Planar Microstrip Ring Resonators for Microwave-Based Gas Sensing: Design Aspects and Initial Transducers for Humidity and Ammonia Sensing
Abstract
:1. Introduction
2. Principle of Planar Microwave Transducers
3. Sensor Design
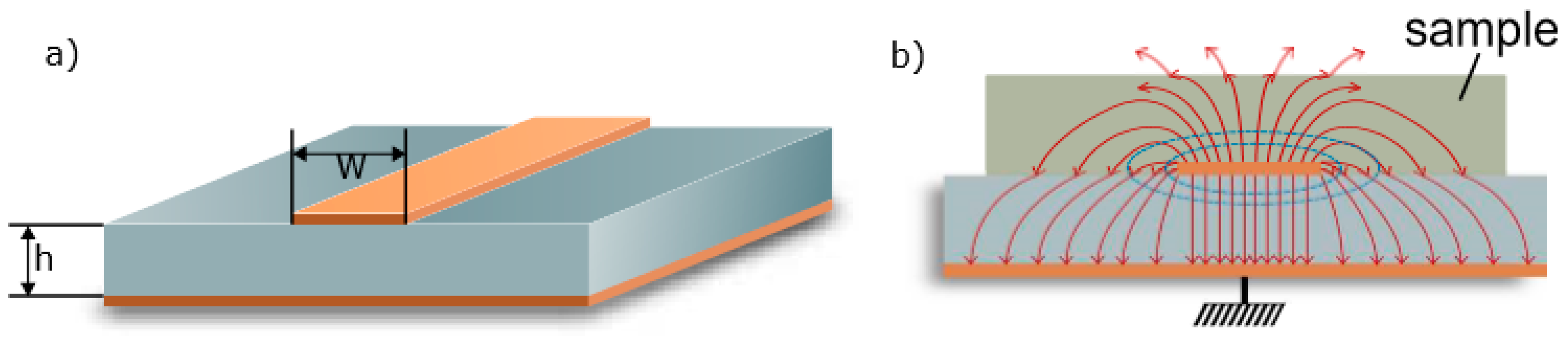
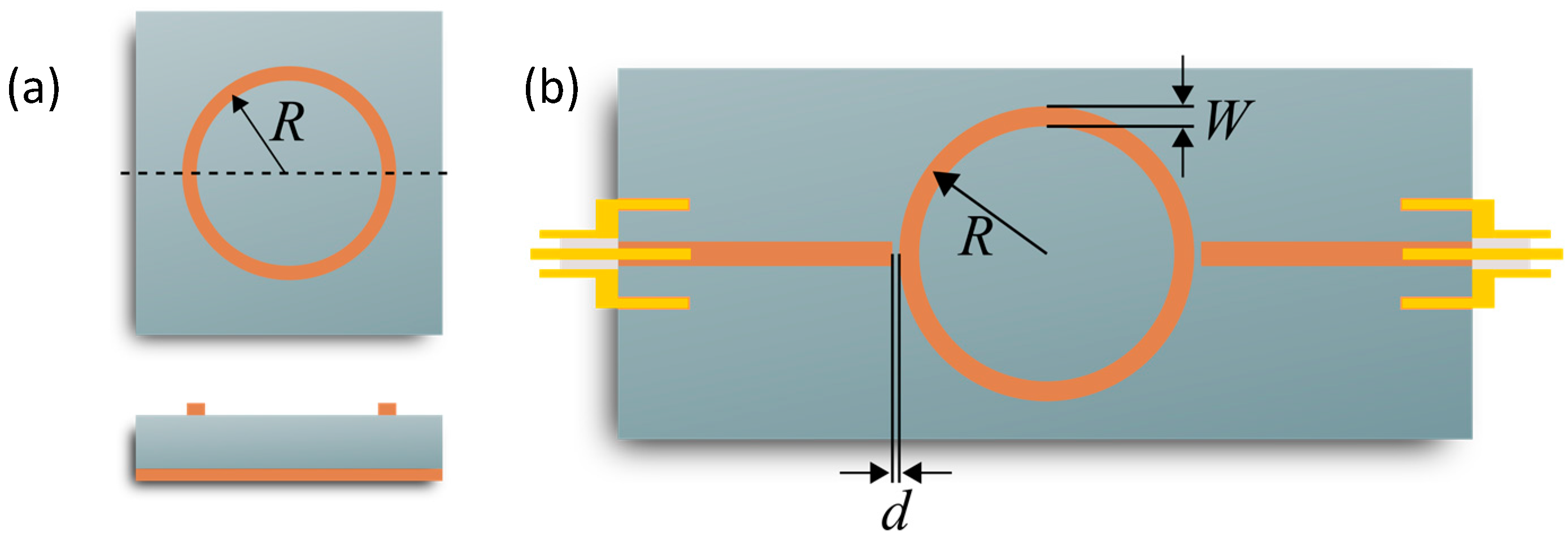
3.1. Microstrip Ring Resonator Design
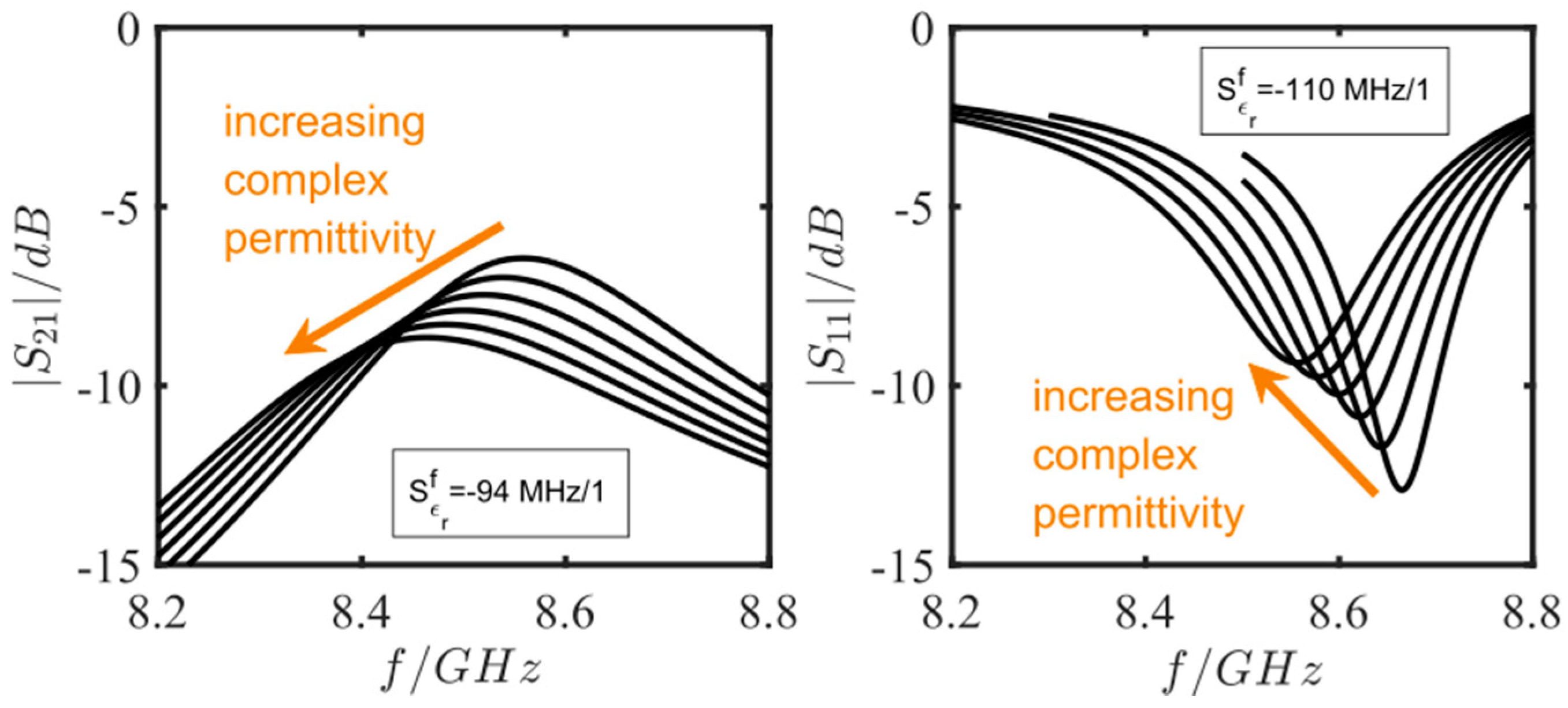
3.2. Simulation Studies
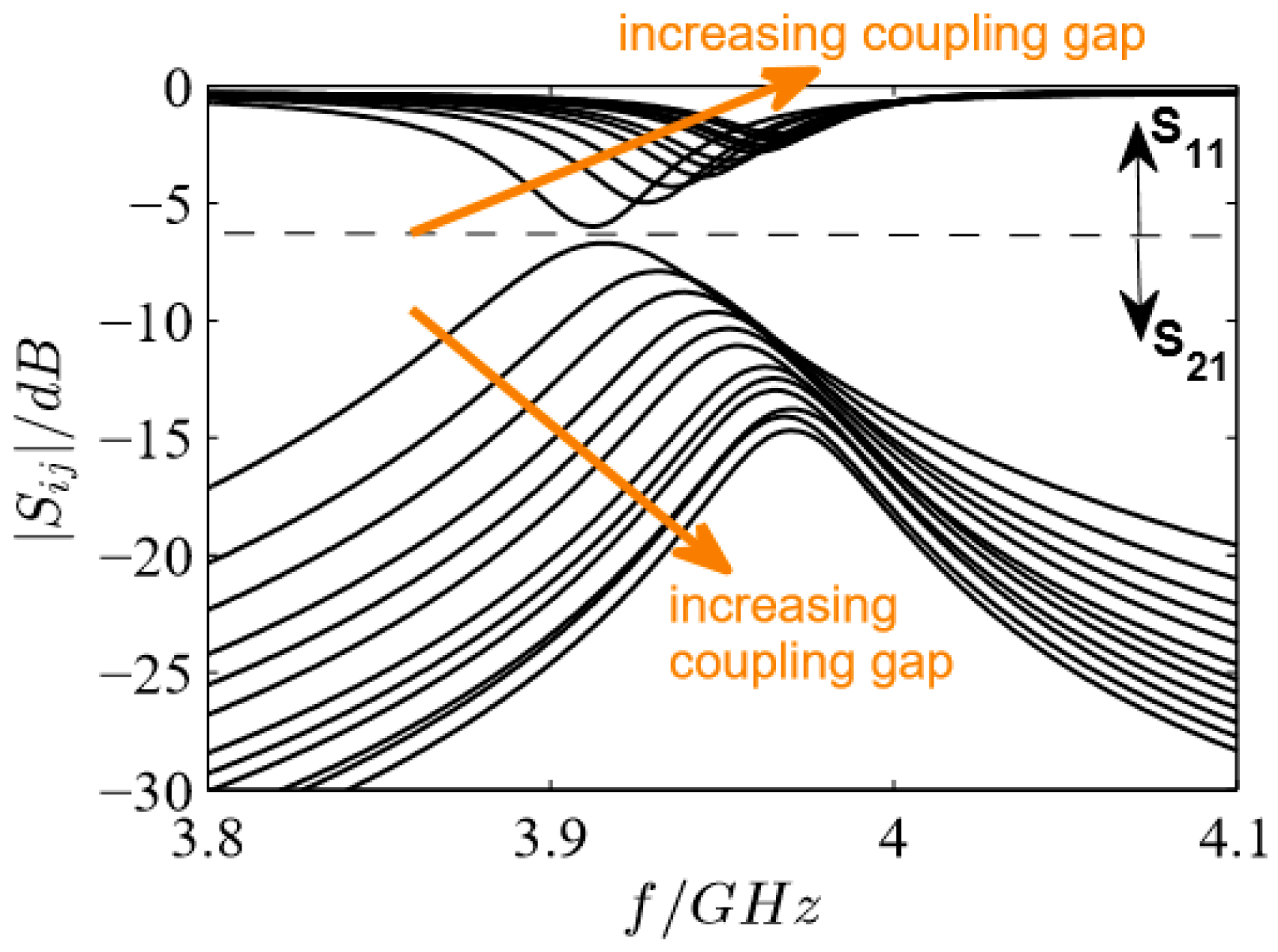
3.3. Influence of the Coupling Gap
3.4. Miniaturization
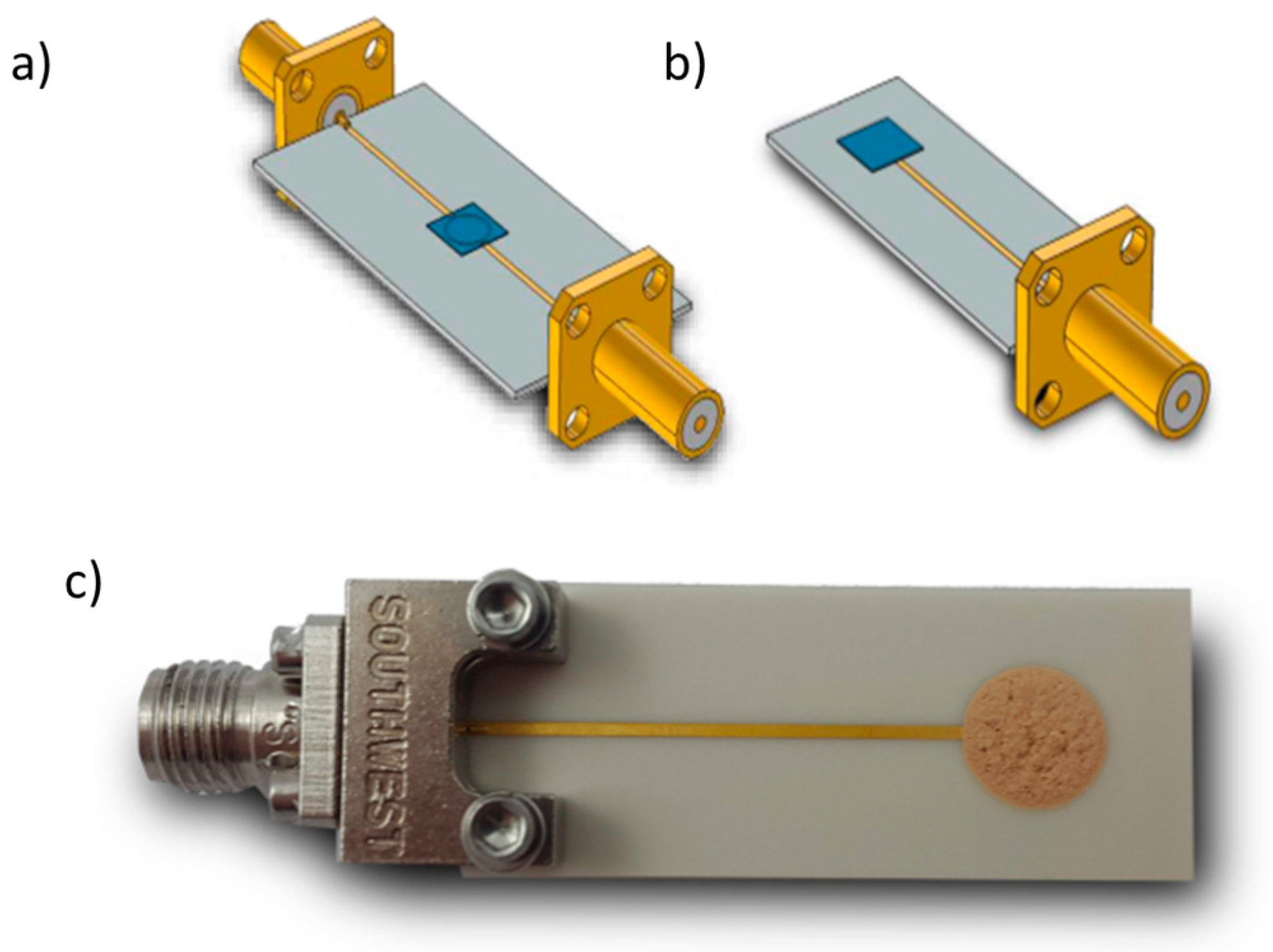
3.5. Final Microwave Gas-Sensing Device
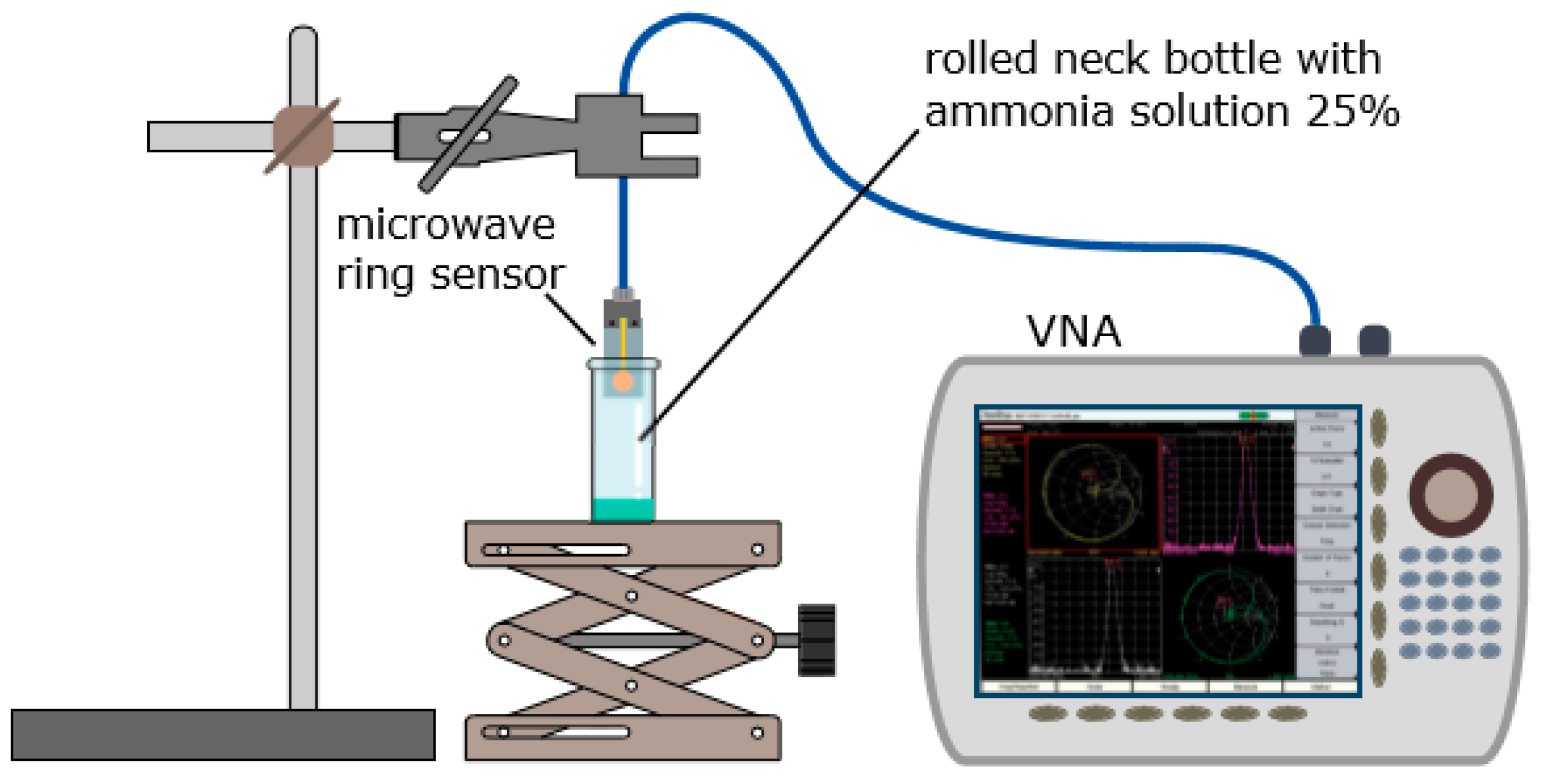
4. Experiment and Methods
5. Results and Discussion
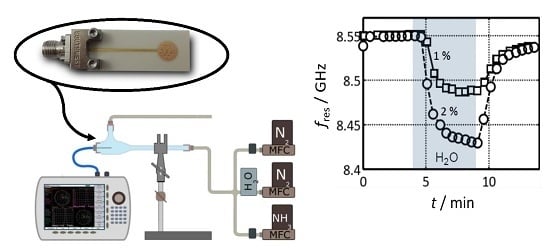
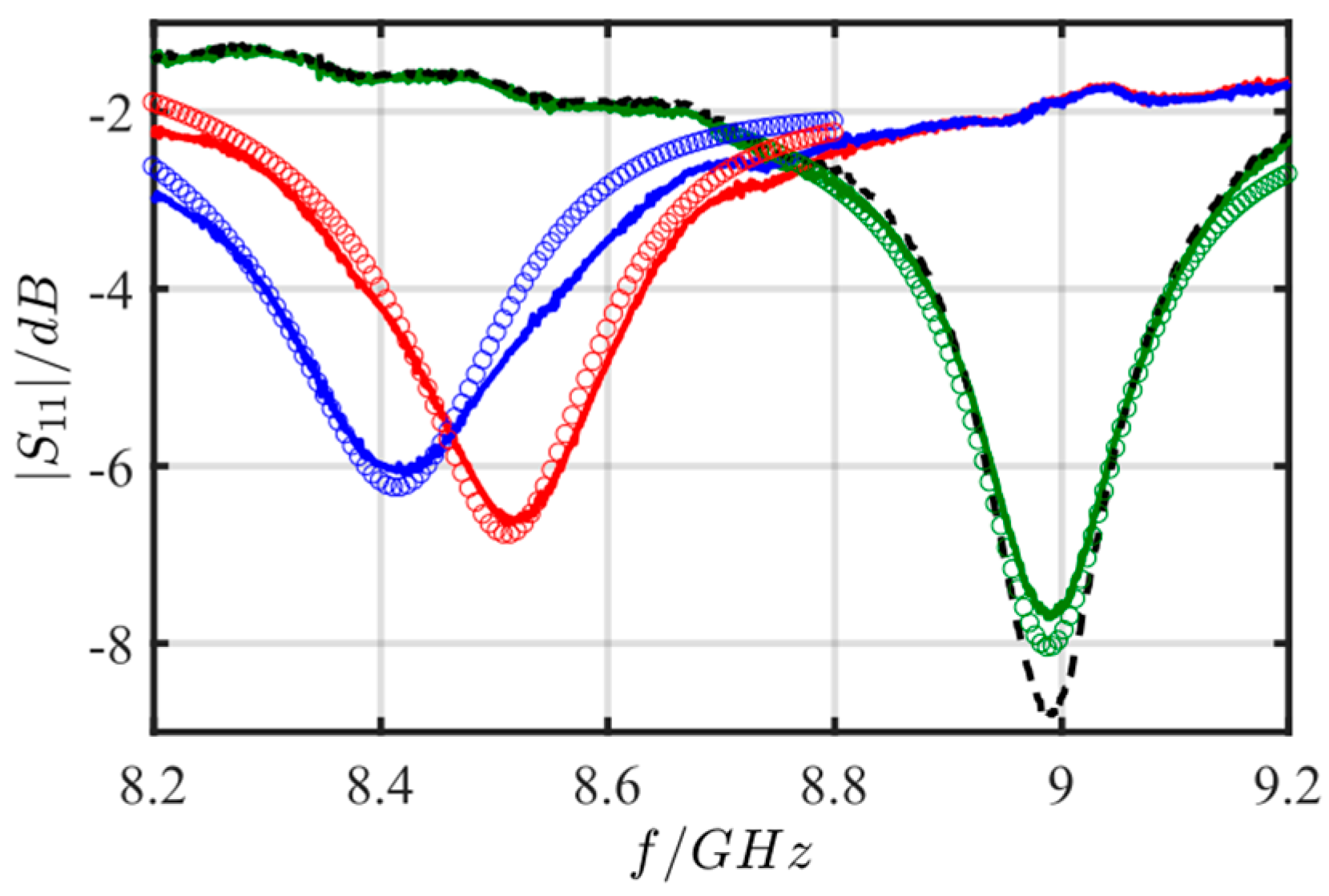
5.1. Initial Tests: Proof of Concept
- Sensor outside the glass (i.e., in air) without zeolite layer.
- Sensor inside the glass (ammonia-in-air ambience) without zeolite layer.
- Sensor outside the glass (i.e., in air) with zeolite layer.
- Sensor inside the glass (ammonia-in-air ambience) with zeolite layer.
5.2. Tests under Defined Gas Exposure
6. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Comini, E.; Faglia, G.; Sberveglieri, G. Solid State Gas Sensing; Springer International Publishing AG: Heidelberg, Germany, 2009. [Google Scholar]
- Bănică, F.-G. Chemical Sensors and Biosensors: Fundamentals and Applications; John Wiley & Sons, Ltd.: Chichester, UK, 2012; pp. 246–257. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Fundamentals of Semiconductor Gas Sensors. In Semiconductor Gas Sensors; Jaaniso, R., Tan, O.K., Eds.; Woodhead Publishing Ltd.: Cambridge, UK, 2013; pp. 3–34. [Google Scholar]
- Barsan, N.; Koziej, D.; Weimar, U. Metal Oxide-Based Gas Sensor Research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Shariaty, P.; Hashisho, Z.; Daneshmand, M. A Non-Contact Microwave Sensor for Monitoring the Interaction of Zeolite 13X with CO2 and CH4 in Gaseous Streams. Sens. Actuators B Chem. 2017, 238, 1240–1247. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Sohrabi, A.; Shaibani, P.M.; Daneshmand, M.; Thundat, T. Detection of Volatile Organic Compounds Using Microwave Sensors. IEEE Sens. J. 2015, 15, 248–254. [Google Scholar] [CrossRef]
- Rossignol, J.; Barochi, G.; de Fonseca, B.; Brunet, J.; Bouvet, M.; Pauly, A.; Markey, L. Microwave-based gas sensor with phthalocyanine film at room temperature. Sens. Actuators B Chem. 2013, 189, 213–216. [Google Scholar] [CrossRef]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Stuerga, D.; Pribetich, P. Microwave Gas Sensing with a Microstrip Interdigital Capacitor: Detection of NH3 with TiO2 nanoparticles. Sens. Actuators B Chem. 2016, 236, 554–564. [Google Scholar] [CrossRef]
- Bailly, G.; Rossignol, J.; de Fonseca, B.; Pribetich, P.; Stuerga, D. Microwave Gas Sensing with Hematite: Shape Effect on Ammonia Detection Using Pseudocubic, Rhombohedral, and Spindlelike Particles. ACS Sens. 2016, 1, 656–662. [Google Scholar] [CrossRef]
- Chen, L.F.; Ong, C.K.; Neo, C.P.; Varadan, V.V.; Varadan, V.K. Microwave Electronics: Measurement and Materials Characterization; John Wiley & Sons, Ltd.: Chichester, UK, 2004. [Google Scholar]
- Yeow, Y.; Abbas, Z.; Khalid, K. Application of Microwave Moisture Sensor for Determination of Oil Palm Fruit Ripeness. Meas. Sci. Rev. 2010, 10, 714. [Google Scholar] [CrossRef] [Green Version]
- Yogi, R.A.; Parolia, R.S.; Karekar, R.N.; Aiyer, R.C. Microwave Microstrip Ring Resonator as a Paper Moisture Sensor: Study with Different Grammage. Meas. Sci. Technol. 2002, 13, 1558–1562. [Google Scholar] [CrossRef]
- Sarabandi, K.; Li, E.S. Microstrip Ring Resonator for Soil Moisture Measurements. IEEE Trans. Geosci. Remote Sens. 1997, 35, 1223–1231. [Google Scholar] [CrossRef]
- Abegaonkar, M.P.; Karekar, R.N.; Aiyer, R.C. A Microwave Microstrip Ring Resonator as a Moisture Sensor for Biomaterials: Application to Wheat Grains. Meas. Sci. Technol. 1999, 10, 195–200. [Google Scholar] [CrossRef]
- Schwerthoeffer, U.; Weigel, R.; Kissinger, D. A Highly Sensitive Glucose Biosensor Based on a Microstrip Ring Resonator. In Proceedings of the IEEE MTT-S International Microwave Workshop Series on RF and Wireless Technologies for Biomedical and Healthcare Applications (IMWS-BIO), Singapore, 9–11 December 2013; pp. 1–3. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Thundat, T.; Daneshmand, M. High resolution microwave microstrip resonator for sensing applications. Sens. Actuators A Phys. 2015, 233, 224–230. [Google Scholar] [CrossRef]
- Yi, F.-Y.; Chen, D.; Wu, M.-K.; Han, L.; Jiang, H.-L. Chemical Sensors Based on Metal–Organic Frameworks. ChemPlusChem 2016, 81, 675–690. [Google Scholar] [CrossRef]
- Marr, I.; Reiß, S.; Hagen, G.; Moos, R. Planar Zeolite film-Based Potentiometric Gas Sensors Manufactured by a Combined Thick-film and Electroplating Technique. Sensors 2011, 11, 7736–7748. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Schönebaum, S.; Simons, T.; Rauch, D.; Dietrich, M.; Moos, R.; Simon, U. Correlating the Integral Sensing Properties of Zeolites with Molecular Processes by Combining Broadband Impedance and DRIFT Spectroscopy—A New Approach for Bridging the Scales. Sensors 2015, 15, 28915–28941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.; Wang, J.; Long, Y. Zeolite-based Materials for Gas Sensors. Sensors 2006, 6, 1751–1764. [Google Scholar] [CrossRef]
- Bahoumina, P.; Hallil, H.; Lachaud, J.L.; Rebiere, D.; Dejous, C.; Abdelghani, A.; Frigui, K.; Bila, S.; Baillargeat, D.; Zhang, Q.; et al. Chemical Gas Sensor Based on a Novel Capacitive Microwave Flexible Transducer and Composite Polymer Carbon Nanomaterials. In Proceedings of the Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), Cannes, France, 1–4 April 2017. [Google Scholar] [CrossRef]
- Zarifi, M.H.; Gholidoustb, A.; Abdolrazzaghic, M.; Shariatyb, P.; Hashisho, Z.; Daneshmand, M. Sensitivity Enhancement in Planar Microwave Active-Resonator Using Metal Organic Framework for CO2 Detection. Sens. Actuators B Chem. 2017, in press. [Google Scholar] [CrossRef]
- Bailly, G.; Harrabi, A.; Rossignol, J.; Michel, M.; Stuerga, D.; Pribetich, P. Microstrip Spiral Resonator For Microwave-Based Gas Sensing. IEEE Sens. Lett. 2017, 1. [Google Scholar] [CrossRef]
- Piekarz, I.; Socki, J.; Wincza, K.; Gruszczynski, S. Microwave Sensors for Dielectric Sample Measurement Based on Coupled-Line Section. IEEE Trans. Microw. Theory Tech. 2017, 65, 1615–1631. [Google Scholar] [CrossRef]
- Yakhlef, Y.; Benhabiles, M.T.; Benkhaoua, L.; Riabi, M.L. Compact Miniature Sensors Based on Tapered Lines Coupled Metamaterial Resonators. In Proceedings of the First IEEE MTT-S International Microwave Bio Conference (IMBIOC), Gothenburg, Sweden, 15–17 May 2017; pp. 1–4. [Google Scholar]
- Denoual, M.; Pouliquen, M.; Jorel, C.; Radu, C.; Robbes, D.; Harnois, M.; de Sagazan, O.; Grand, J.; Awala, H.; Mintova, S.; et al. Zeolite-Based Thermal Mass Gas Sensor with Self-Identification Algorithm. In Proceedings of the Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS (DTIP), Gothenburg, Sweden, 19–21 September 2017; pp. 1–4. [Google Scholar]
- Reinecke, T.; Walter, J.-G.; Kobelt, T.; Ahrens, A.; Scheper, T.; Zimmermann, S. Biosensor Based on a Split-Ring Resonator. In Proceedings of the SENSOR 2017, Nuremberg, Germany, 30 May–1 June 2017; pp. 78–83. [Google Scholar] [CrossRef]
- Rydosz, A.; Maciak, E.; Wincza, K.; Gruszczynski, S. Microwave-Based Sensors with Phthalocyanine Films for Acetone, Ethanol and Methanol Detection. Sens. Actuators B Chem. 2016, 237, 876–886. [Google Scholar] [CrossRef]
- Kröcher, O.; Devadas, M.; Elsener, M.; Wokaun, A.; Söger, N.; Pfeifer, M.; Demel, Y.; Mussmann, L. Investigation of the Selective Catalytic Reduction of NO by NH3 on Fe-ZSM5 Monolith Catalysts. Appl. Catal. B Environ. 2006, 66, 208–216. [Google Scholar] [CrossRef]
- Reiß, S.; Schönauer, D.; Hagen, G.; Fischerauer, G.; Moos, R. Monitoring the Ammonia Loading of Zeolite-Based Ammonia SCR Catalysts by a Microwave Method. Chem. Eng. Technol. 2011, 34, 791–796. [Google Scholar] [CrossRef]
- Pozar, D.M. Microwave Engineering, 4th ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Dietrich, M.; Rauch, D.; Porch, A.; Moos, R. A Laboratory Test Setup for in Situ Measurements of the Dielectric Properties of Catalyst Powder Samples under Reaction Conditions by Microwave Cavity Perturbation: Set up and Initial Tests. Sensors 2014, 14, 16856–16868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heine, C.; Girgsdies, F.; Truschke, A.; Schlögl, R.; Eichelbaum, M. The Model Oxidation Catalyst α-V2O5: Insights from Contactless in Situ Microwave Permittivity and Conductivity Measurements. Appl. Phys. A 2013, 112, 289–296. [Google Scholar] [CrossRef]
- Beulertz, G.; Votsmeier, M.; Moos, R. Effect of Propene, Propane, and Methane on Conversion and Oxidation State of Three-Way Catalysts: A Microwave Cavity Perturbation Study. Appl. Catal. B 2015, 165, 369–377. [Google Scholar] [CrossRef]
- Dietrich, M.; Rauch, D.; Simon, U.; Porch, A.; Moos, R. Ammonia Storage Studies on H-ZSM-5 Zeolites by Microwave Cavity Perturbation: Correlation of Dielectric Properties with Ammonia Storage. J. Sens. Sens. Syst. 2015, 4, 263–269. [Google Scholar] [CrossRef]
- Feulner, M.; Seufert, F.; Müller, A.; Hagen, G.; Moos, R. Influencing Parameters on the Microwave-Based Soot Load Determination of Diesel Particulate Filters. Top. Catal. 2017, 60, 374–380. [Google Scholar] [CrossRef]
- Moos, R. Microwave-Based Catalyst State Diagnosis—State of the Art and Future Perspectives. SAE Int. J. Engines 2015, 8, 1240–1245. [Google Scholar] [CrossRef]
- Hoffmann, R.K. Integrierte Mikrowellenschaltungen; Springer: Berlin/Heidelberg, Germany, 1983. [Google Scholar]
- Dietrich, M.; Steiner, C.; Hagen, G.; Moos, R. Radio-Frequency-Based Urea Dosing Control for Diesel Engines with Ammonia SCR Catalysts. SAE Int. J. Engines 2017, 10, 1638–1645. [Google Scholar] [CrossRef]
- Rauch, D.; Dietrich, M.; Simons, T.; Simon, U.; Porch, A.; Moos, R. Microwave Cavity Perturbation Studies on H-form and Cu Ion-Exchanged SCR Catalyst Materials: Correlation of Ammonia Storage and Dielectric Properties. Top. Catal. 2017, 60, 243–249. [Google Scholar] [CrossRef]
- Simon, U.; Flesch, U.; Maunz, W.; Müller, R.; Plog, C. The Effect of NH3 on the Ionic Conductivity of Dehydrated Zeolites Na Beta and H Beta. Microporous Mesoporous Mater. 1998, 21, 111–116. [Google Scholar] [CrossRef]
- Anderson, P.A.; Armstrong, A.R.; Porch, A.; Edwards, P.P.; Woodall, L.J. Structure and Electronic Properties of Potassium-Loaded Zeolite L. J. Phys. Chem. B 1997, 101, 9892–9900. [Google Scholar] [CrossRef]
- Kraus, M.; Kopinke, F.-D.; Roland, U. Influence of Moisture Content and Temperature on the Dielectric Permittivity of Zeolite NaY. Phys. Chem. Chem. Phys. 2011, 13, 4119–4125. [Google Scholar] [CrossRef] [PubMed]
- Niwa, M.; Iwamoto, M.; Segawa, K. Temperature-Programmed Desorption of Ammonia on Zeolites. Influence of the Experimental Conditions on the Acidity Measurement. BCSJ 1986, 59, 3735–3739. [Google Scholar] [CrossRef]
- Marr, I.; Groß, A.; Moos, R. Overview on Conductometric Solid-State Gas Dosimeters. J. Sens. Sens. Syst. 2014, 3, 29–46. [Google Scholar] [CrossRef] [Green Version]



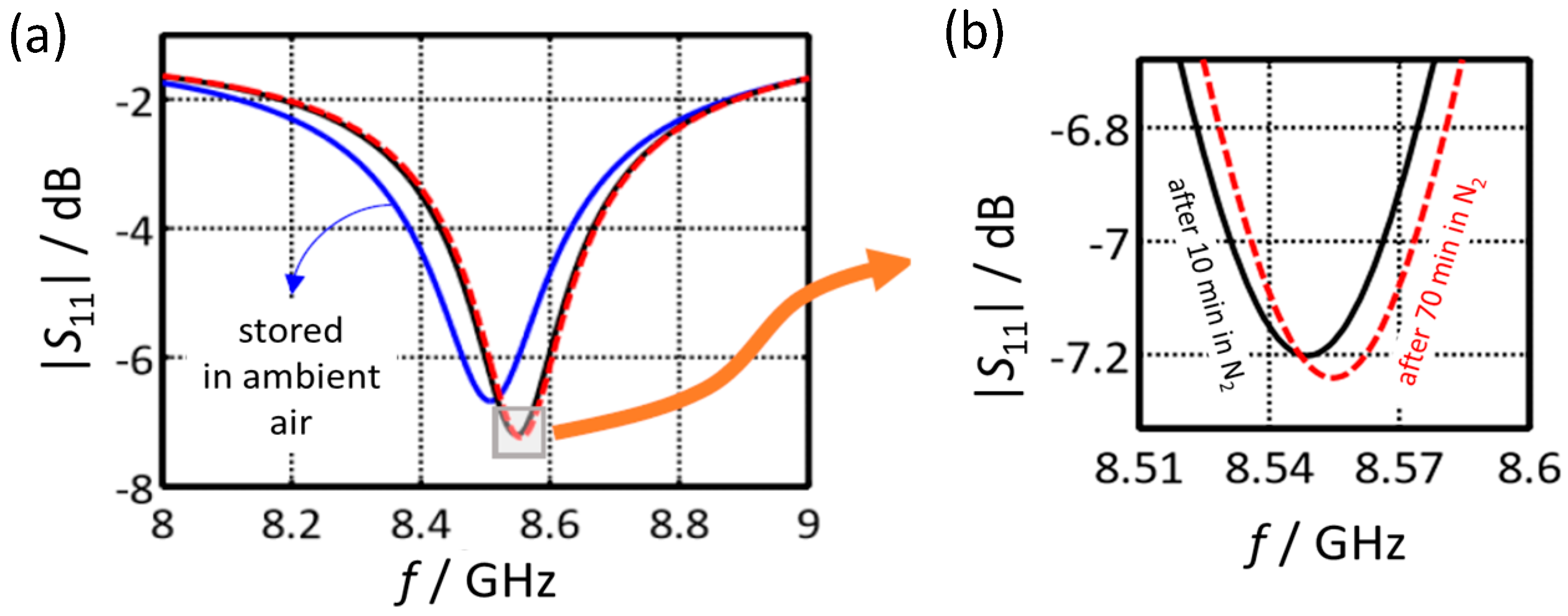
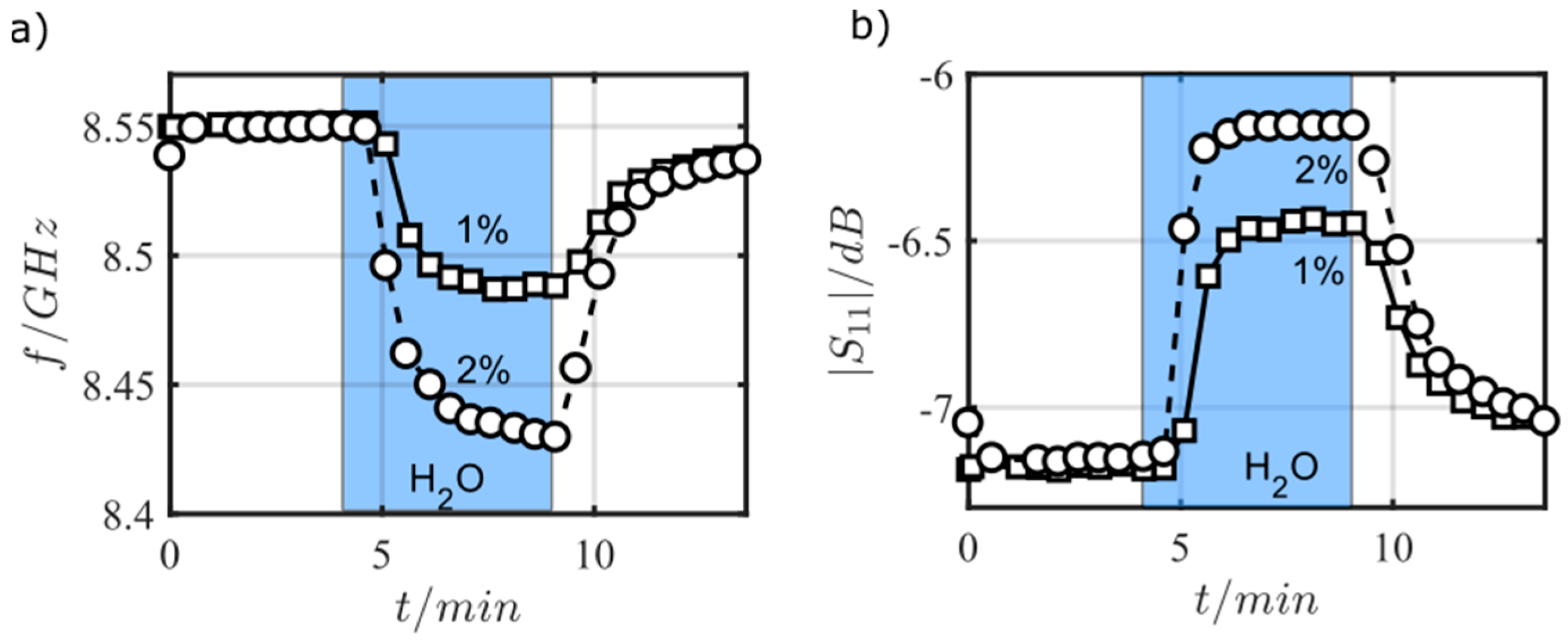
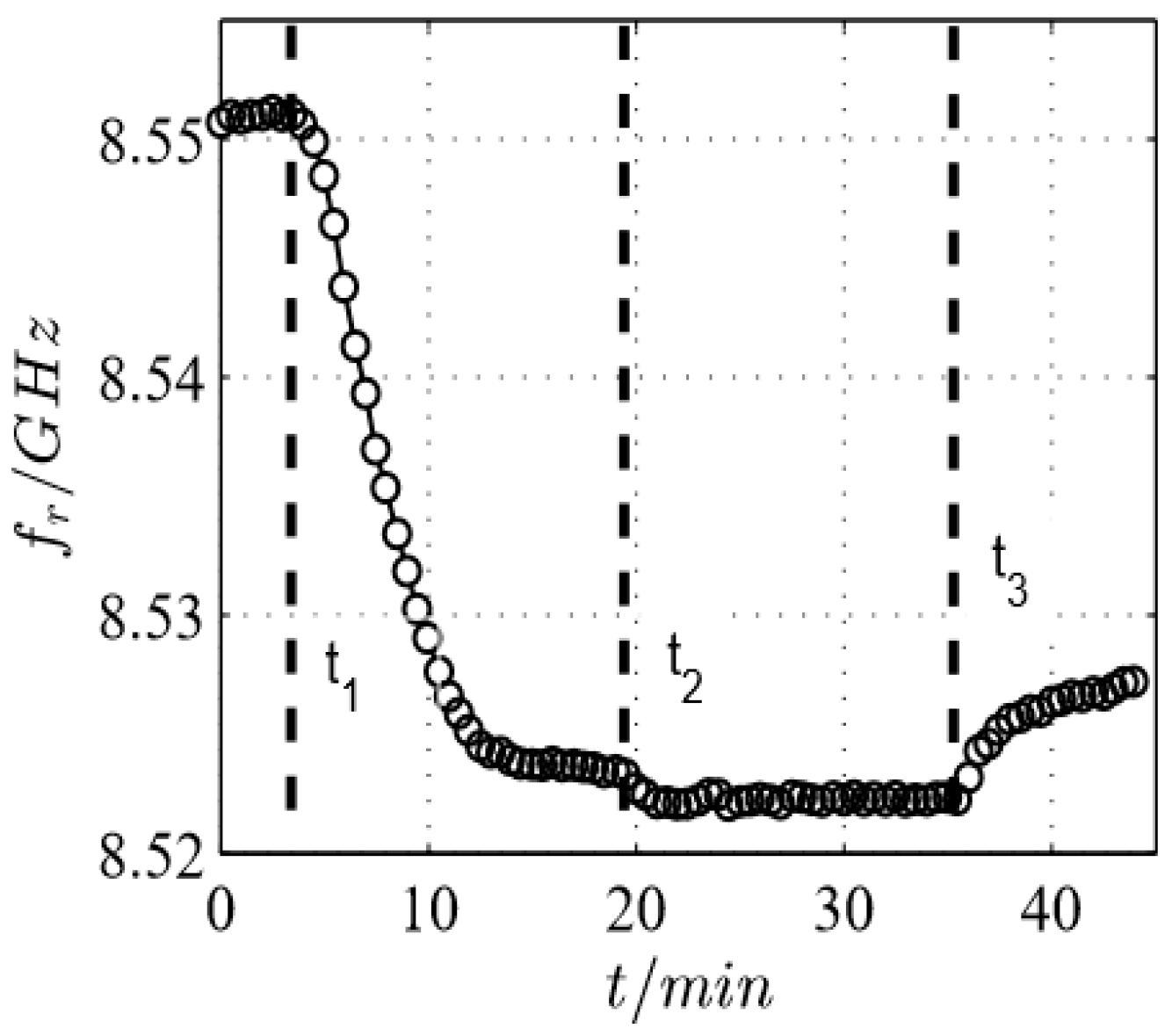
| /GHz | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| R/mm | 29.2 | 14.6 | 9.72 | 7.28 | 5.81 | 4.84 | 4.14 | 3.62 | 3.21 | 2.88 |
| Step | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| ε’ | 3 + j0 | 3.2 + j0.1 | 3.4 + j0.2 | 3.6 + j0.3 | 3.8 + j0.4 | 4 + j0.5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogner, A.; Steiner, C.; Walter, S.; Kita, J.; Hagen, G.; Moos, R. Planar Microstrip Ring Resonators for Microwave-Based Gas Sensing: Design Aspects and Initial Transducers for Humidity and Ammonia Sensing. Sensors 2017, 17, 2422. https://doi.org/10.3390/s17102422
Bogner A, Steiner C, Walter S, Kita J, Hagen G, Moos R. Planar Microstrip Ring Resonators for Microwave-Based Gas Sensing: Design Aspects and Initial Transducers for Humidity and Ammonia Sensing. Sensors. 2017; 17(10):2422. https://doi.org/10.3390/s17102422
Chicago/Turabian StyleBogner, Andreas, Carsten Steiner, Stefanie Walter, Jaroslaw Kita, Gunter Hagen, and Ralf Moos. 2017. "Planar Microstrip Ring Resonators for Microwave-Based Gas Sensing: Design Aspects and Initial Transducers for Humidity and Ammonia Sensing" Sensors 17, no. 10: 2422. https://doi.org/10.3390/s17102422