Sensitivity to Heavy-Metal Ions of Unfolded Fullerene Quantum Dots
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Apparatus
2.2. Preparation of UFQDs
2.3. Instrumentation and Methods for the Response of UFQDs to Heavy-Metal Ions
3. Results and Discussion
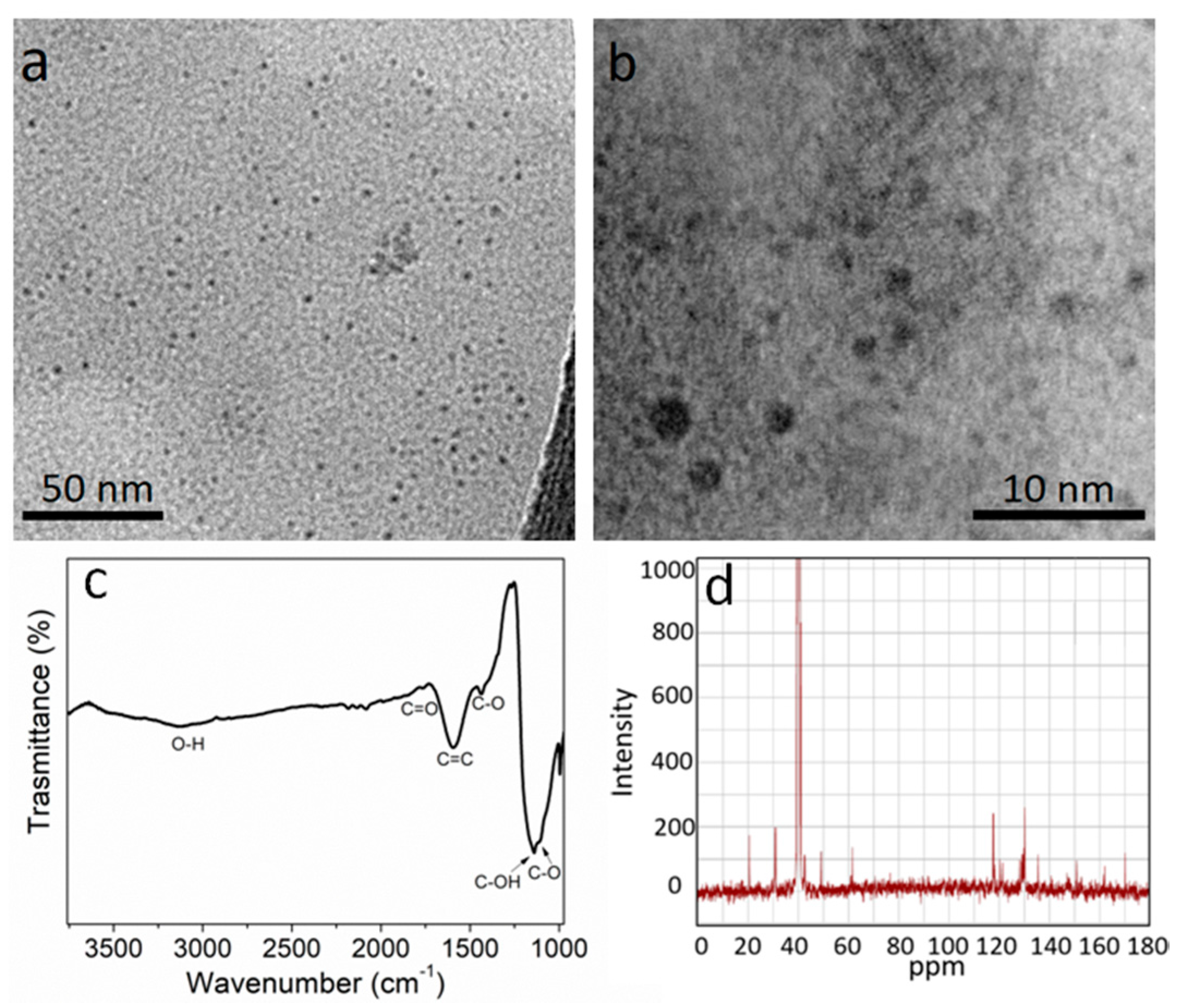
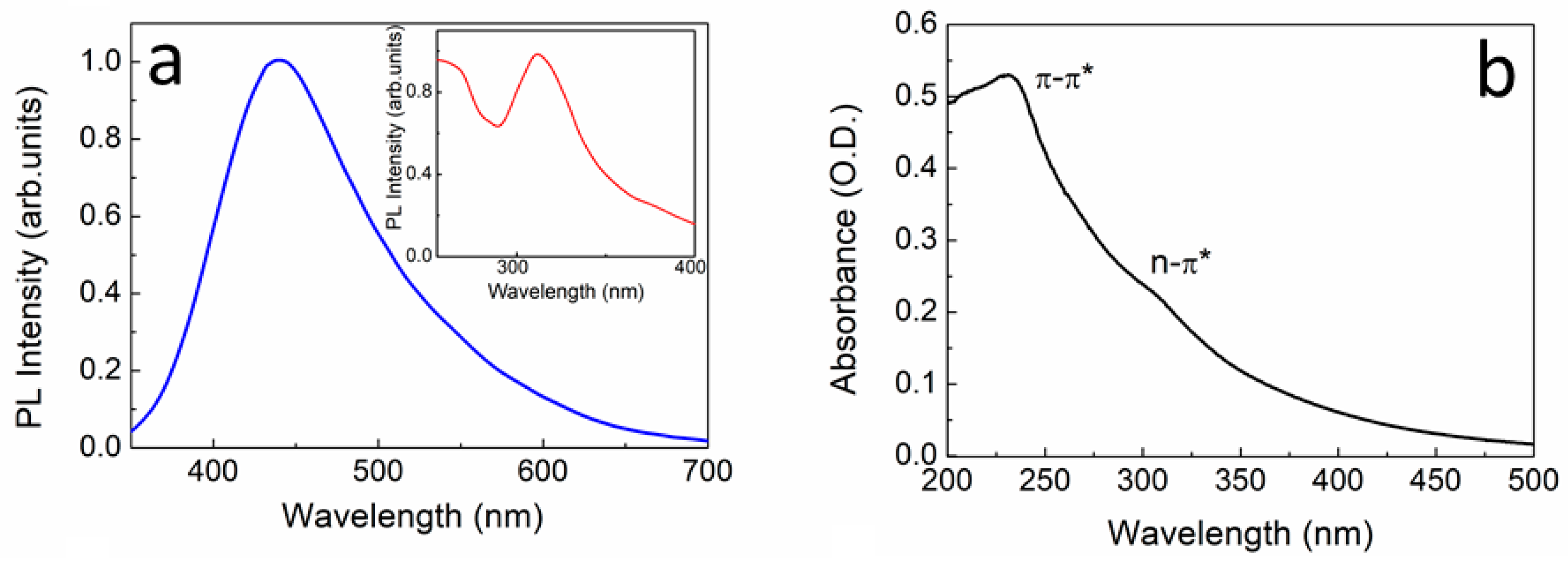
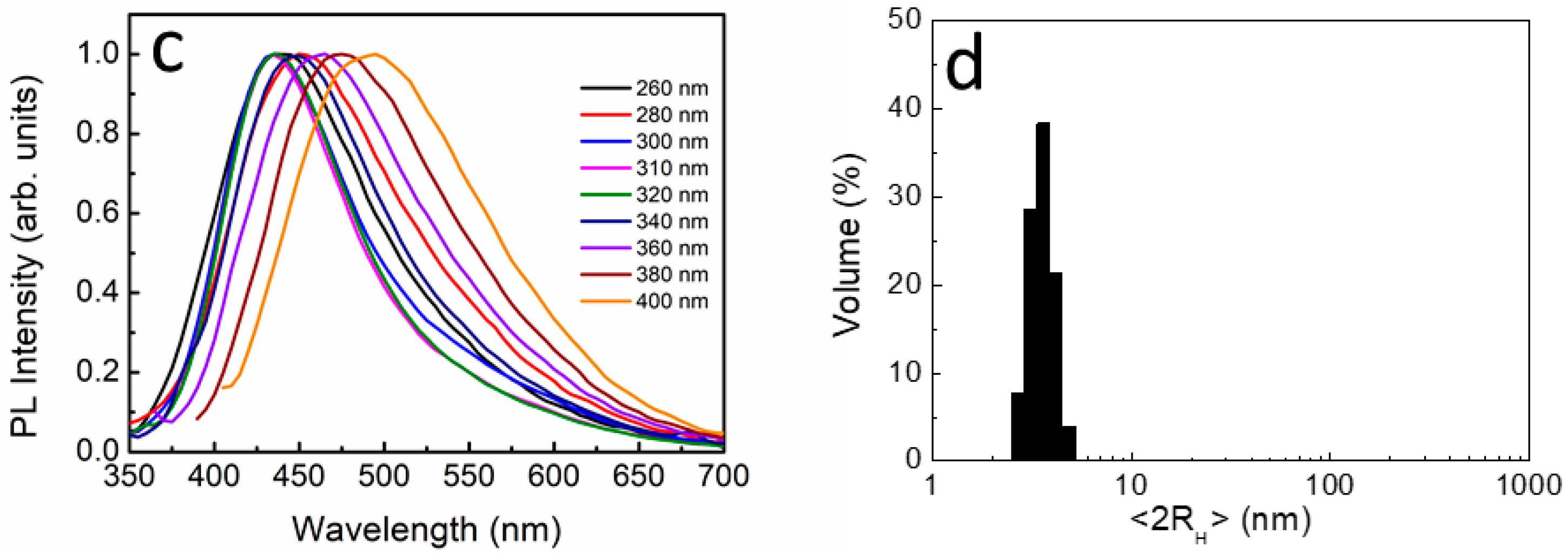
3.1. Optical Characterization of UFQDs
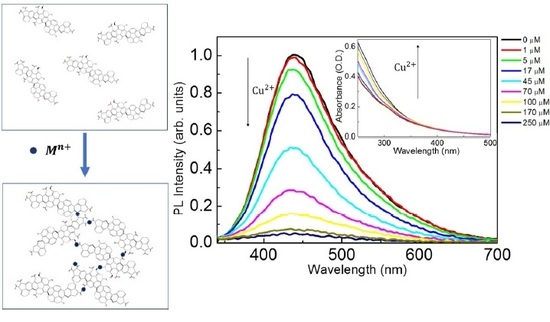
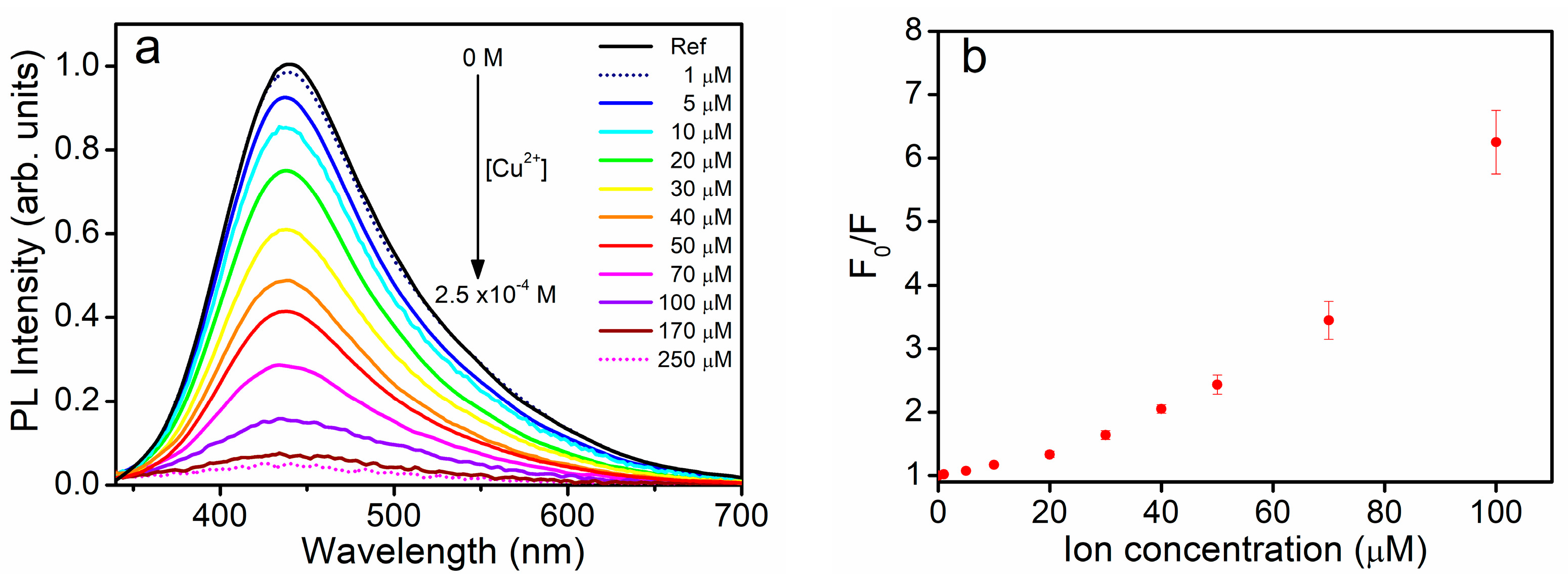
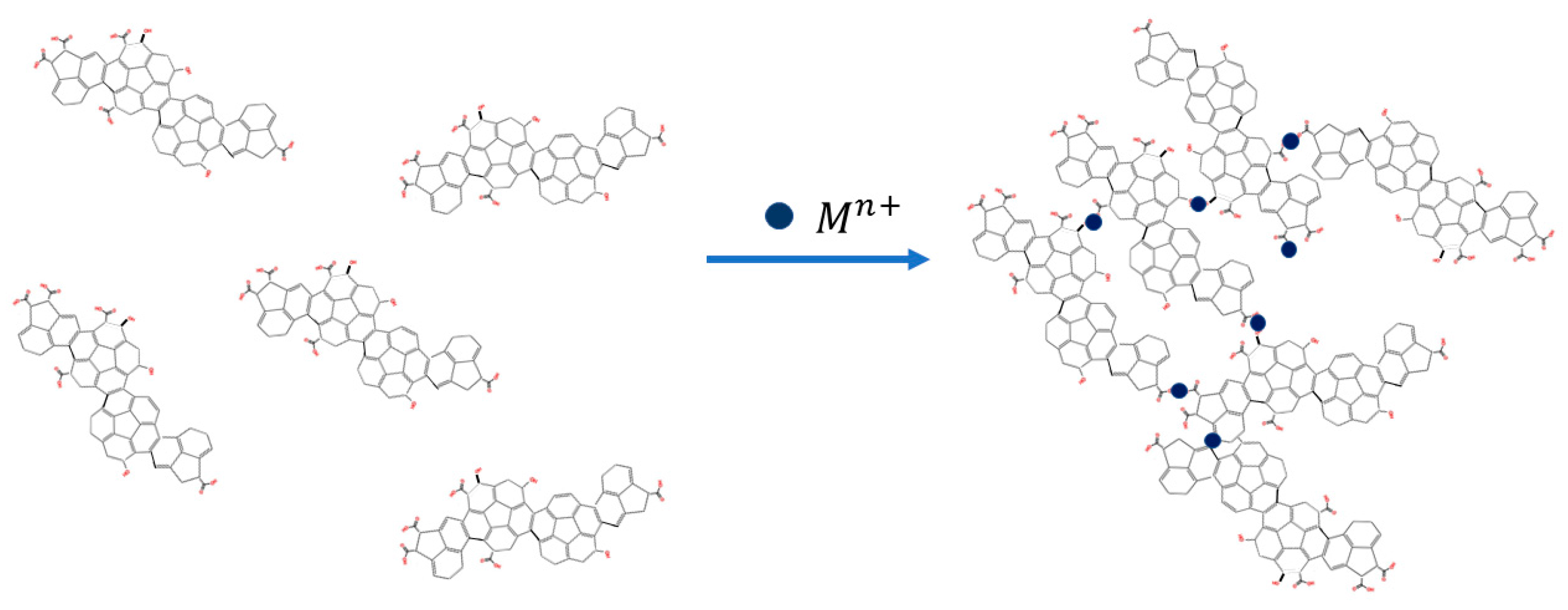
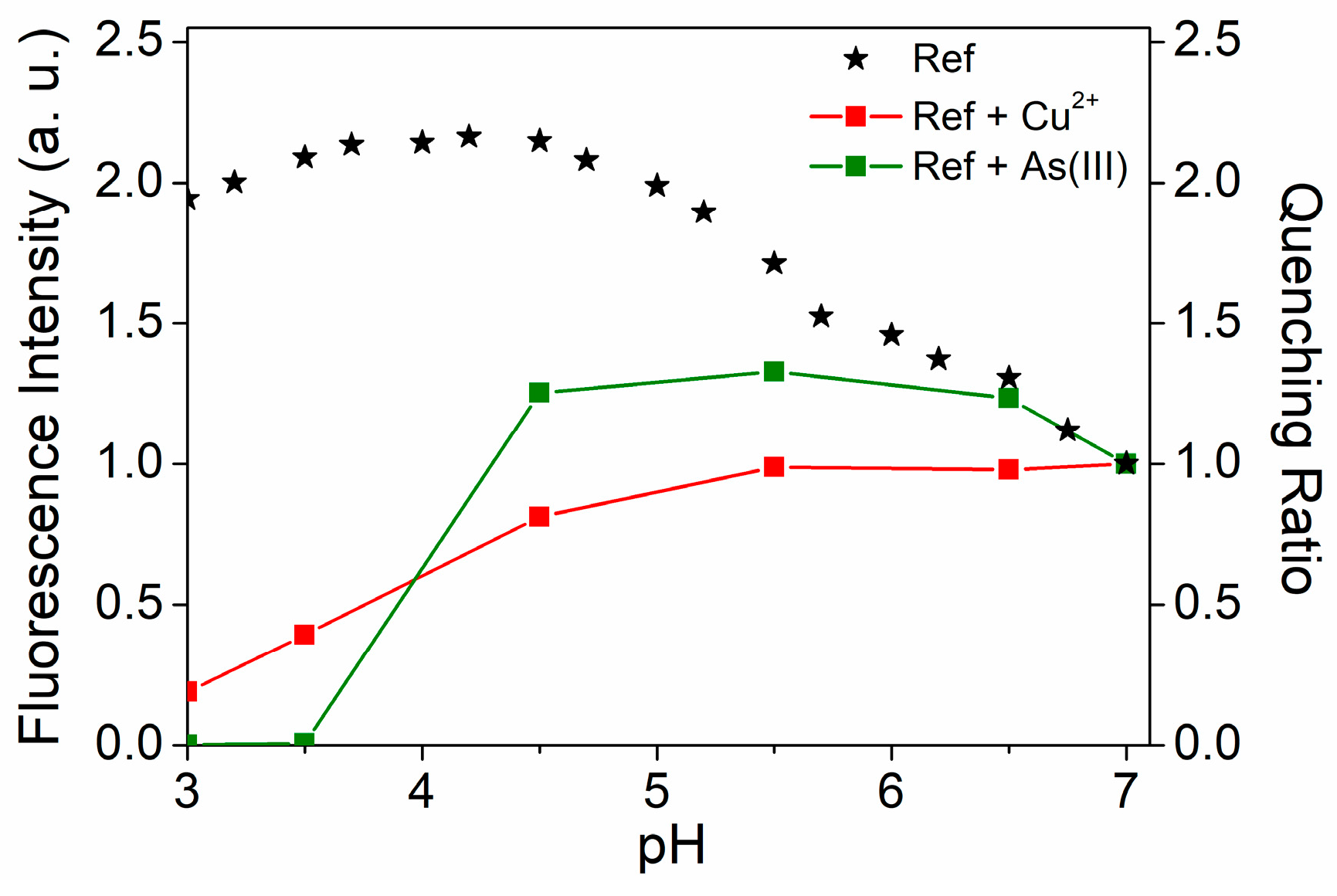
3.2. The Quenching Effect of Heavy Metal Ions on the PL of UFQDs
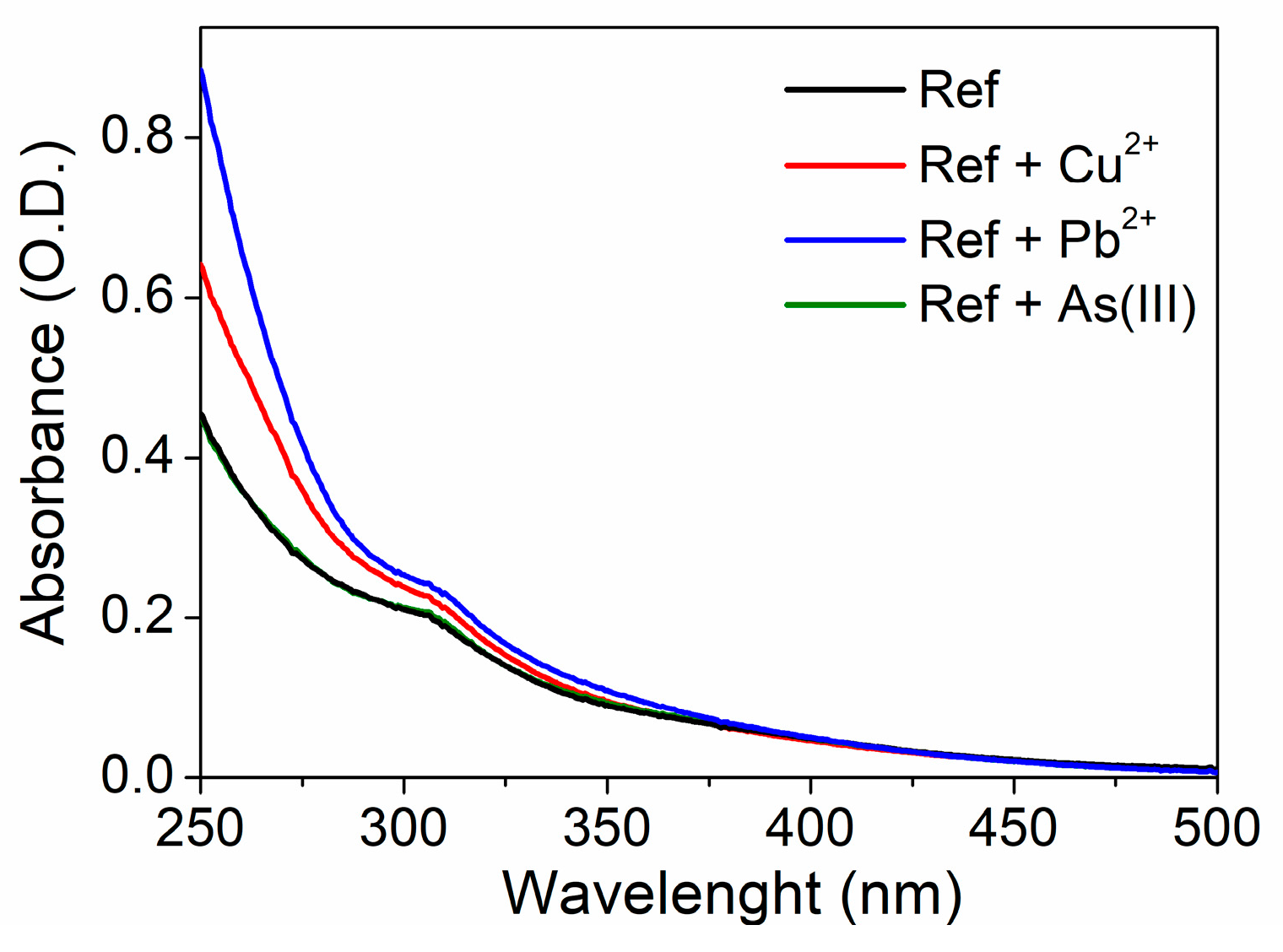
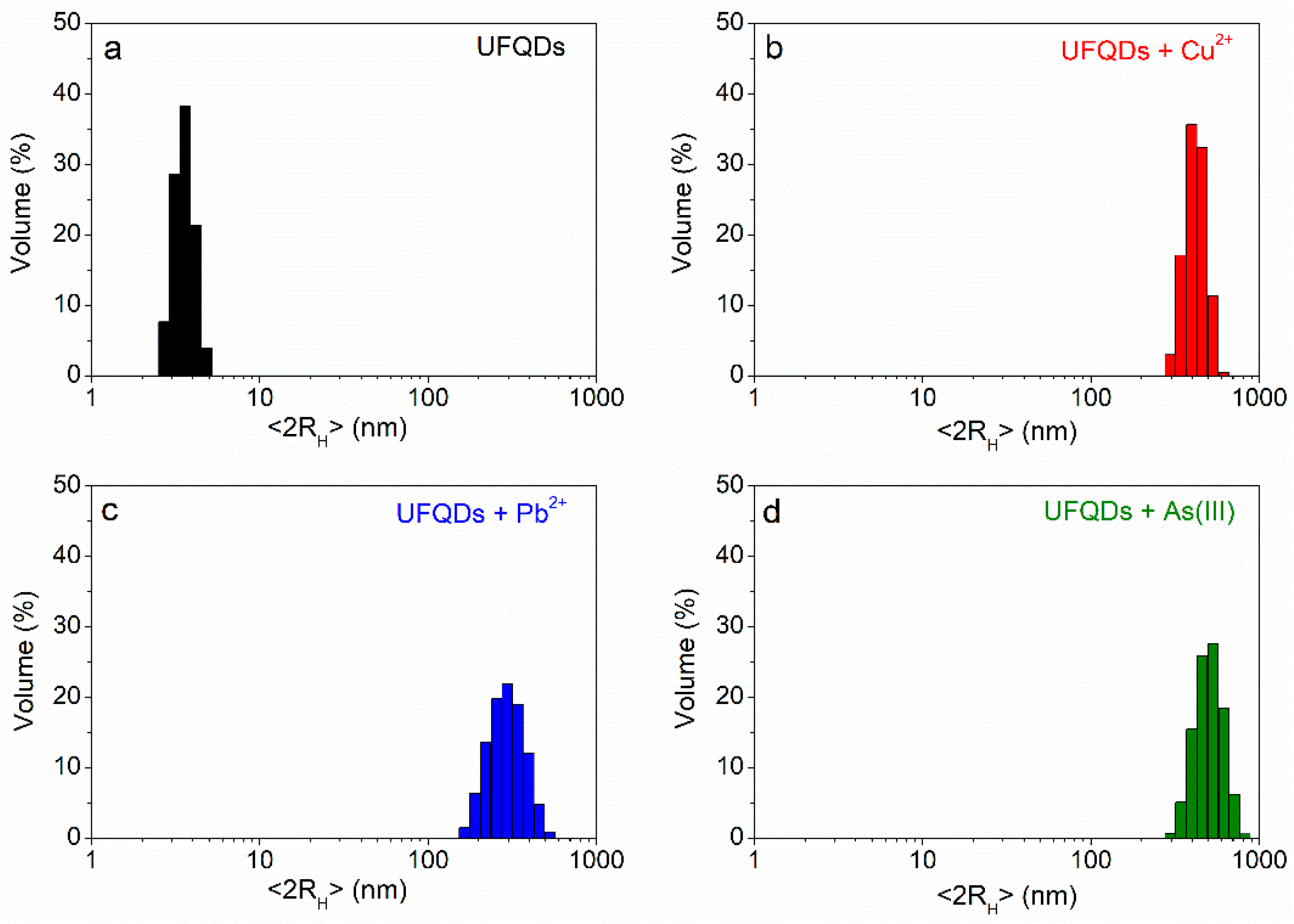
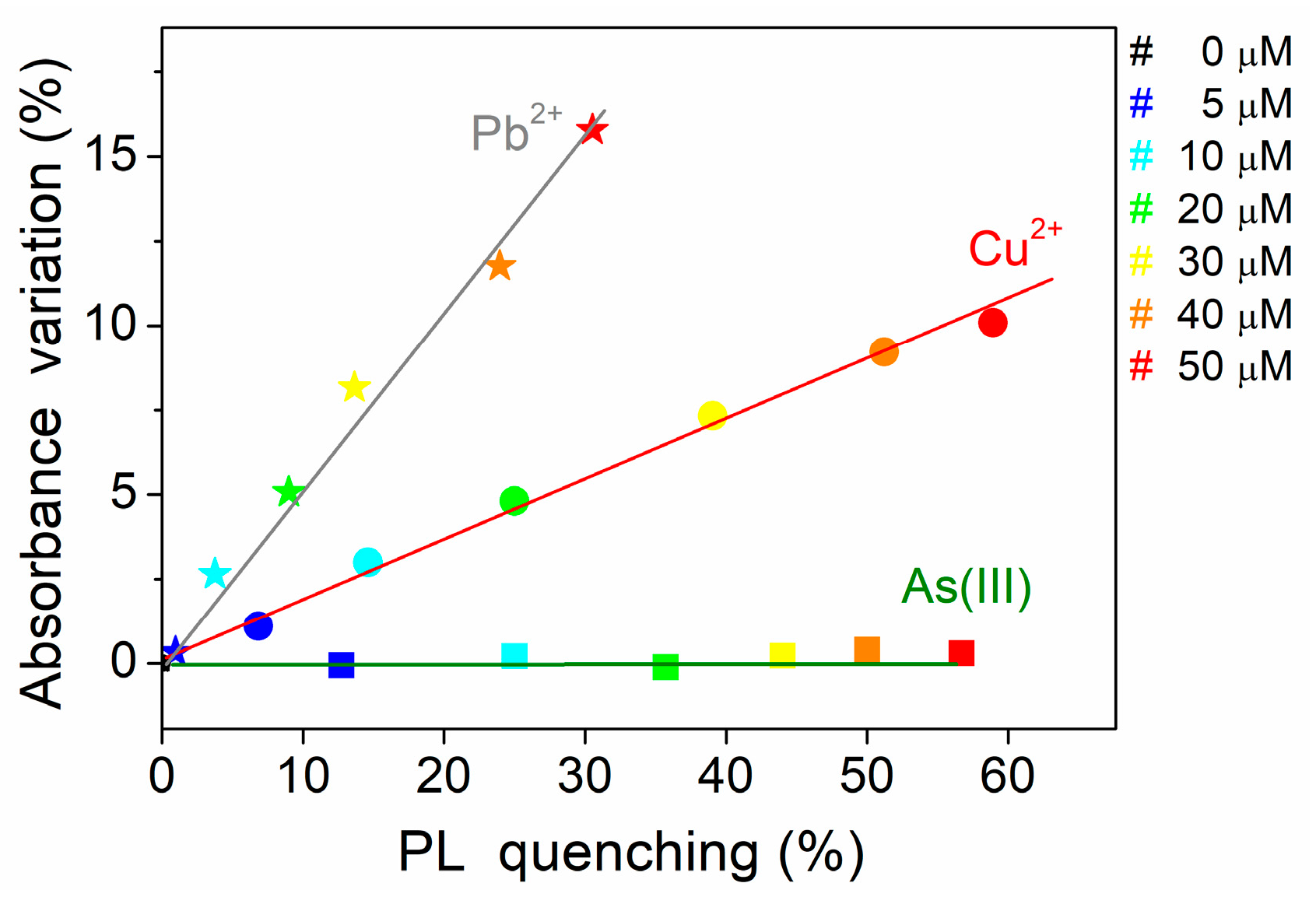
3.3. The Effect of Heavy-Metal ions on the Transmittance Spectrum and DLS Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Barreto, J.A.; O’Malley, W.; Kubeil, M.; Graham, B.; Stephan, H.; Spiccia, L. Nanomaterials: Applications in cancer imaging and therapy. Adv. Mater. 2011, 23, H18–H40. [Google Scholar] [CrossRef] [PubMed]
- Lin, N.; Huang, J.; Dufresne, A. Preparation, properties and applications of polysaccharide nanocrystals in advanced functional nanomaterials: A review. Nanoscale 2012, 4, 3274–3294. [Google Scholar] [CrossRef] [PubMed]
- Prosposito, P.; Mochi, F.; Ciotta, E.; Casalboni, M.; De Matteis, F.; Venditti, I.; Fontana, L.; Testa, G.; Fratoddi, I. Hydrophilic silver nanoparticles with tunable optical properties: Application for the detection of heavy metals in water. Beilstein J. Nanotechnol. 2016, 7, 1654–1661. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Wang, E. Noble metal nanomaterials: Controllable synthesis and application in fuel cells and analytical sensors. Nanotoday 2011, 6, 240–264. [Google Scholar] [CrossRef]
- De Angelis, R.; D’Amico, L.; Casalboni, M.; Hatami, F.; Masselink, W.T.; Prosposito, P. Photoluminescence sensitivity to methanol vapours of surface InP quantum dot: Effect of dot size and coverage. Sens. Actuators B Chem. 2013, 189, 113–117. [Google Scholar] [CrossRef]
- Orsini, A.; Medaglia, P.G.; Scarpellini, D.; Pizzoferrato, R.; Falconi, C. Towards high-performance, low-cost quartz sensors with high-density, well-separated, vertically aligned ZnO nanowires by low-temperature, seed-less, single-step, double-sided growth. Nanotechnology 2013, 24, 355503. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Li, C. Graphene quantum dots: Emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem. Commun. 2012, 48, 3686–3699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, S. Carbon dots: Large-scale synthesis, sensing and bioimaging. Biochem. Pharmacol. 2016, 19, 382–393. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots for sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Zheng, X.T.; Ananthanarayanan, A.; Luo, K.Q.; Chen, P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small 2015, 11, 1620–1636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Bai, L.; Shang, W.; Xie, W.; Ma, H.; Fu, Y.; Fang, D.; Sun, H. Facile synthesis of water-soluble, highly fluorescent graphene quantum dots as a robust biological label for stem cells. J. Mater. Chem. 2012, 22, 7461–7467. [Google Scholar] [CrossRef]
- Bak, S.; Kim, D.; Lee, H. Graphene quantum dots and their possible energy applications: A review. Curr. Appl. Phys. 2016, 16, 1192–1201. [Google Scholar] [CrossRef]
- Zor, E.; Morales-narva, E.; Zamora-ga, A.; Bingol, H.; Ersoz, M. Graphene Quantum Dots-based Photoluminescent Sensor: A Multifunctional Composite for Pesticide Detection. Appl. Mater. Interfaces 2015, 7, 20272–20279. [Google Scholar] [CrossRef] [PubMed]
- Naumov, A.V. Optical Properties of Graphene Oxide, in Graphene Oxide: Fundamentals and Applications; Dimiev, A.M., Eigler, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016. [Google Scholar]
- Sk, M.A.; Ananthanarayanan, A.; Huang, L.; Lim, H. Revealing the tunable photoluminescence properties of graphene quantum dots. J. Mater. Chem. C Mater. Opt. Electron. Devices 2014, 2, 6954–6960. [Google Scholar] [CrossRef]
- Cao, L.I.; Meziani, M.J.; Sahu, S. Photoluminescence Properties of Graphene versus Other Carbon Nanomaterials. Acc. Chem. Res. 2012, 46, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Cushing, S.K.; Zhou, X.; Wu, N. Fingerprinting photoluminescence of functional groups in graphene oxide. J. Mater. Chem. 2012, 22, 23374–23379. [Google Scholar] [CrossRef]
- Galande, C.; Mohite, A.D.; Naumov, A.V.; Gao, W.; Ci, L.; Ajayan, A.; Gao, H.; Srivastava, A.; Weisman, R.B.; Ajayan, P.M. Quasi-Molecular Fluorescence from Graphene Oxide. Sci. Rep. 2011, 85, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Shao, J.; Chen, C.; Li, H.; Wang, R.; Chi, Y.; Lin, X.; Chen, G. Blue luminescent graphene quantum dots and graphene oxide prepared by tuning the carbonization degree of citric acid. Carbon 2012, 50, 4738–4743. [Google Scholar] [CrossRef]
- Eda, B.G.; Lin, Y.; Mattevi, C.; Yamaguchi, H.; Chen, H.; Chen, I.; Chen, C.; Chhowalla, M. Blue Photoluminescence from Chemically Derived Graphene Oxide. Adv. Mater. 2010, 22, 505–509. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhu, Y.; Yang, X.; Zong, J.; Zhang, J. One-pot hydrothermal synthesis of graphene quantum dots surface-passivated by polyethylene glycol and their photoelectric conversion under near-infrared light. New J. Chem. 2012, 36, 97–101. [Google Scholar] [CrossRef]
- Tang, L.; Ji, R.; Cao, X.; Lin, J.; Jiang, H.; Li, X.; Teng, K.S. Deep Ultraviolet Photoluminescence of Water-Soluble Self-Passivated Graphene Quantum Dots. ACS Nano 2012, 6, 5102–5110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Tang, S.; Qiao, C.; Wang, L.; Wang, H. Surface Chemistry Routes to Modulate the Photoluminescence of Graphene Quantum Dots: From Fluorescence Mechanism to Up-Conversion Bioimaging Applications. Adv. Funct. Mater. 2012, 22, 4732–4740. [Google Scholar] [CrossRef]
- Jin, S.H.; Jun, G.H.; Hong, S.H.; Jeon, S.; Science, M.; Technology, I. Tuning the Photoluminescence of Graphene Quantum Dots through the Charge Transfer E ff ect of Functional. ACS Nano 2013, 7, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Liu, F.; Wang, C.; Xin, X.; Liu, J.; Guo, S.; Zhang, J. Effect of Lateral Size of Graphene Quantum Dots on Their Properties and Application. ACS Appl. Mater. Interfaces 2016, 8, 2104–2110. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cui, X.; Li, L. Synthesis of Large, Stable Colloidal Graphene Quantum Dots with Tunable Size. JACS 2010, 132, 5944–5945. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Tang, T.; Feng, Q.; Li, M.; Liu, Y.; Tang, N.; Zhong, W.; Du, Y. Tuning photoluminescence of reduced graphene oxide quantum dots from blue to purple. J. Appl. Phys. 2014, 164307, 1–5. [Google Scholar] [CrossRef]
- Jian, J.; Chen, W. Graphene Quantum Dots as a Fluorescence Probes for Sensing Metal Ions: Synthesis and Applications. Curr. Org. Chem. 2015, 19, 1150–1162. [Google Scholar]
- Wang, D.; Wang, L.; Dong, X.; Shi, Z.; Jin, J. Chemically tailoring graphene oxides into fluorescent nanosheets for Fe3+ ion detection. Carbon 2012, 50, 2147–2154. [Google Scholar] [CrossRef]
- Wang, F.; Gu, Z.; Lei, W.; Wang, W.; Xia, X.; Hao, Q. Graphene quantum dots as a fluorescent sensing platform for highly efficient detection of copper(II) ions. Sens. Actuators B. Chem. 2014, 190, 516–522. [Google Scholar] [CrossRef]
- Chakraborti, H.; Sinha, S.; Ghosh, S.; Kalyan, S. Interfacing water soluble nanomaterials with fluorescence chemosensing: Graphene quantum dot to detect Hg2+ in 100% aqueous solution. Mater. Lett. 2013, 97, 78–80. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Ni, Y.; Kokot, S. A rapid and label-free dual detection of Hg(II) and cysteine with the use of fluorescence switching of graphene quantum dots. Sens. Actuators B. Chem. 2015, 207, 490–497. [Google Scholar] [CrossRef]
- Huang, H.; Liao, L.; Xu, X.; Zou, M.; Liu, F.; Li, N. The electron-transfer based interaction between transition metal ions and photoluminescent graphene quantum dots (GQDs): A platform for metal ion sensing. Talanta 2013, 117, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Qiu, H.; Zhu, F.; Lu, S.; Xiao, Q. Graphene quantum dots as on-off-on fluorescent probes for chromium(VI) and ascorbic acid. Microchim. Acta 2015, 182, 1723–1731. [Google Scholar] [CrossRef]
- Liu, X.; Gao, W.; Zhou, X. Pristine graphene quantum dots for detection of copper ions. J. Mater. Res. 2014, 29, 1401–1407. [Google Scholar] [CrossRef]
- Length, F. Heavy metal pollution and human biotoxic effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Gordon, B.; Callan, P.; Vickers, C. WHO Guidelines for Drinking-Water Quality, 2008th ed.; WHO: Geneve, Switzerland, 2008; Volume 38, ISBN 978-92-4-154761-1. [Google Scholar]
- Ackerman, A.H.; Creed, P.A.; Parks, A.N.; Fricke, M.W.; Schwegel, C.A.; Creed, J.T.; Heitkemper, D.T.; Vela, N.P. Comparison of a chemical and enzymatic extraction of arsenic from rice and an assessment of the arsenic absorption from contaminated water by cooked rice. Environ. Sci. Technol. 2005, 39, 5241–5246. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gou, H.; Al-Ogaidi, I.; Wu, N. Nanostructured sensors for detection of heavy metals: A review. ACS Sustain. Chem. Eng. 2013, 1, 713–723. [Google Scholar] [CrossRef]
- Afkhami, A.; Soltani-Felehgari, F.; Madrakiana, T.; Ghaedi, H.; Rezaeivala, M. Fabrication and application of a new modified electrochemical sensor using nano-silica and a newly synthesized Schiff base for simultaneous determination of Cd2+, Cu2+ and Hg2+ ions in water and some foodstuff samples. Anal. Chim. Acta 2013, 771, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Bonyár, A.; Nagy, P.; Mayer, V.; Vitéz, A.; Gerecs, A.; Sántha, H.; Harsányi, G. A colorimetry based, semi-automated portable sensor device for the detection of arsenic in drinking water. Sens. Actuators B Chem. 2017, 251, 1042–1049. [Google Scholar] [CrossRef]
- Zhu, D.; Ren, A.; He, X.; Luo, Y.; Duan, Z.; Yan, X.; Xiong, Y.; Zhong, X. A novel ratiometric fluorescent probe for selective and sensitive detection of Cu2+ in complete aqueous solution. Sens. Actuators B Chem. 2017, 252, 134–141. [Google Scholar] [CrossRef]
- Ciotta, E.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Venditti, I.; Fratoddi, I.; Pizzoferrato, R. Sensitivity to Heavy-Metal Ions of Cage-Opening Fullerene Quantum Dots. Multidiscip. Digit. Publ. Inst. Proc. 2017, 1, 475. [Google Scholar] [CrossRef]
- Chua, C.K.; Sofer, Z.; Šimek, P.; Jankovský, O.; Klímová, K.; Bakardjieva, S.; Hrdličková Kučková, Š.; Pumera, M. Synthesis of strongly fluorescent graphene quantum dots by cage-opening buckminsterfullerene. ACS Nano 2015, 9, 2548–2555. [Google Scholar] [CrossRef] [PubMed]
- Innocenzi, P.; Brusatin, G.; Abbotto, A.; Beverina, L.; Pagani, G.A.; Casalboni, M.; Sarcinelli, F.; Pizzoferrato, R. Entrapping of push-pull zwitterionic chromophores in hybrid matrices for photonic applications. J. Sol-Gel Sci. Technol. 2003, 26, 967–970. [Google Scholar] [CrossRef]
- D’Amato, R.; Venditti, I.; Russo, M.V.; Falconieri, M. Growth control and long-range self-assembly of poly(methyl methacrylate) nanospheres. J. Appl. Polym. Sci. 2006, 102, 4493–4499. [Google Scholar] [CrossRef]
- Venditti, I.; Testa, G.; Sciubba, F.; Carlini, L.; Porcaro, F.; Meneghini, C.; Mobilio, S.; Battocchio, C.; Fratoddi, I. Hydrophilic Metal Nanoparticles Functionalized by 2-Diethylaminoethanethiol: A Close Look at the Metal–Ligand Interaction and Interface Chemical Structure. J. Phys. Chem. C 2017, 121, 8002–8013. [Google Scholar] [CrossRef]
- De Angelis, R.; Venditti, I.; Fratoddi, I.; De Matteis, F.; Prosposito, P.; Cacciotti, I.; D’Amico, L.; Nanni, F.; Yadav, A.; Casalboni, M.; et al. From nanospheres to microribbons: Self-assembled Eosin Y doped PMMA nanoparticles as photonic crystals. J. Colloid Interface Sci. 2014, 414, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.J.; Rong, M.Z.; Zhang, M.Q. Preparation of graphene oxide and polymer-like quantum dots and their one- and two-photon induced fluorescence properties. Phys. Chem. Chem. Phys. 2016, 18, 4800–4806. [Google Scholar] [CrossRef] [PubMed]
- Yun, L.; Kyusik, Z. Graphene oxide-modified ZnO particles: Synthesis, characterization, and antibacterial properties. Int. J. Nanomed. 2015, 10, 79–92. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. Compendium of Chemical Terminology: The “Gold Book”, 2nd ed.; Blackwell Science: Oxford, UK, 2014; ISBN 0-9678550-9-8. [Google Scholar]
- Peak, D.; Werner, T.C.; Dennin, R.M.; Baird, J.K. Fluorescence quenching at high quencher concentrations. J. Chem. Phys. 1983, 79, 3328. [Google Scholar] [CrossRef]
- Campbell, K.; Zappas, A.; Bunz, U.; Thio, Y.S.; Bucknall, D.G. Fluorescence quenching of a poly(para-phenylene ethynylenes) by C 60 fullerenes. J. Photochem. Photobiol. A Chem. 2012, 249, 41–46. [Google Scholar] [CrossRef]
- Keizer, J. Nonlinear Fluorescence Quenching and the Origin of Positive Curvature in Stern-Volmer Plots. J. Am. Chem. Soc. 1983, 105, 1494–1498. [Google Scholar] [CrossRef]
- Qian, Z.S.; Shan, X.Y.; Chai, L.J.; Chen, J.R.; Feng, H. A fluorescent nanosensor based on graphene quantum dots—Aptamer probe and graphene oxide platform for detection of lead(II) ion. Biosens. Bioelectron. 2015, 68, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.-X.; Zhang, M.; Fu, Q.-Q.; Liu, R.; Shi, G.-Y. Highly sensitive and selective fluorescent detection of cerebral lead(II) based on graphene quantum dot conjugates. Chem. Commun. 2013, 49, 10599–10601. [Google Scholar] [CrossRef] [PubMed]
- Compton, O.C.; Nguyen, S.T. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Liu, L.; Gao, B.; Munoz-Carpena, R.; Zhang, M.; Chen, H.; Zhou, Z.; Wang, H. Aggregation kinetics of graphene oxides in aqueous solutions: Experiments, mechanisms, and modeling. Langmuir 2013, 29, 15174–15181. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Chen, B.; Xing, B. Aggregation Kinetics and Self-Assembly Mechanisms of Graphene Quantum Dots in Aqueous Solutions: Cooperative Effects of pH and Electrolytes. Environ. Sci. Technol. 2017, 51, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene Oxide Papers Modified by DivalentIons—Enhancing Mechanical Properties via Chemical Cross-Linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Szabò, T.; Tombacz, E.; Illès, E.; Dèkàny, I. Enhanced acidity and pH-dependent surface charge characterization of successively oxidized graphite oxides. Carbon 2006, 44, 537–545. [Google Scholar] [CrossRef]
- Albrecht, T.W.J.; Addai-Mensah, J.; Fornasiero, D. Effect of pH, Concentration and Temperature on Copper and Zinc Hydroxide Formation/Precipitation in Solution. In CHEMECA 2011—“Engineering a Better World”; Institution of Engineers Australia: Barton, Australia, 2011; pp. 1–10. [Google Scholar]
- Kochmann, S.; Hirsch, T.; Wolfbeis, O.S. The pH dependence of the total fluorescence of graphite oxide. J. Fluoresc. 2012, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.P.; Lu, C.; Su, F. Sorption of divalent metal ions from aqueous solution by carbon nanotubes: A review. Sep. Purif. Technol. 2007, 58, 224–231. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciotta, E.; Paoloni, S.; Richetta, M.; Prosposito, P.; Tagliatesta, P.; Lorecchio, C.; Venditti, I.; Fratoddi, I.; Casciardi, S.; Pizzoferrato, R. Sensitivity to Heavy-Metal Ions of Unfolded Fullerene Quantum Dots. Sensors 2017, 17, 2614. https://doi.org/10.3390/s17112614
Ciotta E, Paoloni S, Richetta M, Prosposito P, Tagliatesta P, Lorecchio C, Venditti I, Fratoddi I, Casciardi S, Pizzoferrato R. Sensitivity to Heavy-Metal Ions of Unfolded Fullerene Quantum Dots. Sensors. 2017; 17(11):2614. https://doi.org/10.3390/s17112614
Chicago/Turabian StyleCiotta, Erica, Stefano Paoloni, Maria Richetta, Paolo Prosposito, Pietro Tagliatesta, Chiara Lorecchio, Iole Venditti, Ilaria Fratoddi, Stefano Casciardi, and Roberto Pizzoferrato. 2017. "Sensitivity to Heavy-Metal Ions of Unfolded Fullerene Quantum Dots" Sensors 17, no. 11: 2614. https://doi.org/10.3390/s17112614