Fabrication and Characterization of a Stabilized Thin Film Ag/AgCl Reference Electrode Modified with Self-Assembled Monolayer of Alkane Thiol Chains for Rapid Biosensing Applications
Abstract
:1. Introduction
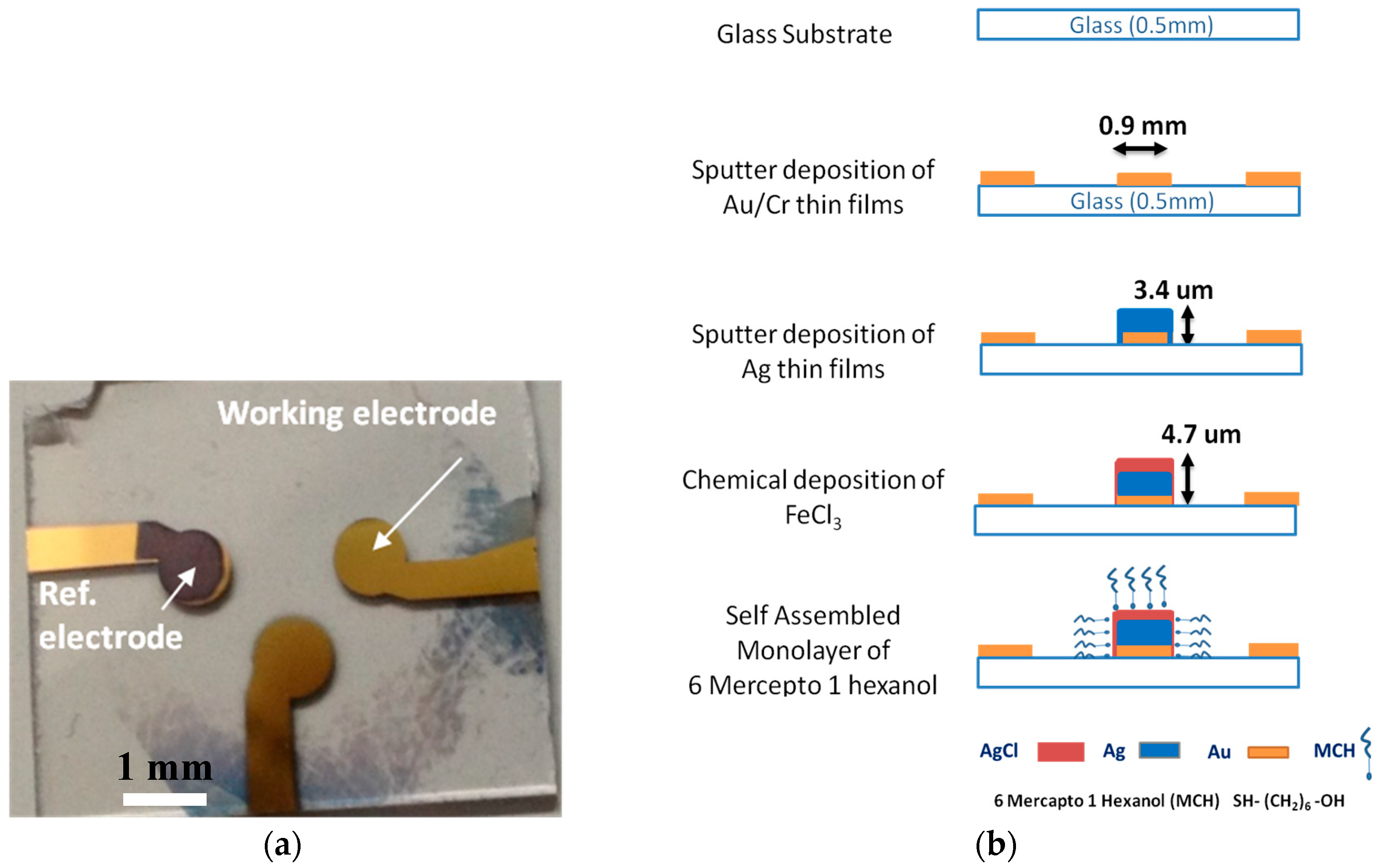
2. Materials and Methods
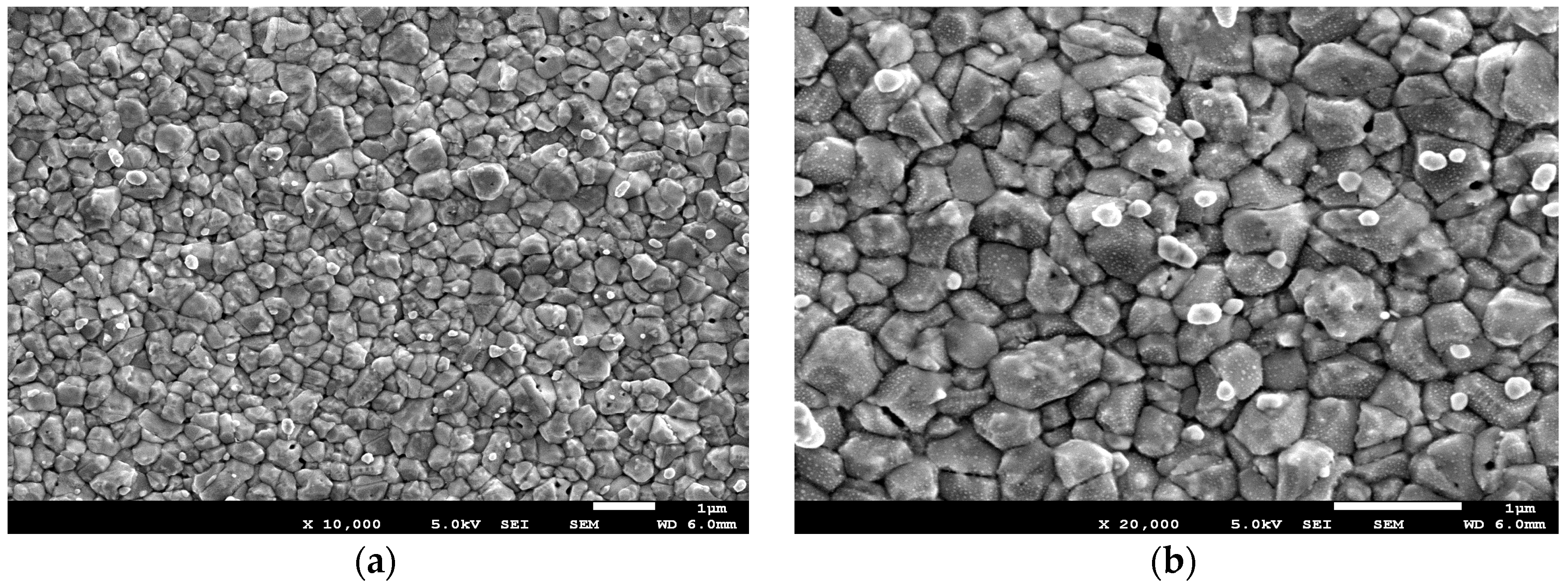
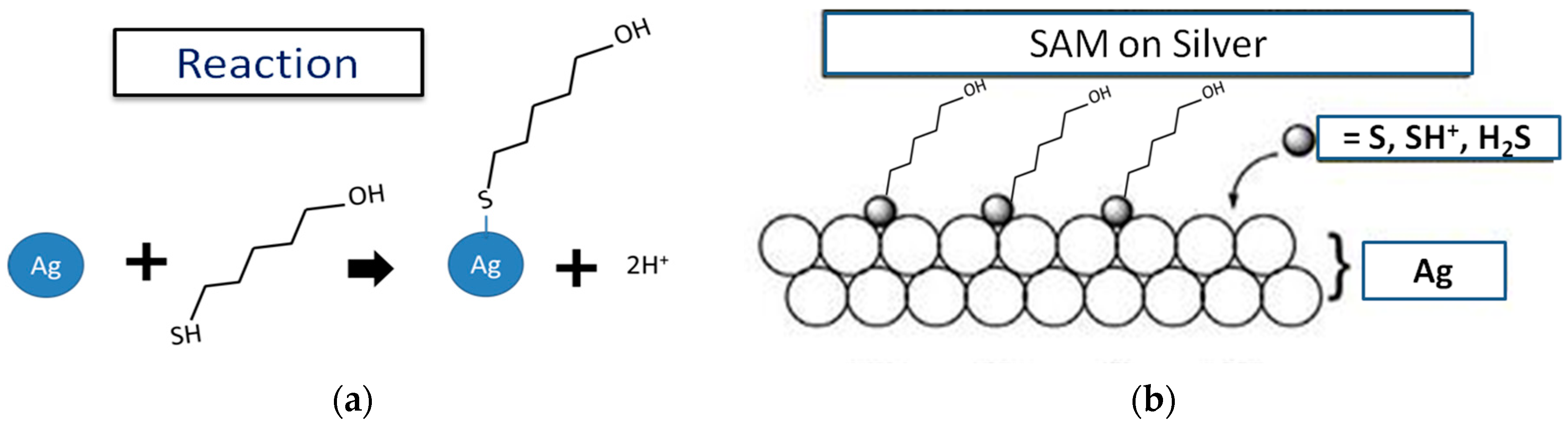
3. Results and Discussion
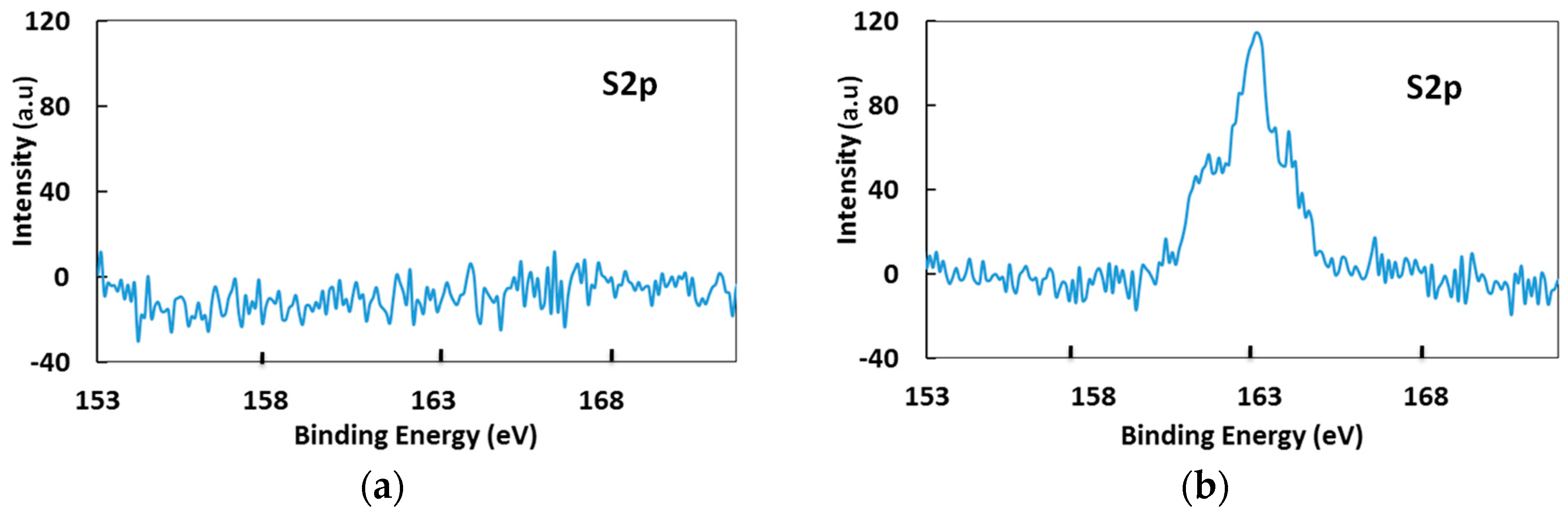
3.1. Surface Analysis of RE through XPS
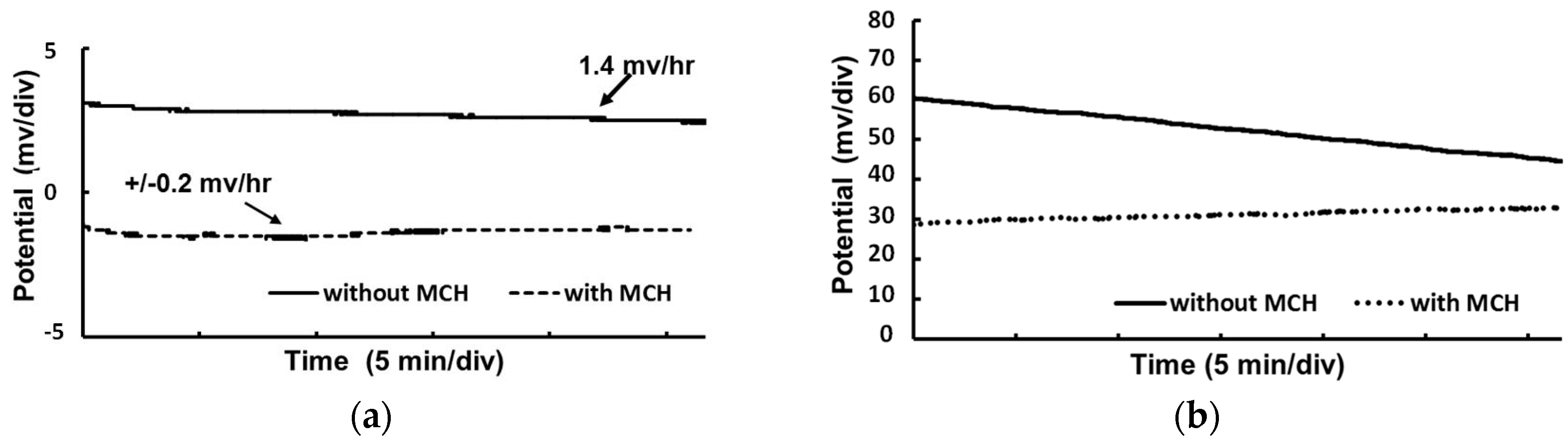
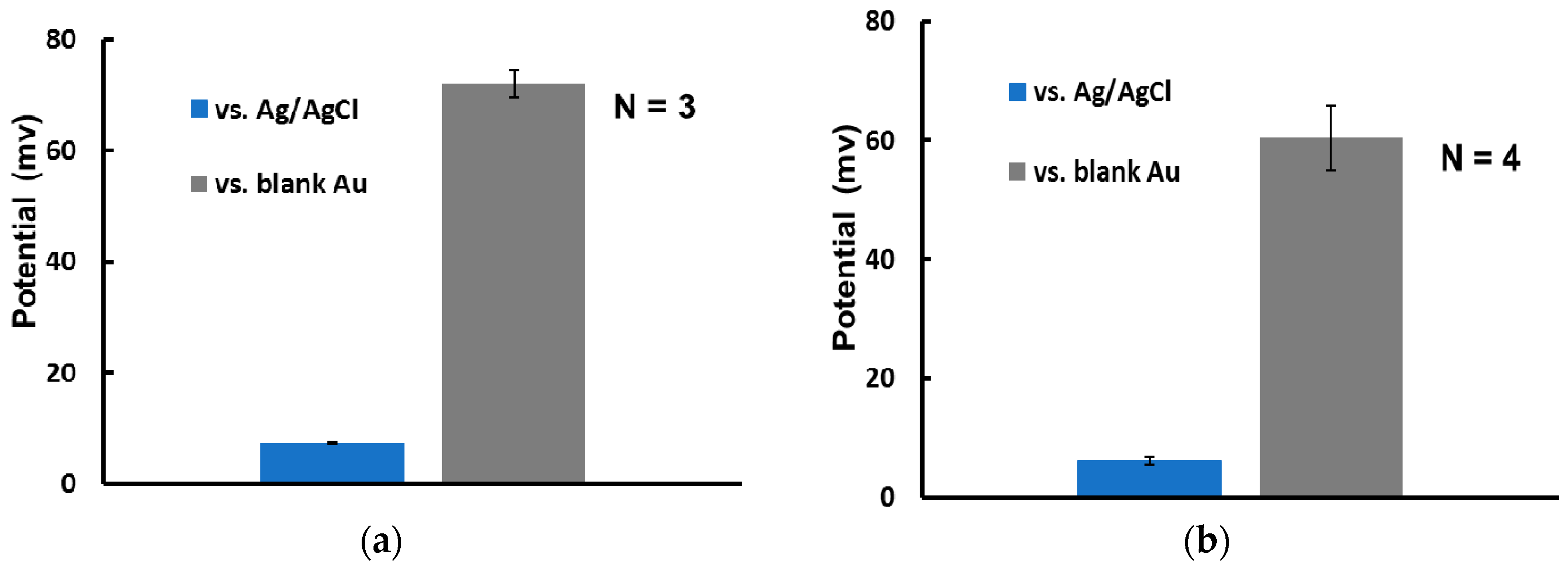
3.2. Electrical Characterization of RE
3.3. miRNA Sensing Using MCH Coated Thin Film RE
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegansheterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Chua, J.H.; Chee, R.; Agarwal, A.; Wong, S.M. Label-free direct detection of MiRNAs with silicon nanowire biosensors. Biosens. Bioelectron. 2009, 24, 2504–2508. [Google Scholar] [CrossRef] [PubMed]
- Pankaj, R.; Ramnani, P.; Gao, Y.; Ozsoz, M.; Mulchandani, A. Electronic Detection of MicroRNA at Attomolar Level with High Specificity. Anal. Chem. 2013, 85, 8061–8064. [Google Scholar] [CrossRef]
- Cai, Z.; Song, Y.; Wu, Y.; Zhu, Z.; Yang, C.J.; Chen, X. An electrochemical sensor based on label-free functional allosteric molecular beacons for detection target DNA/miRNA. Biosens. Bioelectron. 2013, 41, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Kilic, T.; Topkaya, S.N.; Ariksoysal, D.O.; Ozsoz, M.; Ballar, P.; Eracd, Y.; Gozene, O. Electrochemical based detection of microRNA, mir21 in breast cancer cells. Biosens. Bioelectron. 2012, 38, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yi, G.; Gao, Z. A highly sensitive microRNA biosensor based on ruthenium oxide nanoparticle-initiated polymerization of aniline. Chem. Commun. 2010, 46, 9131–9133. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Chai, Y.; Yuan, R.; Su, H.; Han, J. A novel label-free electrochemical microRNA biosensor using Pd nanoparticles as enhancer and linker. Analyst 2013, 138, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Meng, X.; Yin, H.; Ai, S. Amplified electrochemical microRNA biosensor using a hemin-G-quadruplex complex as the sensing element. RSC Adv. 2012, 2, 7140–7145. [Google Scholar] [CrossRef]
- Goda, T.; Masuno, K.; Nishida, J.; Kosaka, N.; Ochiya, T.; Matsumoto, A.; Miyahara, Y. A label-free electrical detection of exosomal microRNAs using microelectrode array. Chem. Commun. 2012, 48, 11942–11944. [Google Scholar] [CrossRef] [PubMed]
- Safari-Mohsenabad, S.; Selvaganapathy, P.R.; Deen, M.J. Microfluidic Reference Electrode for Applications inBiosensing. In Proceedings of the 14th International Conference on Miniaturized Systems for Chemistry and Life Sciences, Groningen, The Netherlands, 2010; pp. 596–598. [Google Scholar]
- Smith, R.L.; Scott, D.C. Miniature Liquid Junction Reference Electrode and an Integrated Solid State Electrochemical Sensor Including The Same. U.S. Patent 4592824, 3 June 1986. [Google Scholar]
- Yee, S.; Jin, H.; Lam, L.K.C. Miniature liquid junction reference electrode with micromachined silicon cavity. Sens. Actuators B Chem. 1988, 15, 337–345. [Google Scholar] [CrossRef]
- Berg, V.D.; Grisel, A.; Vlekkert, H.H.V.D.; Rooij, N.F.D. A micro-volume open liquid-junction reference electrode for pH-ISFETs. Sens. Actuators B Chem. 1990, 1, 425–432. [Google Scholar] [CrossRef]
- Suzuki, H.; Hiratsuka, A.; Sasaki, S.; Karubeb, I. Problems associated with the thin-film Ag/AgCl reference electrode and a novel structure with improved durability. Sens. Actuators B Chem. 1998, 46, 104–113. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M. Fabrication and characterization of planar reference electrode for on-chip electroanalysis. Electrochim. Acta 2006, 52, 427–433. [Google Scholar] [CrossRef]
- Huang, I.-Y.; Huang, R.-S. Fabrication and characterization of a new planar solid-state reference electrode for ISFET sensors. Thin Solid Films 2002, 406, 255–261. [Google Scholar] [CrossRef]
- Shinwari, M.W.; Zhitomirsky, D.; Deen, I.A.; Selvaganapathy, P.R.; Deen, M.J.; Landheer, D. Microfabricated reference electrodes and their biosensing applications. Sensors 2010, 10, 1679–1715. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Hong, S.A.; Yang, S. A solid-state thin-film Ag/AgCl reference electrode coated with graphene oxide and its use in a pH sensor. Sensors 2015, 15, 6469–6482. [Google Scholar] [CrossRef] [PubMed]
- Simonis, A.; Lüth, H.; Wang, J.; Schöning, M.J. New concepts of miniaturised reference electrodes in silicontechnology for potentiometric sensor systems. Sens. Actuators B Chem. 2004, 103, 429–435. [Google Scholar] [CrossRef]
- Lee, J.S.; Lee, S.D.; Cui, G.; Lee, H.J.; Shin, J.H.; Cha, G.S.; Nam, H. Hydrophilic polyurethane coated silver/silver chloride electrode for the determination of chloride in blood. Electroanalysis 1999, 11, 260–267. [Google Scholar] [CrossRef]
- Huang, I.Y.; Huang, R.-S.; Lo, L.-H. Improvement of integrated Ag/AgCl thin-film electrodes by KCl-gel coating for ISFET applications. Sens. Actuators B Chem. 2003, 94, 53–64. [Google Scholar] [CrossRef]
- Kim, H.R.; Kim, Y.D.; Kim, K.I.; Shim, J.H.; Nam, H.; Kang, B.K. Enhancement of physical and chemical properties of thin film Ag/AgCl reference electrode using a Ni buffer layer. Sens. Actuators B Chem. 2004, 97, 348–354. [Google Scholar] [CrossRef]
- Arata, H.; Komatsu, H.; Han, A.; Hosokawa, K.; Maeda, M. Rapid microRNA detection using power-free microfluidic chip: Coaxial stacking effect enhances the sandwich hybridization. Analyst 2012, 137, 3234. [Google Scholar] [CrossRef] [PubMed]
- Bashir, R. BioMEMS: State-of-the-art in detection, opportunities and prospects. Adv. Drug Deliv. Rev. 2004, 56, 1565–1586. [Google Scholar] [CrossRef] [PubMed]
- Vancea, J.; Reiss, G.; Schneider, F.; Bauer, K.; Hoffmann, H. Substrate effects on the surface topography of evaporated gold films—Ascanning tunnelling microscopy investigation. Surf. Sci. 1989, 218, 108–126. [Google Scholar] [CrossRef]
- Polk, B.J.; Stelzenmuller, A.; Mijares, G.; MacCrehan, W.; Gaitan, M. Ag/AgCl microelectrodes with improved stability for microfluidics. Sens. Actuators B Chem. 2006, 114, 239–247. [Google Scholar] [CrossRef]
- Feldheim, D.L.; Foss, C.A. Metal Nanoparticles: Synthesis, Characterization, and Applications; Marcel Dekker Inc.: New York, NY, USA, 2001. [Google Scholar]
- Schimd, G. Large Clusters and Colloids. Metals in the embryonic state. Chem. Rev. 1992, 92, 1709–1727. [Google Scholar] [CrossRef]
- Giersig, M.; Mulvaney, P. Preparation of ordered colloid monolayers by electrophoretic deposition. Langumir 1993, 9, 3408–3413. [Google Scholar] [CrossRef]
- Brust, M.; Walker, M.; Bethell, D.; Schiffrin, D.J.; Whyman, R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J. Chem. Soc. Chem. Commun. 1994, 801–802. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physic, 82nd ed.; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Rougvie, A.E. Control of developmental timing in animals. Nat. Rev. Genet. 2001, 2, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Yang, C.; Yang, Q.; Ding, H.; Jia, J.; Guo, J.; Wang, J.; Wang, Z. Deregulation of let-7e in epithelial ovarian cancer promotes the development of resistance to cisplatin. Oncogenesis 2003, 2, e75. [Google Scholar] [CrossRef] [PubMed]

| Type of Molecule (Name) | Bases in Length | Sequences |
|---|---|---|
| Capture Probe DNA (let-7a) | 75 | ATGGATCTCAACTGGATCCAGTGTAATTACTGGATCCAGTTGAGATCCATAACTATACAACCTACTACC TCA ACA—C6-SH |
| Target miRNA (let-7a) | 22 | UGA GGU AGU AGG UUG UAU AGU U |
| Non Target miRNA (let-7c) | 22 | UGA GGU AGU AGG UUG UAU GGU U |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, T.; Ichiki, T. Fabrication and Characterization of a Stabilized Thin Film Ag/AgCl Reference Electrode Modified with Self-Assembled Monolayer of Alkane Thiol Chains for Rapid Biosensing Applications. Sensors 2017, 17, 2326. https://doi.org/10.3390/s17102326
Rahman T, Ichiki T. Fabrication and Characterization of a Stabilized Thin Film Ag/AgCl Reference Electrode Modified with Self-Assembled Monolayer of Alkane Thiol Chains for Rapid Biosensing Applications. Sensors. 2017; 17(10):2326. https://doi.org/10.3390/s17102326
Chicago/Turabian StyleRahman, Tanzilur, and Takanori Ichiki. 2017. "Fabrication and Characterization of a Stabilized Thin Film Ag/AgCl Reference Electrode Modified with Self-Assembled Monolayer of Alkane Thiol Chains for Rapid Biosensing Applications" Sensors 17, no. 10: 2326. https://doi.org/10.3390/s17102326




