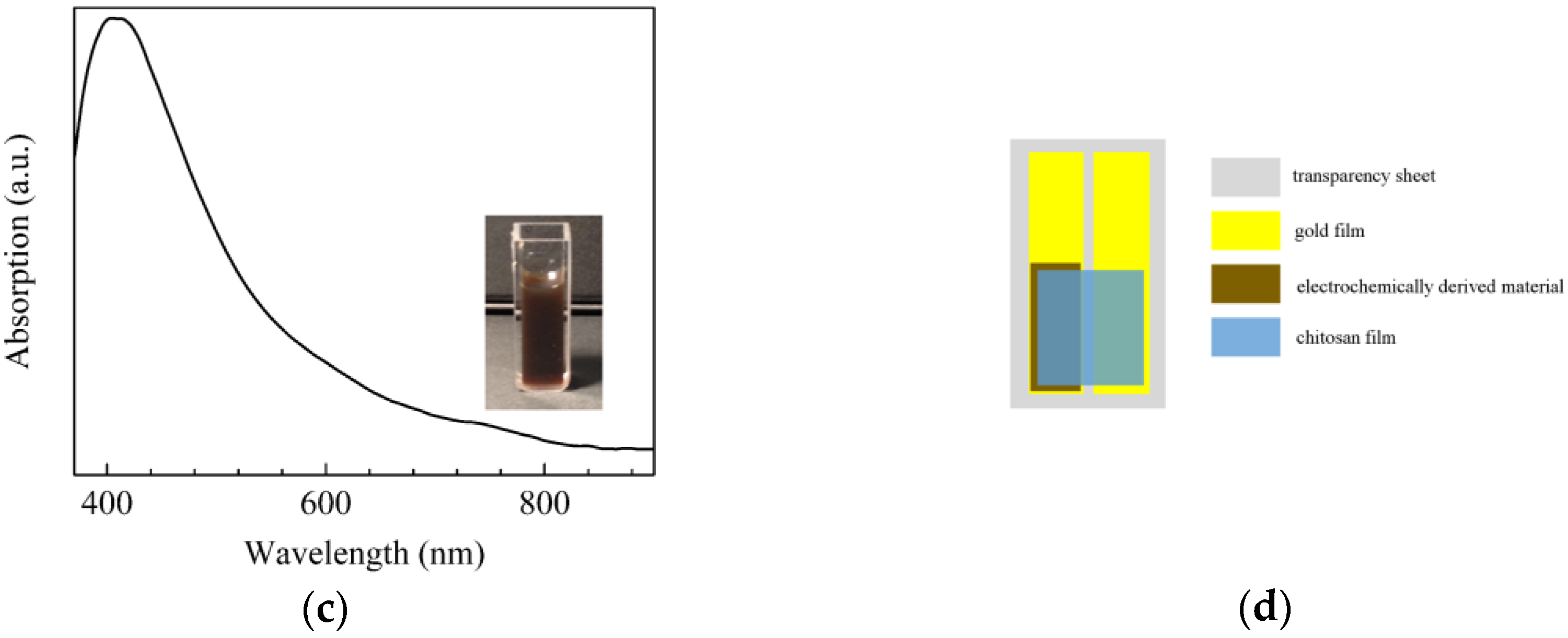
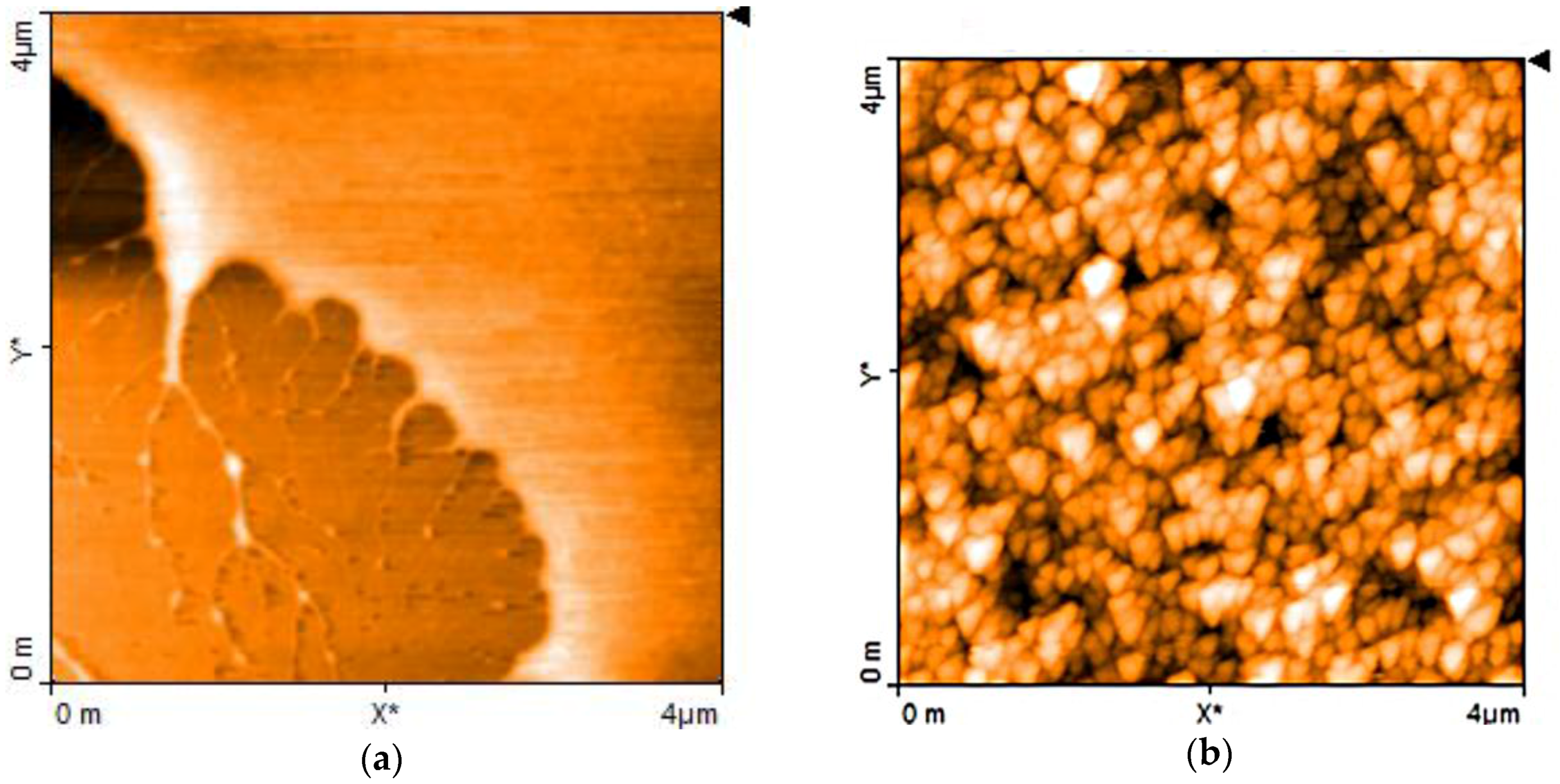
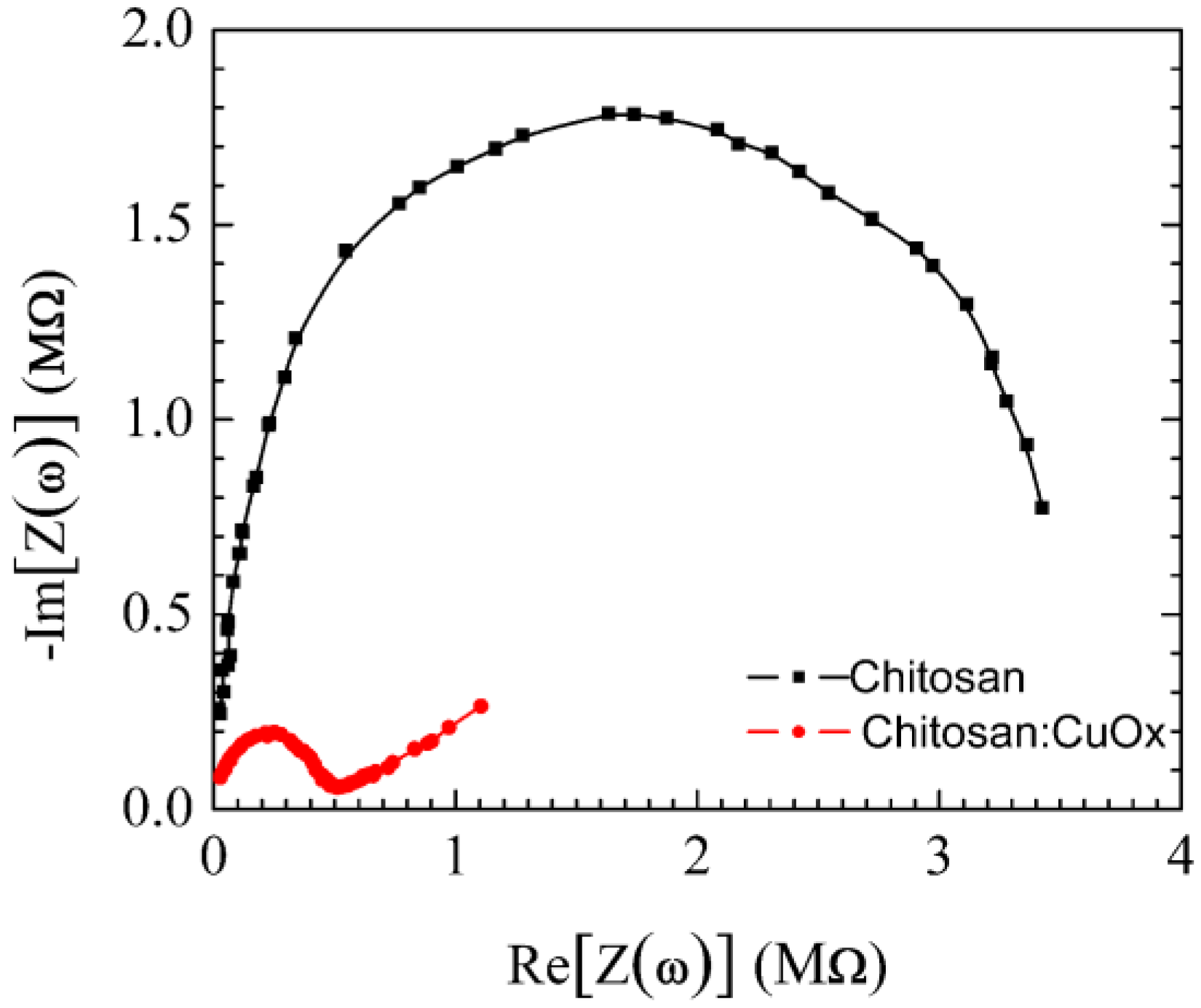

| Sensor | Sensitivity (μA·mM−1 cm−2) | Ref. |
|---|---|---|
| Au/chitosan:CuOx/chitosan/Au | 10,000 | This work |
| Glassy carbon electrode modified with copper nanoparticles decorated silver nanoleaves | 6190 | [42] |
| Glassy carbon electrode modified with FeS nanosheets | 36.4 | [43] |
| Carbon paste electrode modified Ni-Al/layered double hydroxide/Ag nanoparticles | 1.836 | [44] |
| Glassy carbon electrode modified with Ag@C core-shell nanomaterials | 22.94 | [45] |
| Graphene oxide electrode modified with Binary Mn-Co Oxides | 53.65 | [46] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arena, A.; Scandurra, G.; Ciofi, C. Copper Oxide Chitosan Nanocomposite: Characterization and Application in Non-Enzymatic Hydrogen Peroxide Sensing. Sensors 2017, 17, 2198. https://doi.org/10.3390/s17102198
Arena A, Scandurra G, Ciofi C. Copper Oxide Chitosan Nanocomposite: Characterization and Application in Non-Enzymatic Hydrogen Peroxide Sensing. Sensors. 2017; 17(10):2198. https://doi.org/10.3390/s17102198
Chicago/Turabian StyleArena, Antonella, Graziella Scandurra, and Carmine Ciofi. 2017. "Copper Oxide Chitosan Nanocomposite: Characterization and Application in Non-Enzymatic Hydrogen Peroxide Sensing" Sensors 17, no. 10: 2198. https://doi.org/10.3390/s17102198






