A Quartz Crystal Microbalance Immunosensor for Stem Cell Selection and Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
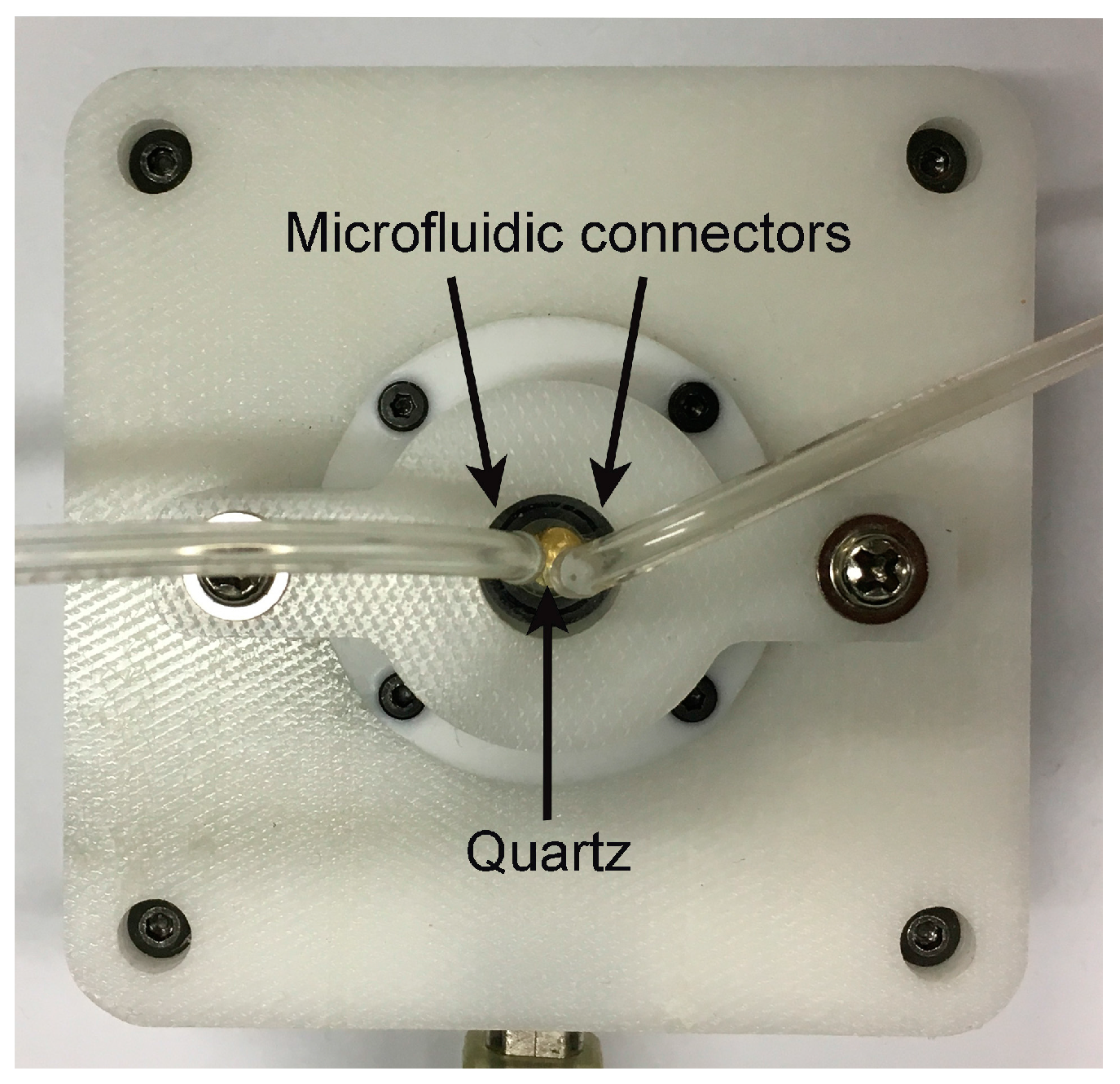
2.2. Instrumentation
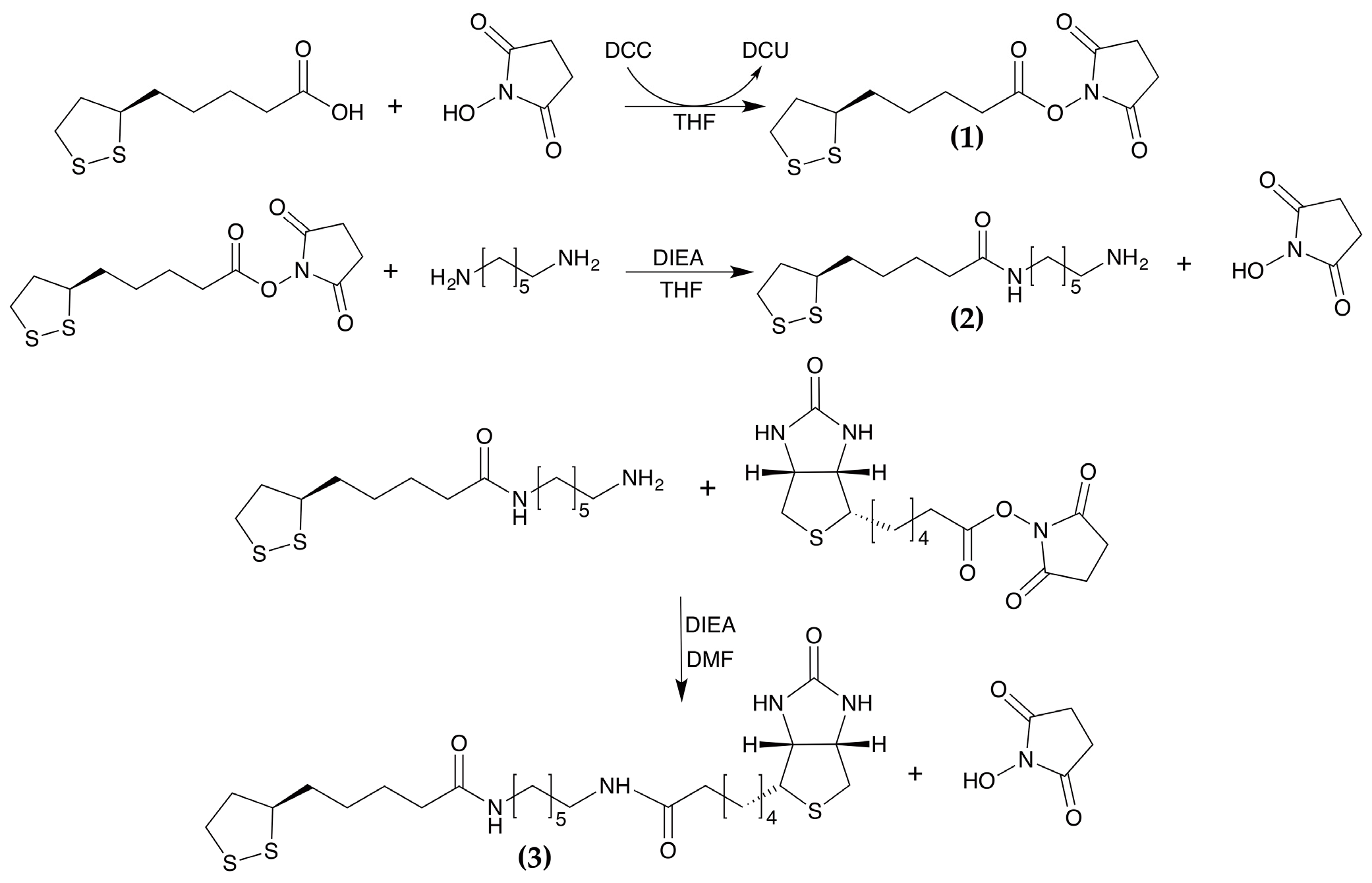
2.3. Synthesis of Biotinylated Linker: N-Dithiooctanoylamidohexyl-5-Biotinylpentanamide (LHB)
2.4. QCM Setup and Detection Test
2.4.1. QCM Immunosensor Assembly
2.4.2. Procedure for the Detection of DPSCs
3. Results and Discussion
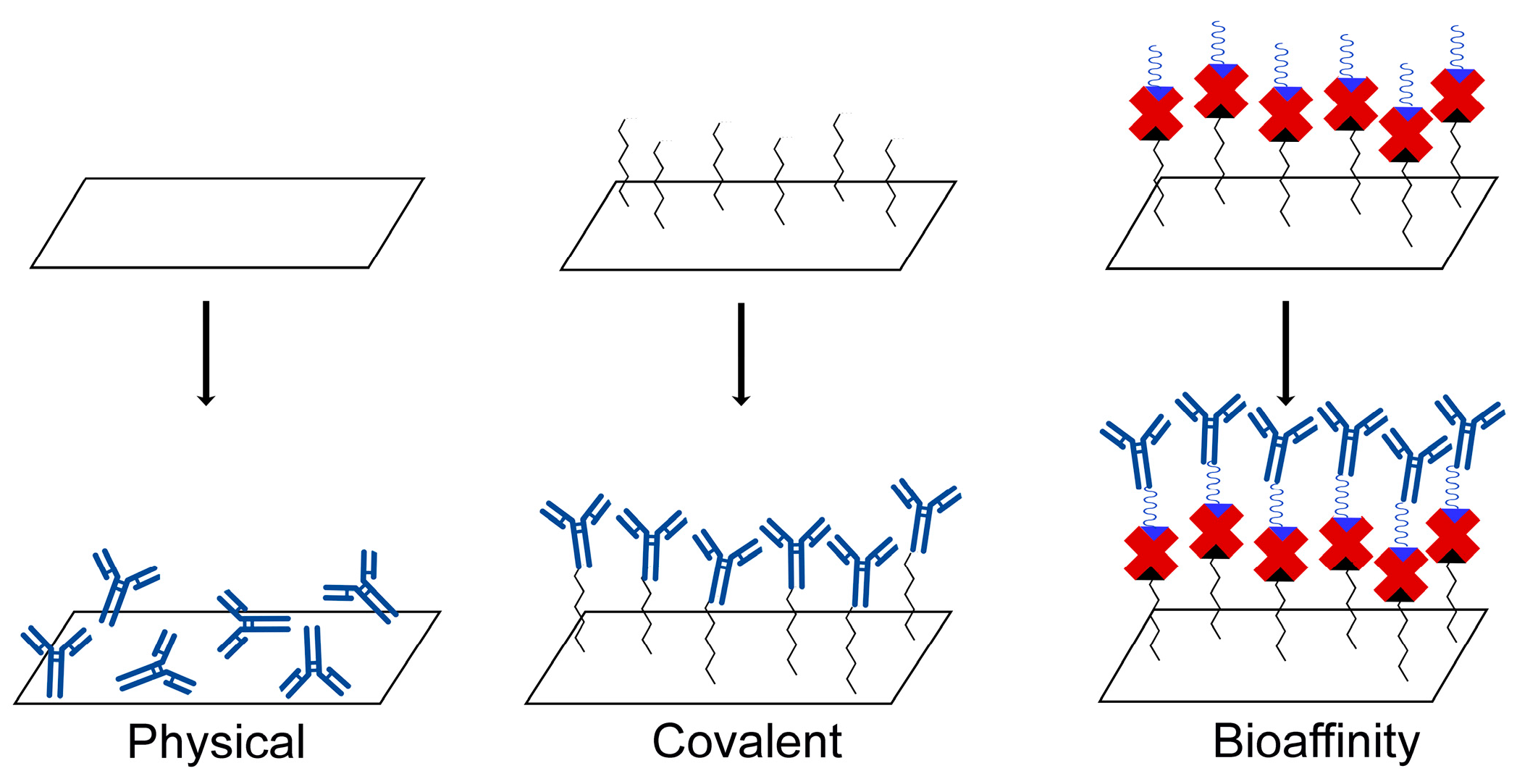
3.1. Derivatization of the Gold Surface for QCM Immunosensor Construction
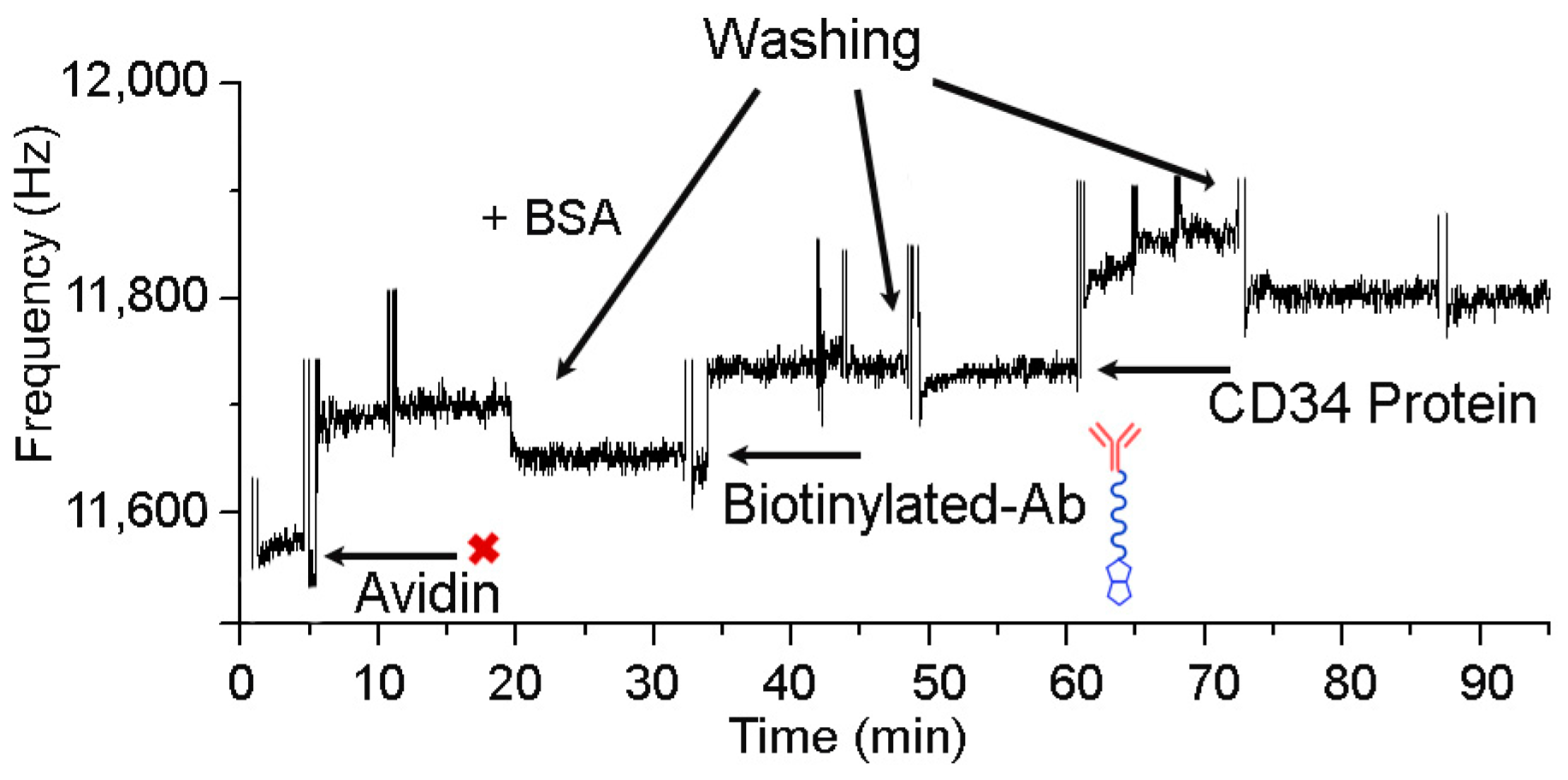
3.2. Immobilization of Anti-CD34 Antibodies on QCM Surface and Test for CD34 Protein Binding
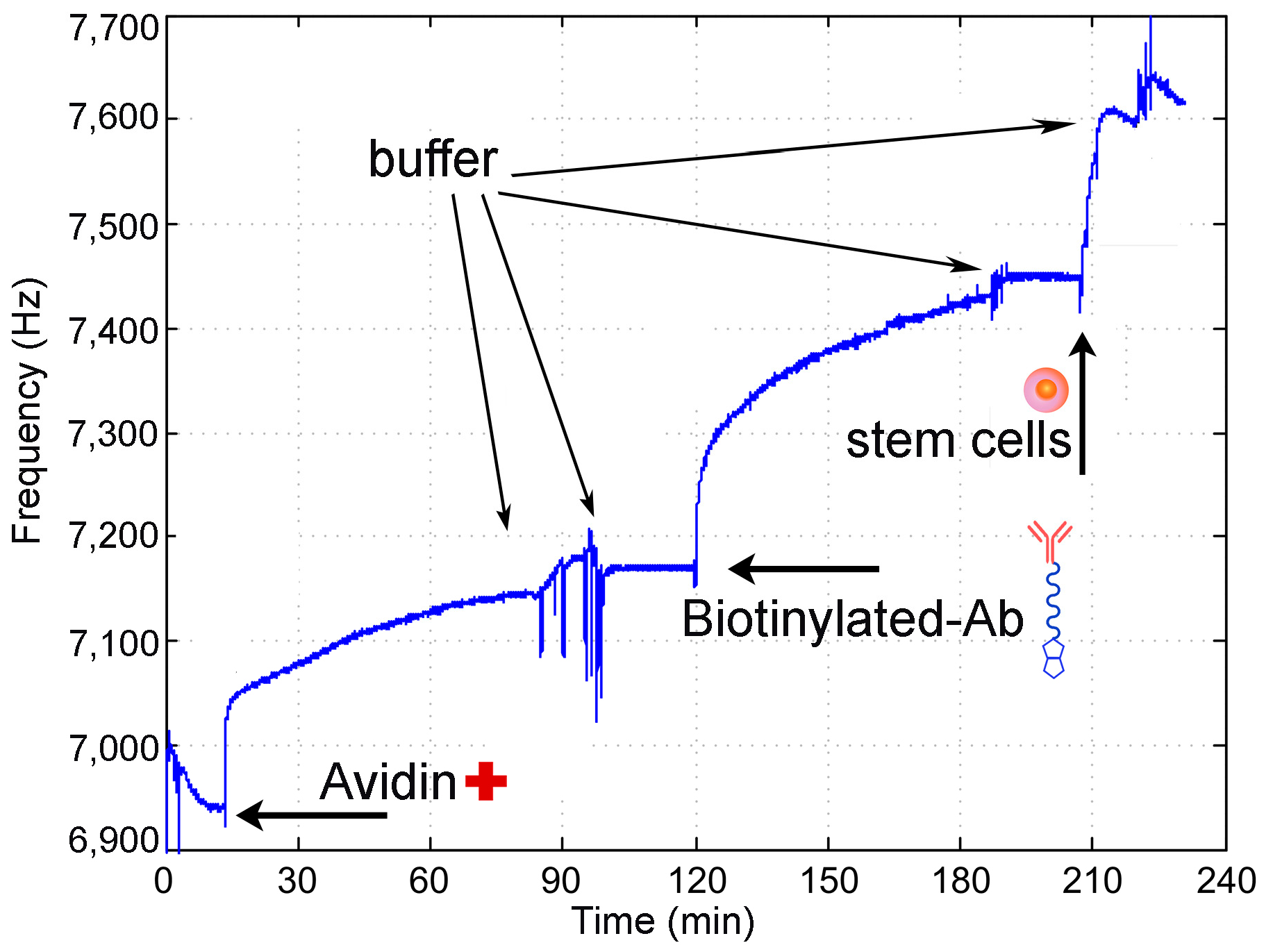
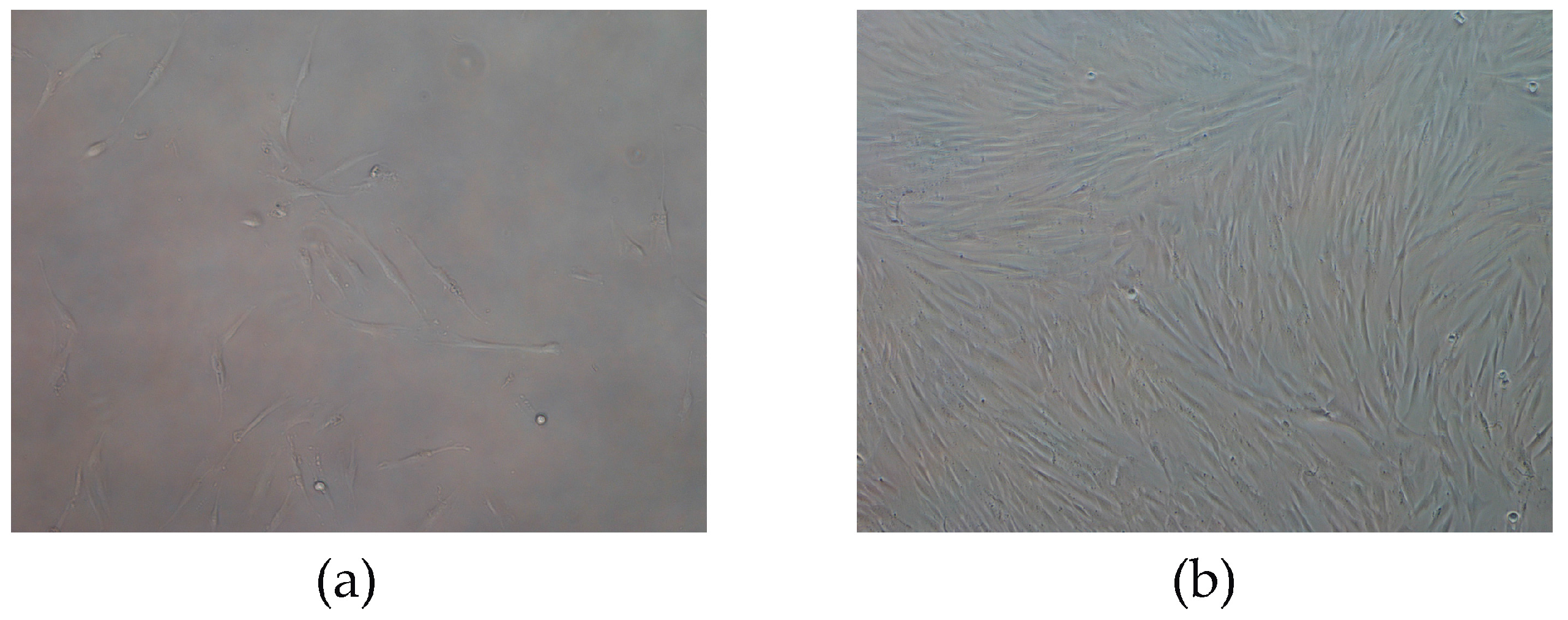
3.3. Stem Cell Detection by QCM-Immunosensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tang, D.; Yuan, R.; Chai, Y. Quartz crystal microbalance immunoassay for carcinoma antigen 125 based on gold nanowire-functionalized biomimetic interface. Analyst 2008, 133, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, K.; Ikebukuro, K.; Tamiya, E.; Karube, I.; Ichiki, N.; Arikawa, Y. Highly sensitive quartz crystal immunosensors for multisample detection of herbicides. Anal. Chim. Acta 1995, 304, 139–145. [Google Scholar] [CrossRef]
- Yakovleva, M.E.; Safina, G.R.; Danielsson, B. A study of glycoprotein–lectin interactions using quartz crystal microbalance. Anal. Chim. Acta 2010, 668, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Li, Q.; Tanga, J.; Sua, B.; Chena, G. An enzyme-free quartz crystal microbalance biosensor for sensitive glucose detection in biological fluids based on glucose/dextran displacement approach. Anal. Chim. Acta 2011, 686, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tu, Y.; Wang, X.; Pan, J.; Ding, Y. A Label-Free Immunosensor for Ultrasensitive Detection of Ketamine Based on Quartz Crystal Microbalance. Sensors 2015, 15, 8540–8549. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, B.; Sakačb, N.; Funaria, R.; Velotta, R. Flexible immunosensor for the detection of salivary α-amylase in body fluids. Talanta 2017, 174, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Fernández, R.; García, P.; García, M.; García, J.V.; Jiménez, Y.; Arnau, A. Design and Validation of a 150 MHz HFFQCM Sensor for Bio-Sensing Applications. Sensors 2017, 17, 2057. [Google Scholar] [CrossRef] [PubMed]
- Della Ventura, B.; Iannaccone, M.; Funaria, R.; Pica Ciamarra, M.; Altucci, C.; Capparelli, R.; Roperto, S.; Velotta, R. Effective antibodies immobilization and functionalized nanoparticles in a quartz-crystal microbalance-based immunosensor for the detection of parathion. PLoS ONE 2017, 12, e0171754. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyka, A.; Haupt, K.; Fellera, K.H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Rixiang, H.; Peng, Y.; Yuanzhi, T. Probing the interactions of organic molecules, nanomaterials, and microbes with solid surfaces using quartz crystal microbalances: Methodology, advantages, and limitations. Environ. Sci. Process. Impacts 2017, 19, 793–811. [Google Scholar] [CrossRef]
- Huang, X.; Bai, Q.; Hu, J.; Hou, D. A Practical Model of Quartz Crystal Microbalance in Actual Applications. Sensors 2017, 17, 1785. [Google Scholar] [CrossRef]
- Dixon, M.C. Quartz Crystal Microbalance with Dissipation Monitoring: Enabling Real-Time Characterization of Biological Materials and Their Interactions. J. Biomol. Tech. 2008, 19, 151–158. [Google Scholar]
- Sauerbrey, G. Verwendung Von Schwingquarzen Zur Wagung Dunner Schichten Und Zur Mikrowagung. Z. Phys. 1959, 155, 206–222. (In German) [Google Scholar] [CrossRef]
- Marx, K.A. Quartz Crystal Microbalance: A Useful Tool for Studying Thin Polymer Films and Complex Biomolecular Systems at the Solution-Surface Interface. Biomacromolecules 2003, 4, 1099–1120. [Google Scholar] [CrossRef] [PubMed]
- Ferhan, A.R.; Jackman, J.A.; Cho, N.J. Integration of Quartz Crystal Microbalance-Dissipation and Reflection-Mode Localized Surface Plasmon Resonance Sensors for Biomacromolecular Interaction Analysis. Anal. Chem. 2016, 88, 12524–12531. [Google Scholar] [CrossRef] [PubMed]
- Dirri, F.; Palomba, E.; Longobardo, A.; Biondi, D.; Boccaccini, A.; Bortolino, S.; Scaccabarozzi, D.; Zampetti, E. QCM-based sensor for volatile organic compounds characterization. In Proceedings of the 2017 IEEE International Workshop on Metrology for AeroSpace (MetroAeroSpace), Padua, Italy, 21–23 June 2017. [Google Scholar] [CrossRef]
- Rizzo, S.; Sannicolò, F.; Benincori, T.; Schiavon, G.; Zecchin, S.; Zotti, G. Calix[4]arene-functionalized poly-cyclopenta[2,1-b;3,4-b′]bithiophenes with good recognition ability and selectivity for small organic molecules for application in QCM-based sensors. J. Mater. Chem. 2004, 14, 1804–1811. [Google Scholar] [CrossRef]
- Becker, B.; Cooper, M.A. A survey of the 2006–2009 quartz crystal microbalance biosensor literature. J. Mol. Recognit. 2011, 24, 754–787. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Vashist, P. Recent advances in quartz crystal microbalance-based sensors. J. Sens. 2011, 571405. [Google Scholar] [CrossRef]
- Speight, R.E.; Cooper, M.A. A Survey of the 2010 Quartz Crystal Microbalance Literature. J. Mol. Recognit. 2012, 25, 451–473. [Google Scholar] [CrossRef] [PubMed]
- Hosu, O.; Selvolini, G.; Cristea, C.; Marrazza, G. Electrochemical Immunosensors for Disease Detection and Diagnosis. Curr. Med. Chem. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Kurosawa, S.; Aizawa, H.; Tozuka, M.; Nakamura, M.; Park, J.-W. Immunosensors using a quartz crystal microbalance. Meas. Sci. Technol. 2003, 14, 1882–1887. [Google Scholar] [CrossRef]
- Muramatsu, H.; Tamiya, E.; Karube, I. Determination of microbes and immunoglobulins using a piezoelectric biosensor. J. Membr. Sci. 1989, 41, 281–290. [Google Scholar] [CrossRef]
- Dultseva, F.N.; Tronin, A.V. Rapid sensing of hepatitis B virus using QCM in the thickness shear mode. Sens. Actuators B 2015, 216, 1–5. [Google Scholar] [CrossRef]
- Su, X.L.; Li, Y. A self-assembled monolayer-based piezoelectric immunosensor for rapid detection of Escherichia coli O157:H7. Biosens. Bioelectron. 2004, 19, 563–574. [Google Scholar] [CrossRef]
- Wang, L.J.; Wu, C.S.; Hu, Z.Y.; Zhang, Y.F.; Li, R.; Wang, P. Sensing Escherichia coli O157:H7 via frequency shift through a self-assembled monolayer based QCM immunosensor. J. Zhejiang Univ. Sci. B 2008, 9, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.K.T.; Nguyen, D.G.; Nguyen, H.P.U.; Tran, V.M.; Nguyen, T.K.M.; Huynh, T.P.; Lam, Q.V.; Huynh, T.D.; Truong, T.N.L. Quartz crystal microbalance (QCM) as biosensor for the detecting of Escherichia coli O157:H7. Adv. Nat. Sci. Nanosci. Nanotechnol. 2014, 5, 045004. [Google Scholar] [CrossRef]
- Mannelli, I.; Minunni, M.; Tombelli, S.; Mascini, M. Quartz crystal microbalance (QCM) affinity biosensor for genetically modified organism (GMOs) detection. Biosens. Bioelectron. 2003, 18, 129–140. [Google Scholar] [CrossRef]
- Lazerges, M.; Perrot, H.; Zeghib, N.; Antoine, E.; Comperec, C. In Situ QCM DNA-biosensor probe modification. Sens. Actuators B 2006, 120, 329–337. [Google Scholar] [CrossRef]
- Kurosawa, S.; Park, J.W.; Aizawa, H.; Wakida, S.I.; Tao, H.; Ishihara, K. Quartz crystal microbalance immunosensors for environmental monitoring. Biosens. Bioelectron. 2006, 22, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhou, T.; Hu, J. A high-throughput QCM chip configuration for the study of living cells and cell-drug interactions. Anal. Bioanal. Chem. 2017, 409, 6463–6473. [Google Scholar] [CrossRef] [PubMed]
- Masdor, N.A.; Altintas, Z.; Tothill, I.E. Sensitive detection of Campylobacter jejuni using nanoparticles enhanced QCM sensor. Biosens. Bioelectron. 2016, 78, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, X.; Yan, H.H.; Xu, S.J.; Tang, D.; Fu, W.L. A novel multi-array immunoassay device for tumor markers based on insert-plug model of piezoelectric immunosensor. Biosens. Bioelectron. 2007, 23, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Tögel, F.; Westenfelder, C. The role of multipotent marrow stromal cells (MSCs) in tissue regeneration. Organogenesis 2011, 7, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Dantuma, E.; Merchant, S.; Sugaya, K. Stem cells for the treatment of neurodegenerative diseases. Stem Cell Res. Ther. 2010, 1, 37. [Google Scholar] [CrossRef] [PubMed]
- Toma, J.G.; Akhavan, M.; Fernandes, K.J.L.; Barnabé-Heider, F.; Sadikot, A.; Kaplan, D.R.; Miller, F.D. Isolation of multipotent adult stem cells from the dermis of mammalian skin. Nat. Cell Biol. 2001, 3, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Henningson, C.T.; Stanislaus, M.A.; Gewirtz, A.M. Embryonic and adult stem cell therapy. J. Allergy Clin. Immunol. 2003, 111, S745–S753. [Google Scholar] [CrossRef] [PubMed]
- Ashri, Y.N.; Ajlan, S.A.; Aldahmash, A.M. Dental pulp stem cells. Saudi Med. J. 2015, 36, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 26, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhang, C.; Tani-Ishii, N.; Shi, S.; Wang, C.Y. NF-kB Activation in human dental pulp stem cells by TNF and LPS. J. Dent. Res. 2005, 84, 994–998. [Google Scholar] [CrossRef] [PubMed]
- Fathi, F.; Rahbarghazi, R.; Rashidi, M.R. Label-free biosensors in the field of stem cell biology. Biosens. Bioelectron. 2018, 101, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Nicodemou, A.; Danisovic, L. Mesenchymal stromal/stem cell separation methods: Concise review. Cell Tissue Bank 2017, 18, 443–460. [Google Scholar] [CrossRef] [PubMed]
- Cagnin, S.; Cimetta, E.; Guiducci, C.; Martini, P.; Lanfranchi, G. Overview of Micro- and Nano-Technology Tools for Stem Cell Applications: Micropatterned and Microelectronic Devices. Sensors 2012, 12, 15947–15982. [Google Scholar] [CrossRef] [PubMed]
- Tucker, H.A.; Bunnell, B.A. Characterization of human adipose-derived stem cells using flow cytometry. Methods Mol. Biol. 2011, 702, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Battye, F.L.; Shortman, K. Flow cytometry and cell-separation procedures. Curr. Opin. Immunol. 1991, 3, 239–241. [Google Scholar] [CrossRef]
- Herzenberg, L.A.; Parks, D.; Sahaf, B.; Perez, O.; Roederer, M.; Herzenberg, L.A. The history and future of the fluorescence activated cell sorter and flow cytometry: A view from Stanford. Clin. Chem. 2002, 48, 1819–1827. [Google Scholar] [PubMed]
- Herzenberg, L.A.; De Rosa, S.C. Monoclonal antibodies and the FACS: Complementary tools for immunobiology and medicine). Immunol. Today 2000, 21, 383–390. [Google Scholar] [CrossRef]
- Collings, A.F.; Caruso, F. Biosensors: Recent advances. Rep. Prog. Phys. 1997, 60, 1397–1445. [Google Scholar] [CrossRef]
- Rusmini, F.; Zhong, Z.; Feijen, J. Protein Immobilization Strategies for Protein Biochips. Biomacromolecules 2007, 8, 1775–1789. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Dixit, C.K.; MacCraith, B.D.; O’Kennedy, R. Effect of antibody immobilization strategies on the analytical performance of a surface plasmon resonance-based immunoassay. Analyst 2011, 136, 4431. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K.; Lam, E.; Hrapovic, S.; Male, K.B.; Luong, J.H.T. Immobilization of Antibodies and Enzymes on 3-Aminopropyltriethoxysilane-Functionalized Bioanalytical Platforms for Biosensors and Diagnostics. Chem. Rev. 2014, 114, 11083–11130. [Google Scholar] [CrossRef] [PubMed]
- Diamandis, E.P.; Christopoulos, T.K. The Biotin-(Strept)Avidin System: Principles and Applications in Biotechnology. Clin. Chem. 1991, 37, 625–636. [Google Scholar] [PubMed]
- Savage, M.D.; Mattson, G.; Desai, S.; Nielander, G.W.; Morgensen, S.; Conklin, E.J. Avidin-Biotin Chemistry: A Handbook; Pierce Chemical Co.: Rockford, IL, USA, 1992; ISBN 978-0935940114. [Google Scholar]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Wink, T.; van Zuilen, S.J.; Bult, A.; Van Bennekom, W.P. Self-assembled monolayers for biosensors. Analyst 1997, 122, 43R–50R. [Google Scholar] [CrossRef] [PubMed]
- Vestergaard, M.C.; Tamiya, E. Introduction to Nanobiosensors and Nanobioanalyses. In Nanobiosensors and Nanobioanalyses; Vestergaard, M.C., Kerman, K., Hsing, I.-M., Tamiya, E., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–20. ISBN 978-4-431-55189-8. [Google Scholar]
- Schwartz, D.K. Mechanisms and kinetics of self-assembled monolayer formation. Annu. Rev. Phys. Chem. 2001, 52, 107–137. [Google Scholar] [CrossRef] [PubMed]
- Seifert, M.; Rinker, M.T.; Galla, H.J. Characterization of Streptavidin Binding to Biotinylated, Binary Self-Assembled Thiol Monolayers. Influence of component ratio and solvent. Langmuir 2010, 26, 6386–6393. [Google Scholar] [CrossRef] [PubMed]
- Yam, C.M.; Pradier, C.M.; Salmain, M.; Marcus, P.; Jaouen, G. Binding of Biotin to Gold Surfaces Functionalized by Self-Assembled Monolayers of Cystamine and Cysteamine: Combined FT-IRRAS and XPS Characterization. J. Colloid Interface Sci. 2001, 235, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Azzaroni, O.; Mir, M.; Knoll, W. Supramolecular architectures of streptavidin on biotinylated self-assembled monolayers. Tracking biomolecular reorganization after bioconjugation. J. Phys. Chem. B 2007, 111, 13499–13503. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Granstaff, V.E.; Frye, G.C. Characterization of a Quartz Crystal Microbalance with Simultaneous Mass and Liquid Loading. Anal. Chem. 1991, 63, 2272–2281. [Google Scholar] [CrossRef]
- Atay, S.; Pişkin, K.; Yılmaz, F.; Çakır, C.; Yavuzd, H.; Denizli, A. Quartz crystal microbalance based biosensors for detecting highly metastatic breast cancer cells via their transferrin receptors. Anal. Methods 2016, 8, 153–161. [Google Scholar] [CrossRef]
- Lodish, H.; Berk, A.; Zipursky, S.L.; Matsudaira, P.; Baltimore, D.; Darnell, J. The Molecules of Life. In Molecular Cell Biology, 4th ed.; Section 1.2; Freeman, W.H.: New York, NY, USA, 2000. Available online: https://www.ncbi.nlm.nih.gov/books/NBK21473/ (accessed on 12 November 2017).
- Vashist, S.K.; Luppa, P.B.; Yeo, L.Y.; Ozcan, Y.; Luong, J.H.T. Emerging Technologies for Next-Generation Point-of-Care Testing. Trends Biotechnol. 2015, 33, 692–705. [Google Scholar] [CrossRef] [PubMed]


| Layer | MW (Da) | Δf (Hz) | Δm (ng) | mol (pmol) | N |
|---|---|---|---|---|---|
| Avidin | 66,000 | 207 | 145 | 2.20 | 1.32 × 1012 |
| Antibody | 150,000 | 261 | 184 | 1.23 | 0.74 × 1012 |
| Starting Frequency | Avidin | Anti-CD34 | Stem Cell | |
|---|---|---|---|---|
| Average value (Hz) | 0 | 203 | 485 | 684 |
| Median (Hz) | −0.12 | 229 | 506 | 676 |
| Standard Deviation (Hz) | 1.2 | 62 | 32 | 40 |
| Mean Absolute Deviation (Hz) | 1.3 | 56 | 30 | 34 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maglio, O.; Costanzo, S.; Cercola, R.; Zambrano, G.; Mauro, M.; Battaglia, R.; Ferrini, G.; Nastri, F.; Pavone, V.; Lombardi, A. A Quartz Crystal Microbalance Immunosensor for Stem Cell Selection and Extraction. Sensors 2017, 17, 2747. https://doi.org/10.3390/s17122747
Maglio O, Costanzo S, Cercola R, Zambrano G, Mauro M, Battaglia R, Ferrini G, Nastri F, Pavone V, Lombardi A. A Quartz Crystal Microbalance Immunosensor for Stem Cell Selection and Extraction. Sensors. 2017; 17(12):2747. https://doi.org/10.3390/s17122747
Chicago/Turabian StyleMaglio, Ornella, Salvatore Costanzo, Rosaria Cercola, Gerardo Zambrano, Marco Mauro, Raffaele Battaglia, Gianluca Ferrini, Flavia Nastri, Vincenzo Pavone, and Angela Lombardi. 2017. "A Quartz Crystal Microbalance Immunosensor for Stem Cell Selection and Extraction" Sensors 17, no. 12: 2747. https://doi.org/10.3390/s17122747






