Chronotropic Competence Indices Extracted from Wearable Sensors for Cardiovascular Diseases Management
Abstract
:1. Introduction
2. Definition of Chronotropic Competence Indices
- Resting Heart Rate : The resting heart rate is defined as the heart rate when a person is awake, in a neutrally temperate environment, and has not been subject to any recent exertion or stimulation, such as stress or surprise. There is increasing evidence that elevated resting heart rate is associated with increased cardiovascular morbidity and mortality, both in the general population and in patients with cardiovascular disease [24]. The normal resting heart rate in adults is 60–80 beats per minute (bpm).
- Chronotropic Rate : Chronotropic rate represents the rate at which the heart rate increase as exercise intensity increases. It is measured as amount of heart rate increase in response to every unit of metabolic equivalent (MET) exercise intensity increase. In practice, it can be measured and calculated as:CR is similar to the “Exercise HR” in the EACPR/AHA Joint Scientific Statement [14], where it provides insight into chronotropic competence and cardiac response to exercise. It normally increases ∽10 beats per MET.CR is an important parameter to provide personalized quantitative relation between HR and exercise intensity so that target heart rate (THR) can be used to prescribe exercise intensity in exercise training. However, a person’s CR may vary due to medication or rehab progress, and it is recommended to measure CR promptly or monitor CR changes in order to keep exercise prescription updated [25].
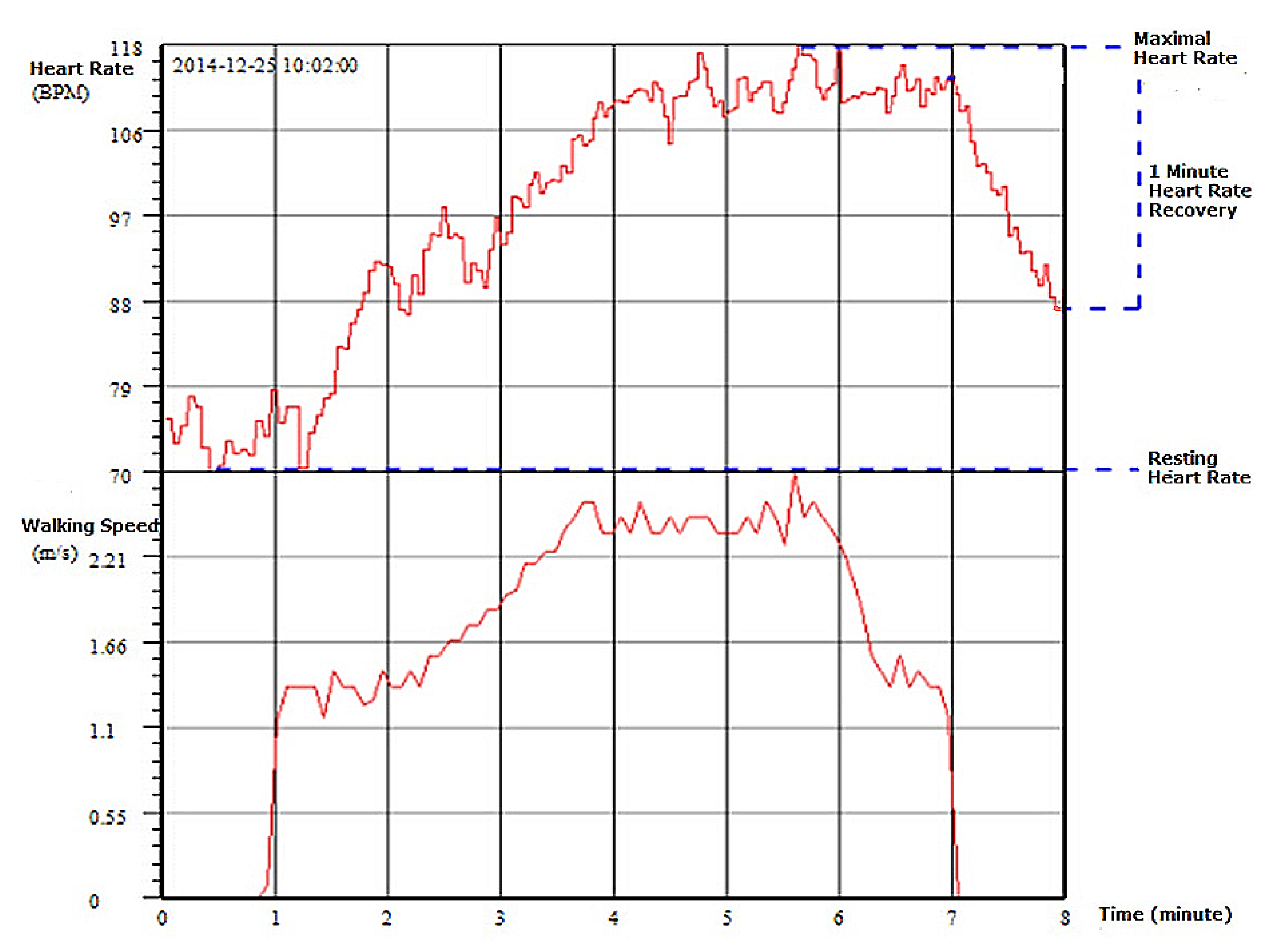
- Chronotropic Limit : Chronotropic limit represents the maximal heart rate an individual can achieve without severe problems through exercise stress. It is measured as Heart Rate Reserve (HRR) and calculated aswhere is the maximal heart rate one achieves during exercise test, and is the predicted maximal heart rate, and is usually calculated as .Maximal heart rate is usually obtained when reaching peak exercise, which is easily identified during CPET testing. In this case, the normal value of CL is 0.8–1.3. However, when CPET testing or peak exercise is not achievable, then CL normal values can be estimated from various types of exercise. For example, in a 6-min walking test, CL for a 60 year old person should be considered normal. With a resting heart rate of 75 bpm, CR would be 10 beats per MET, and the maximal heart rate would be 109 bpm with an exercise intensity of 4.4 MET.
- Heart Rate Recovery at 1 min after Exercise : This measure is defined as the reduction in heart rate at maximum during exercise and the rate as measured at 1 minute after stopping exercise. The measurement of does not require that the exercise intensity reaches one’s maximum capacity. The EACPR/AHA Joint Scientific Statement [14] considers that provides insight into the speed of parasympathetic reactivation, and that the normal value of should be >12 beats.There have been a number of clinical studies on the prognosis value of . For example, Dhoble et al. [19] examined conventional cardiovascular risk factors and exercise test parameters in 6546 individuals (mean age 49 years, 58% men) between 1993 and 2003. A total of 285 patients died during the follow-up period. beats was found independently associated with mortality ().
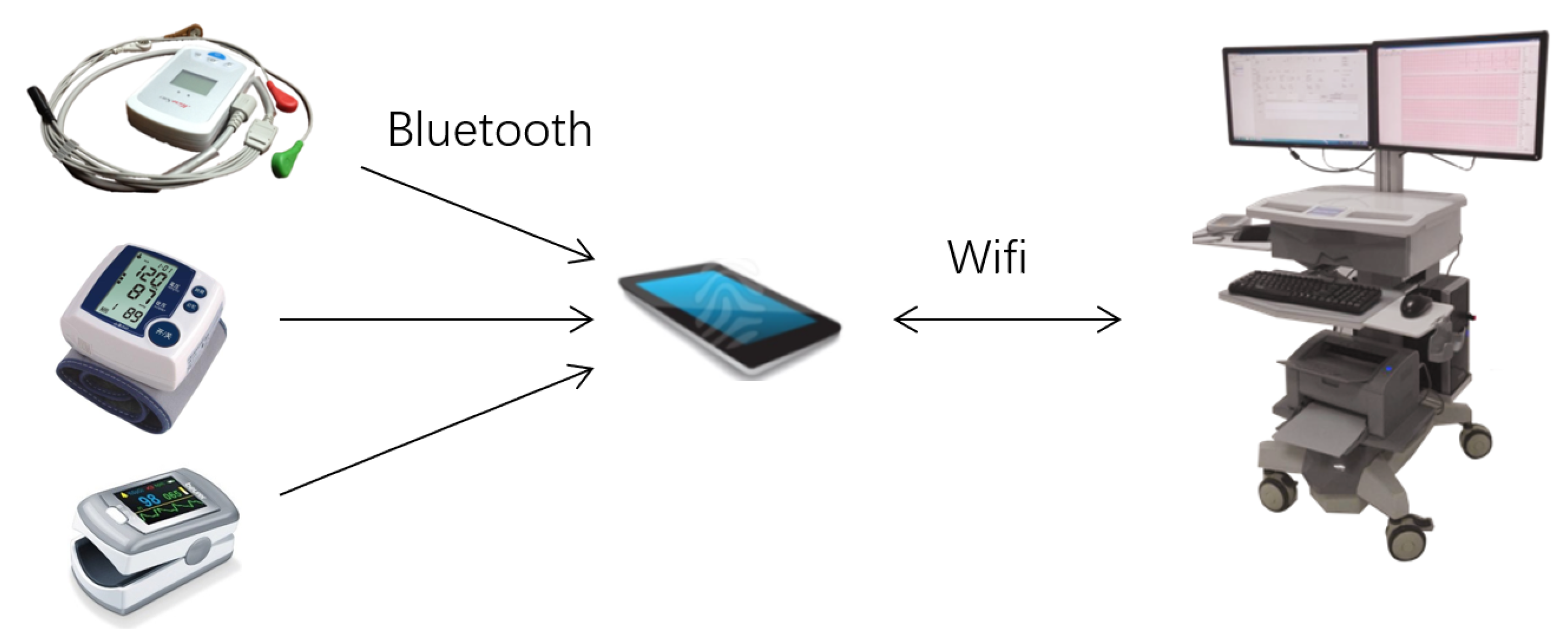
3. Cardiac Chronotropic Competence Test
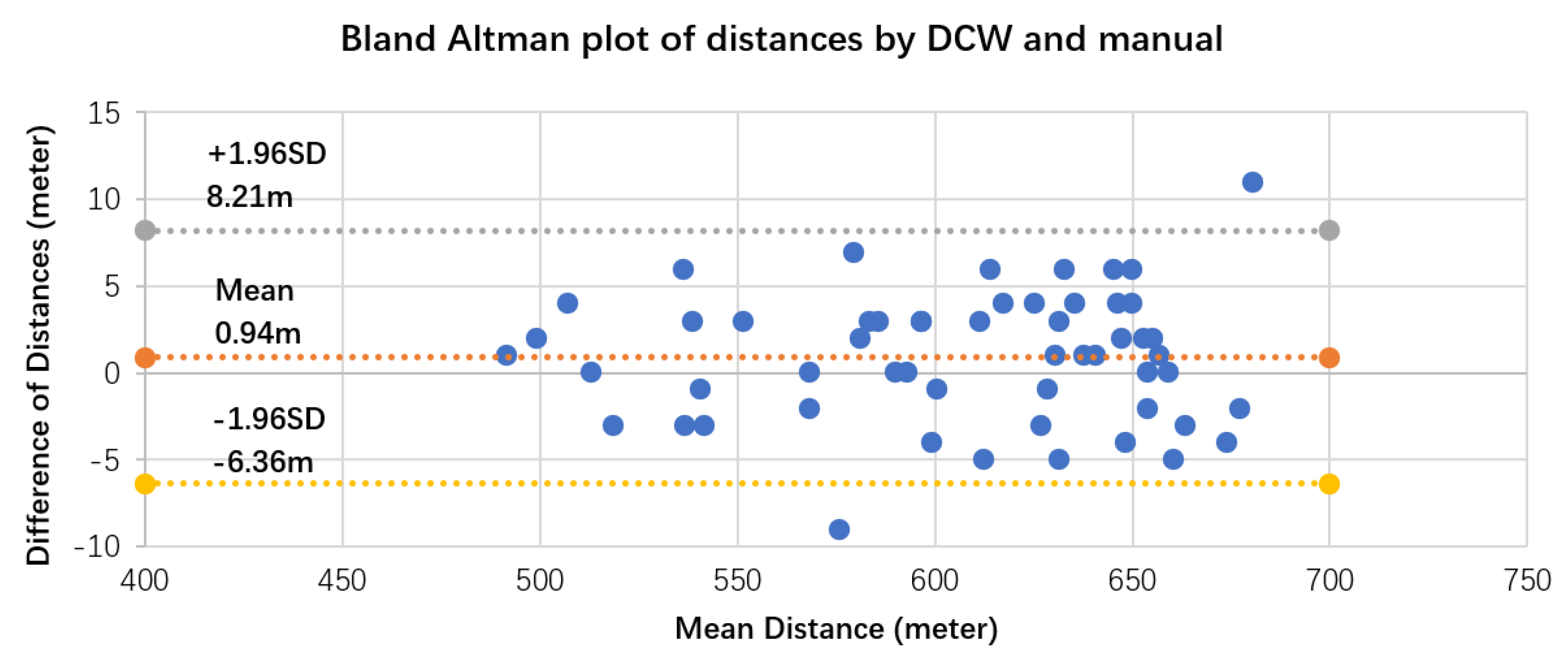
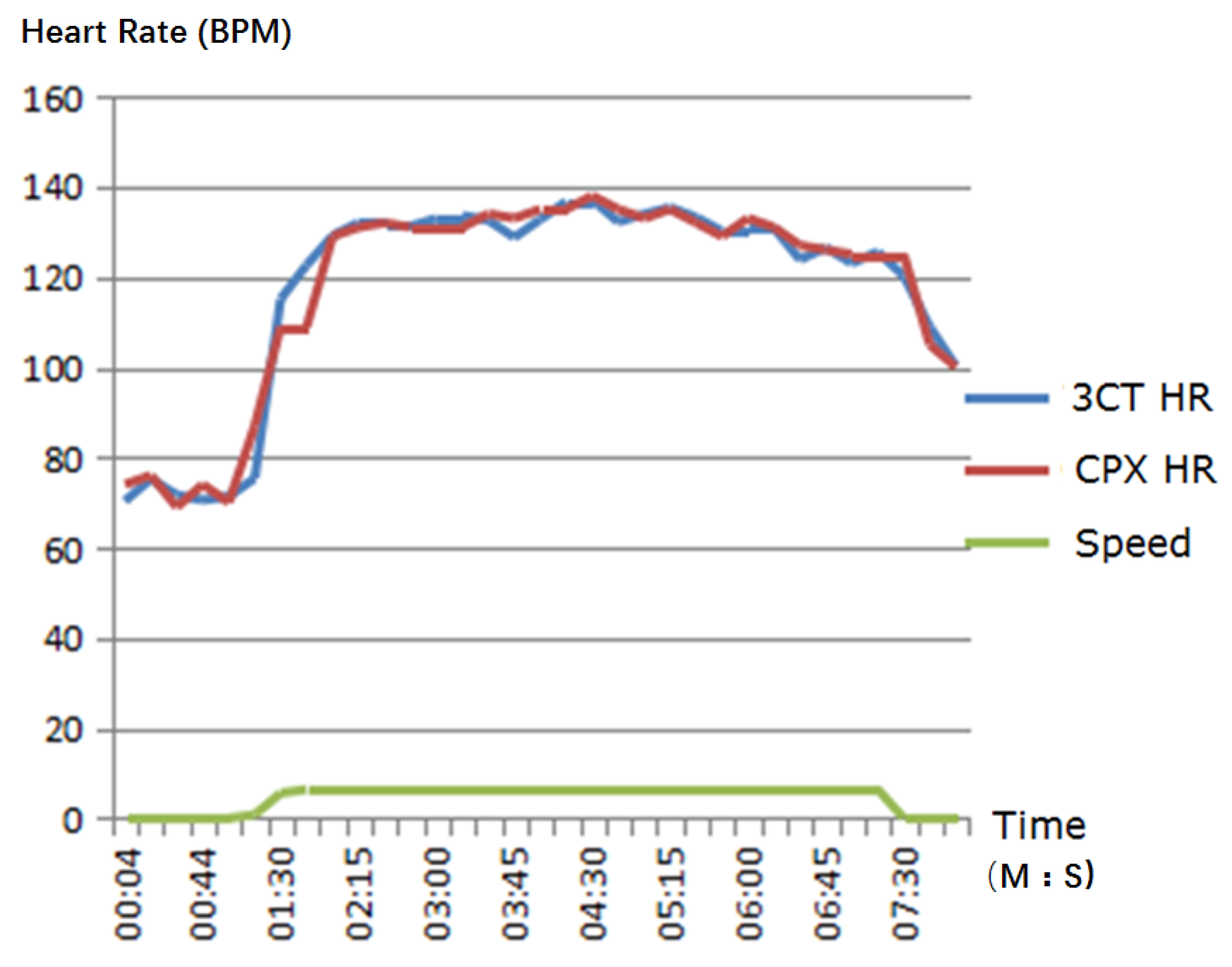
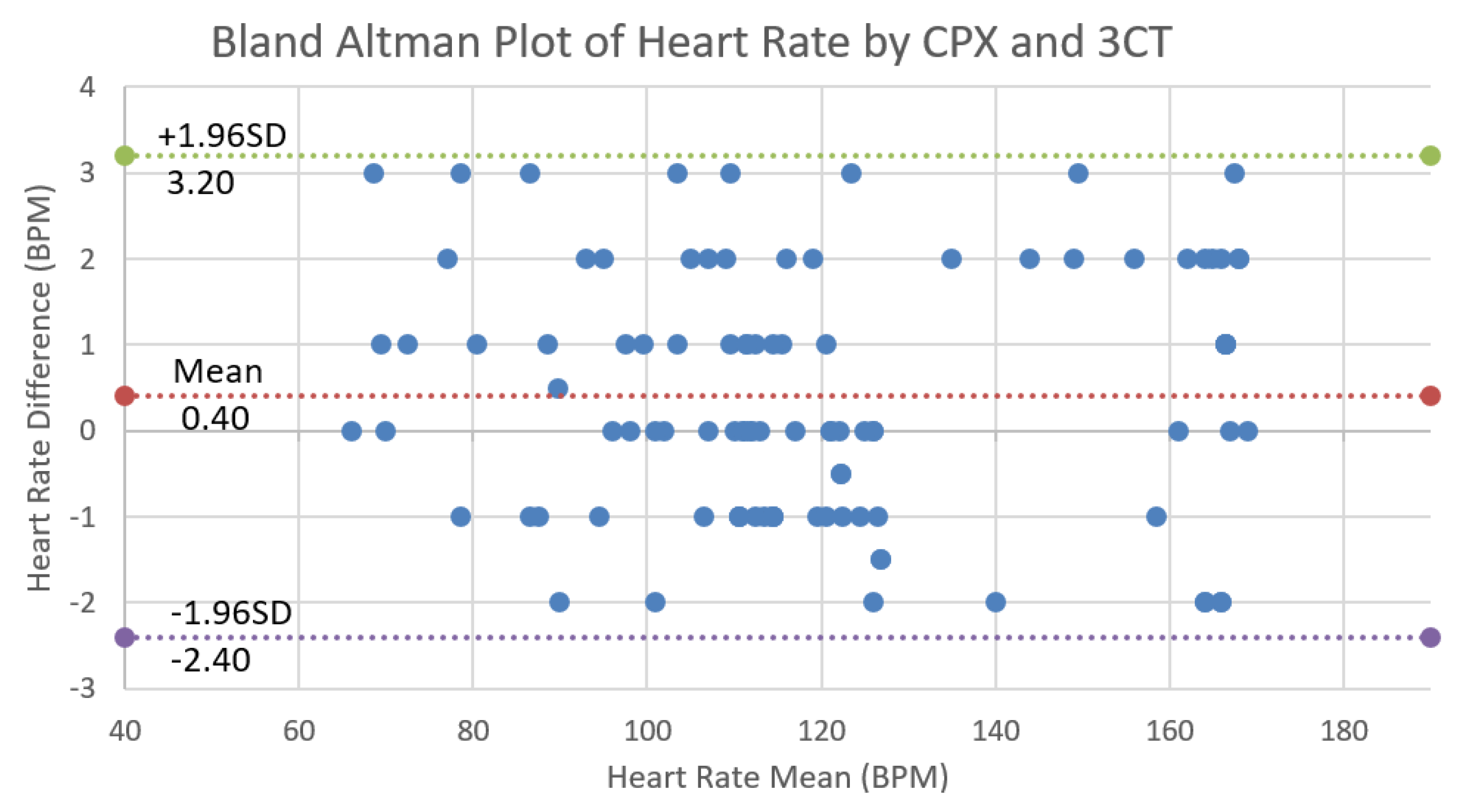
4. Validation of 3CT
Validation Results and Analysis
5. CCI for Cardiac Rehabilitation Validation
6. Conclusions and Remarks
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, Z.; Zheng, J.; Wu, H.; Wang, W.; Wang, B.; Liu, H. Development of a respiratory inductive plethysmography module supporting multiple sensors for wearable systems. Sensors 2012, 12, 13167–13184. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K.; Thomson, S. The role of exercise testing in the modern management of pulmonary arterial hypertension. Diseases 2014, 2, 120–147. [Google Scholar] [CrossRef]
- Balady, G.J.; Arena, R.; Sietsema, K.; Myers, J.; Coke, L.; Fletcher, G.F.; Forman, D.; Franklin, B.; Guazzi, M.; Gulati, M.; et al. Clinician’s Guide to Cardiopulmonary Exercise Testing in Adults. Circulation 2010, 122, 191–225. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.G.; Hu, D.Y. CPET Lab setting requirements and Quality Control in Clinical Practice. China J. Cardiol. 2014, 42, 817–821. [Google Scholar]
- Brubaker, P.H.; Kitzman, D.W. Chronotropic Incompetence. Circulation 2011, 123, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Okin, P.M.; Lauer, M.S.; Kligfield, P. Chronotropic Response to Exercise. Circulation 1996, 94, 3226–3231. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Zhao, S.P.; Cao, Y.; Ye, H.J.; Li, Q.H. Diagnostic value of chronotropic incompetence in coronary heart disease. J. Cent. South Univ. Med. Sci. 2007, 32, 309–311. [Google Scholar]
- Klein, D.A.; Katz, D.H.; Beussink-Nelson, L.; Sanchez, C.L.; Strzelczyk, T.A.; Shah, S.J. Association of Chronic Kidney Disease With Chronotropic Incompetence in Heart Failure With Preserved Ejection Fraction. Am. J. Cardiol. 2015, 116, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Parashar, A.; Kumbhani, D.; Agarwal, S.; Garg, J.; Kitzman, D.; Levine, B.; Drazner, M.; Berry, J.D. Exercise Training in Patients with Heart Failure and Preserved Ejection Fraction: A Meta-analysis of Randomized Control Trials. Circ. Heart Fail. 2014, 8, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.P.; Zurek, M.; Saner, H. Chronotropic incompetence predicts impaired response to exercise training in heart failure patients with sinus rhythm. Eur. J. Prev. Cardiol. 2013, 20, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Yoshitama, T.; Makino, K.; Lee, T.; Saeki, F. Heart Rate Recovery After Exercise Is a Predictor of Silent Myocardial Ischemia in Patients With Type 2 Diabetes. Diabetes Care 2011, 34, 724–726. [Google Scholar] [CrossRef] [PubMed]
- Georgoulias, P.; Demakopoulos, N.; Valotassiou, V.; Orfanakis, A.; Zaganides, A.; Tsougos, I.; Fezoulidis, I. Long-term prognostic value of heart-rate recovery after treadmill testing in patients with diabetes mellitus. Int. J. Cardiol. 2009, 134, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Jae, S.Y.; Kurl, S.; Laukkanen, J.A.; Zaccardi, F.; Choi, Y.H.; Fernhall, B.; Carnethon, M.; Franklin, B.A. Exercise Heart Rate Reserve and Recovery as Predictors of Incident Type 2 Diabetes. Am. J. Med. 2016, 129, 536.e7–536.e12. [Google Scholar] [CrossRef] [PubMed]
- Shin, K.; Shin, K.; Hong, S. Heart rate recovery and chronotropic incompetence in patients with prehypertension. Min. Med. 2015, 106, 87–94. [Google Scholar] [PubMed]
- Aneni, E.; Roberson, L.L.; Shaharyar, S.; Blaha, M.J.; Agatston, A.A.; Blumenthal, R.S.; Meneghelo, R.S.; Conceiçao, R.D.; Nasir, K.; Santos, R.D. Delayed Heart Rate Recovery is Strongly Associated With Early and Late-Stage Prehypertension During Exercise Stress Testing. Am. J. Hypertens. 2014, 27, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Ellestad, M.H. Chronotropic Incompetence. Circulation 1996, 93, 1485–1487. [Google Scholar] [CrossRef] [PubMed]
- Lauer, M.S.; Okin, P.M.; Larson, M.G.; Evans, J.C.; Levy, D. Impaired Heart Rate Response to Graded Exercise. Circulation 1996, 93, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Shaw, L.J.; Thisted, R.A.; Black, H.R.; Bairey Merz, C.N.; Arnsdorf, M.F. Heart Rate Response to Exercise Stress Testing in Asymptomatic Women. Circulation 2010, 122, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Dhoble, A.; Lahr, B.D.; Allison, T.G.; Kopecky, S.L. Cardiopulmonary Fitness and Heart Rate Recovery as Predictors of Mortality in a Referral Population. J. Am. Heart Assoc. 2014, 3, e000559. [Google Scholar] [CrossRef] [PubMed]
- Azarbal, B.; Hayes, S.W.; Lewin, H.C.; Hachamovitch, R.; Cohen, I.; Berman, D.S. The incremental prognostic value of percentage of heart rate reserve achieved over myocardial perfusion single-photon emission computed tomography in the prediction of cardiac death and all-cause mortality. J. Am. Coll. Cardiol. 2004, 44, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Arbit, B.; Azarbal, B.; Hayes, S.W.; Gransar, H.; Germano, G.; Friedman, J.D.; Thomson, L.; Berman, D.S. Prognostic Contribution of Exercise Capacity, Heart Rate Recovery, Chronotropic Incompetence, and Myocardial Perfusion Single-Photon Emission Computerized Tomography in the Prediction of Cardiac Death and All-Cause Mortality. Am. J. Cardiol. 2015, 116, 1678–1684. [Google Scholar] [CrossRef] [PubMed]
- Wilkoff, B.; Miller, R. Exercise testing for chronotropic assessment. Cardiol. Clin. 1992, 10, 705–717. [Google Scholar] [PubMed]
- Wu, J.K. Wearable Cardiac Rehab Equipment and Cloud Service System. Int. Rehabil. Eng. Devices 2016, 6, 16–19. [Google Scholar]
- Cowie, M. Heart rate as a treatable risk factor in cardiovascular disease. Medicographia 2012, 34, 387–394. [Google Scholar]
- ACSM. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: New York, NY, USA, 2013. [Google Scholar]
- Tombaugh, T.N.; Mcintyre, N.J. The mini-mental state examination: A comprehensive review. J. Am. Geriatr. Soc. 1992, 40, 922. [Google Scholar] [CrossRef] [PubMed]
- Jenkinson, C.; Layte, R.; Jenkinson, D.; Lawrence, K.; Petersen, S.; Paice, C.; Stradling, J. A shorter form health survey: Can the SF-12 replicate results from the SF-36 in longitudinal studies? J. Public Health Med. 1997, 19, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61. [Google Scholar] [PubMed]
- Quinn, T.J.; Dawson, J.; Walters, M. Dr John Rankin; his life, legacy and the 50th anniversary of the Rankin Stroke Scale. Scott. Med. J. 2008, 53, 44–47. [Google Scholar] [CrossRef] [PubMed]
- Collin, C.; Wade, D. Assessing motor impairment after stroke: A pilot reliability study. J. Neurol. Neurosurg. Psychiatry 1990, 53, 576–579. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. How to Use the ICF: A Practical Manual for Using The International Classification of Functioning, Disability and Health; Exposure Draft for Comment; International Coach Federation (ICF): Lexington, KY, USA, 2013. [Google Scholar]
| Chronotropic Competence Indices | Measured | Reference Value |
|---|---|---|
| Resting heart rate (HR) | 60–80 bpm | |
| Max HR in Exercise | 220−age | |
| Metabolic equivalent (MET) at Max HR | ||
| Walking distance and grading | I ≤ 300 m < II ≤ 375 m < III ≤ 450 m < IV | |
| Chronotropic rate | ∽10 BPM | |
| Chronotropic limit | ||
| HR recovery after exercise | ≥12 BPM |
| d450 Walking | d640 Doing Housework | d710 Basic Interpersonal Interactions | B730 Muscle Power | ||
|---|---|---|---|---|---|
| Control group | mean before trial | 0.7097 | 0.3548 | 1.2258 | 1.2903 |
| variance before trial | 0.8638 | 0.6082 | 1.0555 | 0.5287 | |
| mean after 3 months | 0.5161 | 0.4194 | 1.2258 | 1.0968 | |
| variance after 3 months | 0.7690 | 0.6204 | 0.9903 | 0.5975 | |
| significance of improvement | 0.0563 | 0.1607 | 1.0000 | 0.0314 | |
| Rehab group | mean before trial | 2.6667 | 3.8000 | 1.0667 | 2.8000 |
| variance before trial | 0.7112 | 1.1552 | 0.7303 | 0.8550 | |
| mean after 3 months | 1.4667 | 3.0000 | 0.4000 | 2.4000 | |
| variance after 3 months | 0.3317 | 0.3371 | 0.2598 | 0.4052 | |
| significance of improvement | 0.0000 | 0.0005 | 0.0007 | 0.0117 | |
| Significance of improvement of rehab group vs. control group | 0.0070 | 0.0209 | 0.0089 | 0.0000 | |
| 6 MWT (meter) | CR (BPM/MET) | (BPM) | ||
|---|---|---|---|---|
| Control group | mean before trial | 225.29 | 12.56 | 24.75 |
| variance before trial | 118.54 | 5.11 | 11.47 | |
| mean after 3 months | 234.14 | 12.37 | 26.64 | |
| variance after 3 months | 129.52 | 4.46 | 12.85 | |
| significance of improvement | 0.152 | 0.753 | 0.557 | |
| Rehab group | mean before trial | 210.12 | 11.03 | 21.21 |
| variance before trial | 139.63 | 3.03 | 11.09 | |
| mean after 3 months | 234.17 | 12.41 | 28.10 | |
| variance after 3 months | 153.81 | 3.82 | 13.40 | |
| significance of improvement | 0.0006 | 0.025 | 0.002 | |
| Significance of improvement of rehab group vs. control group | 0.0445 | 0.0121 | 0.0414 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Li, J.; Seely, A.; Zhu, Y.; Huang, S.; Wang, X.; Zhao, L.; Wang, H.; Christophe, H. Chronotropic Competence Indices Extracted from Wearable Sensors for Cardiovascular Diseases Management. Sensors 2017, 17, 2441. https://doi.org/10.3390/s17112441
Wu J, Li J, Seely A, Zhu Y, Huang S, Wang X, Zhao L, Wang H, Christophe H. Chronotropic Competence Indices Extracted from Wearable Sensors for Cardiovascular Diseases Management. Sensors. 2017; 17(11):2441. https://doi.org/10.3390/s17112441
Chicago/Turabian StyleWu, Jiankang, Jianan Li, Andrew Seely, Yi Zhu, Sisi Huang, Xiaoqin Wang, Lei Zhao, Hongliang Wang, and Herry Christophe. 2017. "Chronotropic Competence Indices Extracted from Wearable Sensors for Cardiovascular Diseases Management" Sensors 17, no. 11: 2441. https://doi.org/10.3390/s17112441




