Investigation of Pristine Graphite Oxide as Room-Temperature Chemiresistive Ammonia Gas Sensing Material
Abstract
:1. Introduction
2. Experimental
3. Results
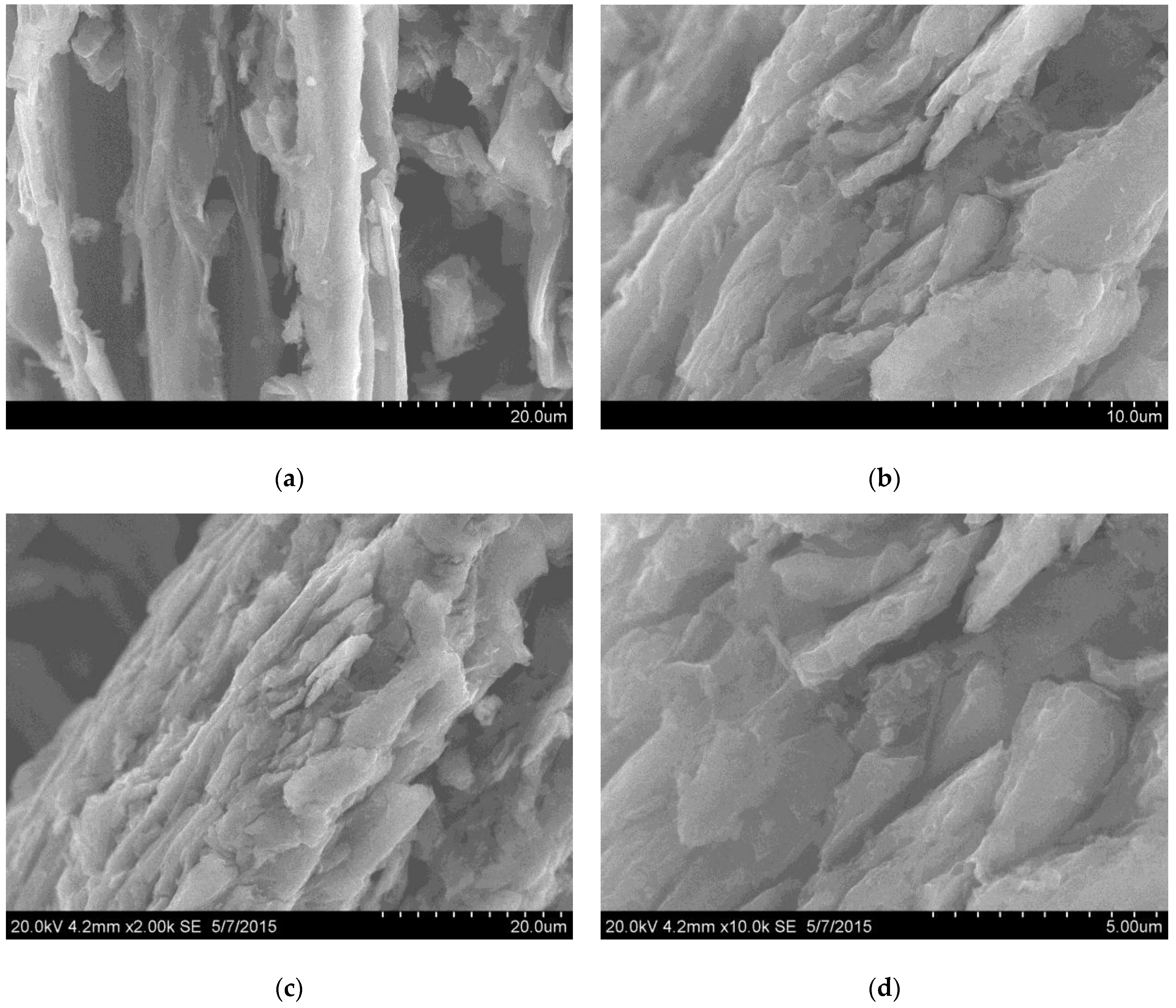
3.1. GO Characterization
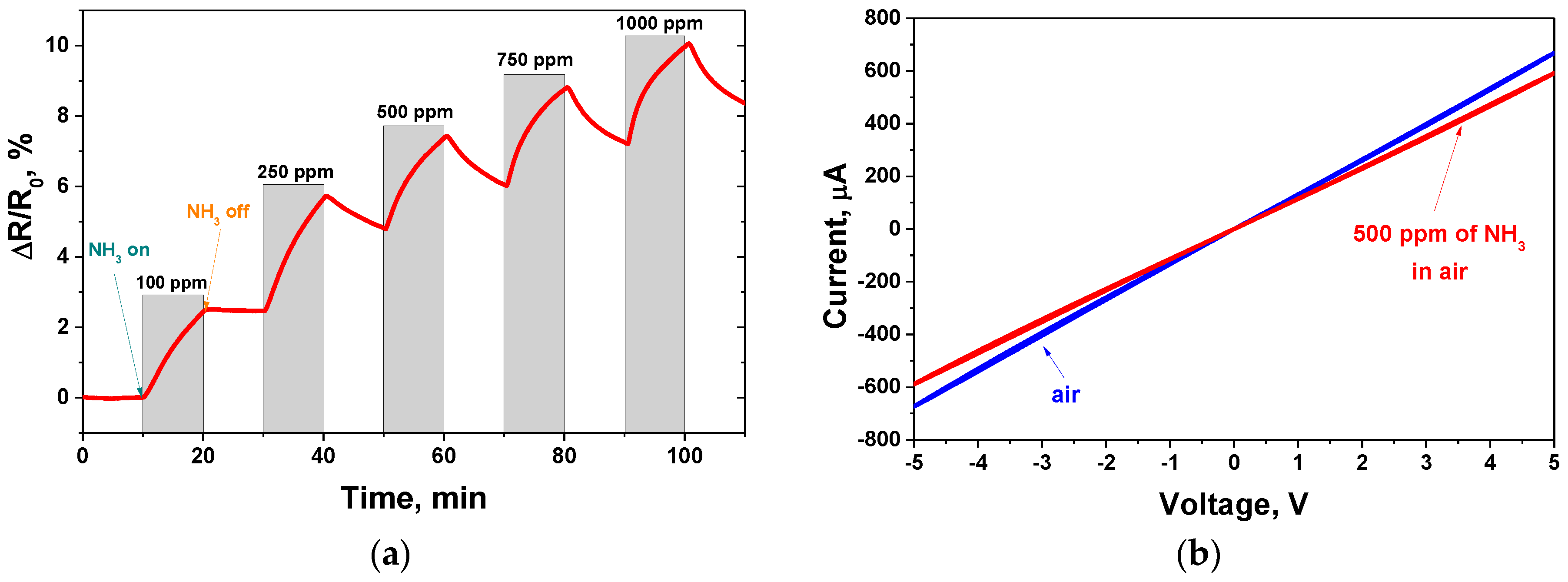
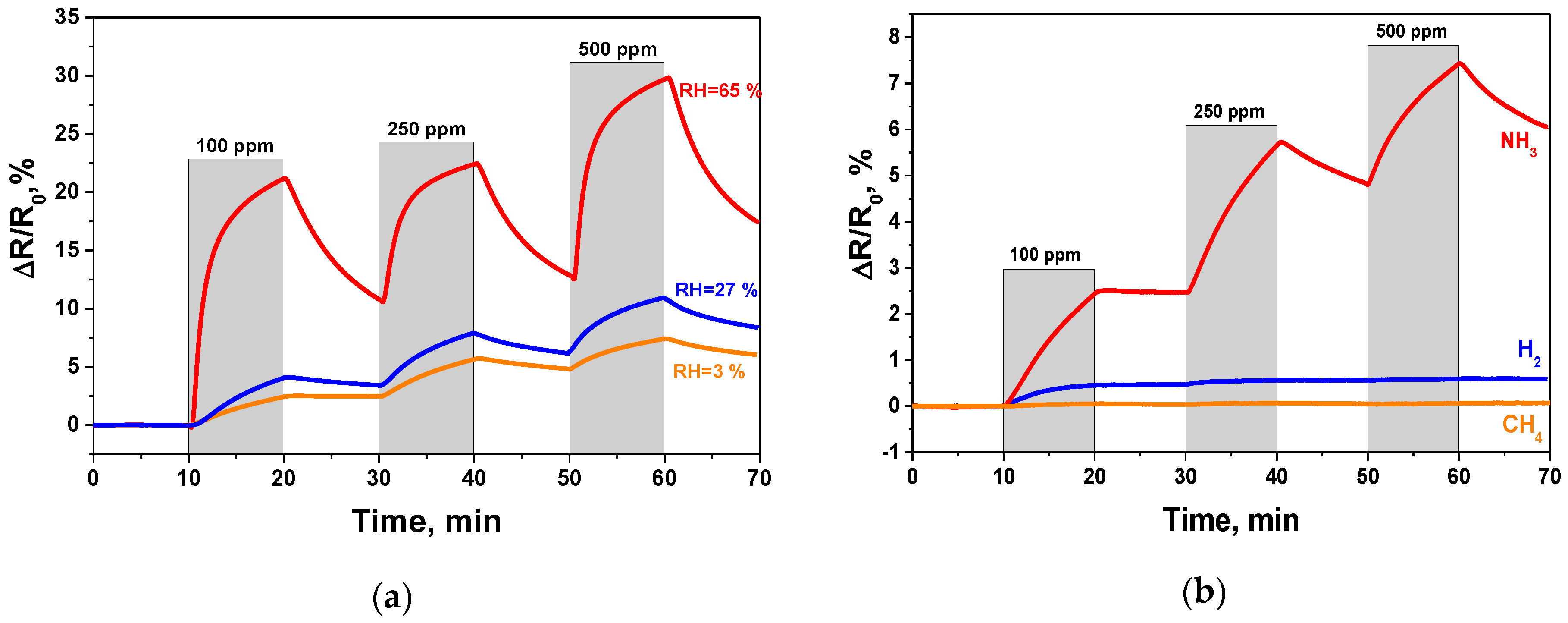
3.2. GO-Based Sensor Testing
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nguyen, H.-Q.; Huh, J.-S. Behavior of single-walled carbon nanotube-based gas sensors at various temperatures of treatment and operation. Sens. Actuators B Chem. 2006, 117, 426–430. [Google Scholar] [CrossRef]
- Travlou, N.A.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Activated carbon-based gas sensors: Effects of surface features on the sensing mechanism. J. Mater. Chem. A 2015, 3, 3821–3831. [Google Scholar] [CrossRef]
- Gedam, N.N.; Padole, P.R.; Rithe, S.K.; Chaudhari, G.N. Ammonia gas sensor based on a spinel semiconductor, Co0.8Ni0.2Fe2O4 nanomaterial. J. Sol-Gel Sci. Technol. 2009, 50, 296–300. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, X.; Su, Q.; Li, Y.; Zhou, Z. Ammonia-sensing characteristics of Pt and SiO2 doped SnO2 materials. Solid-State Electron. 2001, 45, 347–350. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Petit, C.; Seredych, M.; Bandosz, T.J. Revisiting the chemistry of graphite oxides and its effect on ammonia adsorption. J. Mater. Chem. 2009, 19, 9176. [Google Scholar] [CrossRef]
- Brodie, B.C. On the Atomic Weight of Graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 249–259. [Google Scholar] [CrossRef]
- Staudenmaier, L. Verfahren zur Darstellung der Graphitsaure. Ber. Dtsch. Chem. Ges. 1898, 31, 1481–1487. [Google Scholar] [CrossRef]
- Hummers, W.S.J.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Tran, Q.T.; Huynh, T.M.H.; Tong, D.T.; Tran, V.T.; Nguyen, N.D. Synthesis and application of graphene–silver nanowires composite for ammonia gas sensing. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 45012. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, R.; Wang, X.; Feng, W.; Hu, P.; O’Neill, W.; Wang, Z. Fabrication of highly oriented reduced graphene oxide microbelts array for massive production of sensitive ammonia gas sensors. J. Micromech. Microeng. 2013, 23, 95031. [Google Scholar] [CrossRef]
- Seredych, M.; Tamashausky, A.V.; Bandosz, T.J. Graphite oxides obtained from porous graphite: The role of surface chemistry and texture in ammonia retention at ambient conditions. Adv. Funct. Mater. 2010, 20, 1670–1679. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Combined role of water and surface chemistry in reactive adsorption of ammonia on graphite oxides. Langmuir 2010, 26, 5491–5498. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Yao, Y.; Li, N.; Chen, X. High-stability quartz crystal microbalance ammonia sensor utilizing graphene oxide isolation layer. Sens. Actuators B Chem. 2014, 196, 183–188. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Hu, N.; Wang, Y.; Zhang, Y.; Zhou, Z.; Liu, Y.; Shen, S.; Peng, C. Ammonia gas sensors based on chemically reduced graphene oxide sheets self-assembled on Au electrodes. Nanoscale Res. Lett. 2014, 9, 251. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Gunasekaran, S. Electrochemically reduced graphene oxide sheets for use in high performance supercapacitors. Carbon 2013, 51, 36–44. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Jiang, C.; Liu, A.; Xia, B. Quantitative detection of formaldehyde and ammonia gas via metal oxide-modified graphene-based sensor array combining with neural network model. Sens. Actuators B Chem. 2017, 240, 55–65. [Google Scholar] [CrossRef]
- Andre, R.S.; Shimizu, F.M.; Miyazaki, C.M.; Riul, A.; Manzani, D.; Ribeiro, S.J.L.; Oliveira, O.N.; Mattoso, L.H.C.; Correa, D.S. Hybrid layer-by-layer (LbL) films of polyaniline, graphene oxide and zinc oxide to detect ammonia. Sens. Actuators B Chem. 2017, 238, 795–801. [Google Scholar] [CrossRef]
- Travlou, N.A.; Singh, K.; Rodríguez-Castellón, E.; Bandosz, T.J. Cu–BTC MOF–graphene-based hybrid materials as low concentration ammonia sensors. J. Mater. Chem. A 2015, 2, 2445–2460. [Google Scholar] [CrossRef]
- Katkov, M.V.; Sysoev, V.I.; Gusel’nikov, A.V.; Asanov, I.P.; Bulusheva, L.G.; Okotrub, A.V. A backside fluorine-functionalized graphene layer for ammonia detection. Phys. Chem. Chem. Phys. 2014, 17, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Poh, H.L.; Šimek, P.; Sofer, Z.; Pumera, M. Sulfur-doped graphene via thermal exfoliation of graphite oxide in H2S, SO2, or CS2 gas. ACS Nano 2013, 7, 5262–5272. [Google Scholar] [CrossRef] [PubMed]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabo, T.; Szeri, A.; Dékány, I. Graphite Oxide : Chemical Reduction to Graphite and Surface Modification with Primary Aliphatic Amines and Amino Acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Wang, J.-W.; Yan, Q.; Zheng, W.-G.; Chen, C.; Yu, Z.-Z. Vacuum-assisted synthesis of graphene from thermal exfoliation and reduction of graphite oxide. J. Mater. Chem. 2011, 21, 5392. [Google Scholar] [CrossRef]
- Basu, S.; Bhattacharyya, P. Recent developments on graphene and graphene oxide based solid state gas sensors. Sens. Actuators B Chem. 2012, 173, 1–21. [Google Scholar] [CrossRef]
- Hu, N.; Yang, Z.; Wang, Y.Y.; Zhang, L.; Wang, Y.Y.; Huang, X.; Wei, H.; Wei, L.; Zhang, Y. Ultrafast and sensitive room temperature NH3 gas sensors based on chemically reduced graphene oxide. Nanotechnology 2014, 25, 25502. [Google Scholar] [CrossRef] [PubMed]
- Bannov, A.G.; Timofeeva, A.A.; Shinkarev, V.V.; Dyukova, K.D.; Ukhina, A.V.; Maksimovskii, E.A.; Yusin, S.I. Synthesis and studies of properties of graphite oxide and thermally expanded graphite. Prot. Met. Phys. Chem. Surf. 2014, 50, 183–190. [Google Scholar] [CrossRef]
- Zhao, Q.; Cheng, X.; Wu, J.; Yu, X. Sulfur-free exfoliated graphite with large exfoliated volume: Preparation, characterization and its adsorption performance. J. Ind. Eng. Chem. 2014, 20, 4028–4032. [Google Scholar] [CrossRef]
- Yuan, B.; Song, L.; Liew, K.M.; Hu, Y. Mechanism for increased thermal instability and fire risk of graphite oxide containing metal salts. Mater. Lett. 2016, 167, 197–200. [Google Scholar] [CrossRef]
- Ferrari, A.C. Raman spectroscopy of graphene and graphite: Disorder, electron-phonon coupling, doping and nonadiabatic effects. Solid State Commun. 2007, 143, 47–57. [Google Scholar] [CrossRef]
- Gurzęda, B.; Florczak, P.; Kempiński, M.; Peplińska, B.; Krawczyk, P.; Jurga, S. Synthesis of graphite oxide by electrochemical oxidation in aqueous perchloric acid. Carbon 2016, 100, 540–545. [Google Scholar] [CrossRef]
- Gao, W.; Majumder, M.; Alemany, L.B.; Narayanan, T.N.; Ibarra, M.A.; Pradhan, B.K.; Ajayan, P.M. Engineered graphite oxide materials for application in water purification. ACS Appl. Mater. Interfaces 2011, 3, 1821–1826. [Google Scholar] [CrossRef] [PubMed]
- Han, J.-W.; Kim, B.; Li, J.; Meyyappan, M. A carbon nanotube based ammonia sensor on cellulose paper. RSC Adv. 2014, 4, 549. [Google Scholar] [CrossRef]
- Kim, T.; Kim, S.; Min, N.; Pak, J.J.; Lee, C.; Kim, S. NH3 Sensitive Chemiresistor Sensors Using Plasma Functionalized Multiwall Carbon Nanotubes/Conducting Polymer Composites. In Proceedings of the 2008 IEEE Sensors, Lecce, Italy, 26–29 October 2008; pp. 208–211.
- Ghosh, R.; Midya, A.; Santra, S.; Ray, S.K.; Guha, P.K. Chemically reduced graphene oxide for ammonia detection at room temperature. ACS Appl. Mater. Interfaces 2013, 5, 7599–7603. [Google Scholar] [CrossRef] [PubMed]
- Travlou, N.A.; Rodríguez-castell, E. Sensing of NH3 on heterogeneous nanoporous carbons in the presence of humidity. Carbon 2016, 100, 64–73. [Google Scholar] [CrossRef]
- Bekyarova, E.; Davis, M.; Burch, T.; Itkis, M.E.; Zhao, B.; Sunshine, S.; Haddon, R.C. Chemically Functionalized Single-Walled Carbon Nanotubes as Ammonia Sensors. J. Phys. Chem. B 2004, 108, 19717–19720. [Google Scholar] [CrossRef]
- Zhang, T.; Mubeen, S.; Bekyarova, E.; Yoo, B.Y.; Haddon, R.C.; Myung, N.V.; Deshusses, M. A Poly(m-aminobenzene sulfonic acid) functionalized single-walled carbon nanotubes based gas sensor. Nanotechnology 2007, 18, 165504. [Google Scholar] [CrossRef]
- Meyyappan, M. Carbon Nanotube-Based Chemical Sensors. Small 2016, 12, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Jayatissa, A.H. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid State Electron. 2012, 78, 159–165. [Google Scholar] [CrossRef]
- Timmer, B.; Olthuis, W.; Van Den Berg, A. Ammonia sensors and their applications—A review. Sens. Actuators B Chem. 2005, 107, 666–677. [Google Scholar] [CrossRef]
- Teerapanich, P.; Myint, M.T.Z.; Joseph, C.M.; Hornyak, G.L.; Dutta, J. Development and Improvement of Carbon Nanotube-Based Ammonia Gas Sensors Using Ink-Jet Printed Interdigitated Electrodes. IEEE Trans. Nanotechnol. 2013, 12, 255–262. [Google Scholar] [CrossRef]
- Majzlíková, P.; Sedláček, J.; Prášek, J.; Pekárek, J.; Svatoš, V.; Bannov, A.G.; Jašek, O.; Synek, P.; Eliáš, M.; Zajíčková, L.; et al. Sensing Properties of Multiwalled Carbon Nanotubes Grown in MW Plasma Torch: Electronic and Electrochemical Behavior, Gas Sensing, Field Emission, IR Absorption. Sensors 2015, 15, 2644–2661. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Li, X.; Cui, P.; Guo, S.; Liu, W.; Wang, X. Morphology optimization of CVD graphene decorated with Ag nanoparticles as ammonia sensor. Sens. Actuators B Chem. 2017, 244, 124–130. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Wang, X.; Wang, J.; Gaskov, A.M.; Akbar, S.A. Reduced graphene oxide (rGO) decorated TiO2 microspheres for selective room-temperature gas sensors. Sens. Actuators B Chem. 2016, 230, 330–336. [Google Scholar] [CrossRef]


| Active Material for NH3 Detection | NH3 Concentration (ppm) | Sensor Response (%) | RH (%) | Temperature (°C) | Reference |
|---|---|---|---|---|---|
| Graphite oxide | 500 | 30 | 65 | 25 | This work |
| Single-wall carbon nanotubes | 62.5 | 3 | 56 | 25 | [41] |
| Single-wall carbon nanotubes | 100 | 6 | 80 | 25 | [32] |
| Multi-wall carbon nanotubes | 500 | 1.9 | 3 | 25 | [42] |
| Fluorinated graphene | 10,000 | 10.2 | n/a | 25 | [20] |
| CVD graphene decorated Ag nanoparticles | 500 | 12.5 | 80 | 25 | [43] |
| 16 | 100 | 25 | |||
| Reduced graphene oxide decorate by TiO2 microspheres | 30 | 3.2 | 89 | 20 | [44] |
| 30 | 3.5 | 17.8 | 22 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bannov, A.G.; Prášek, J.; Jašek, O.; Zajíčková, L. Investigation of Pristine Graphite Oxide as Room-Temperature Chemiresistive Ammonia Gas Sensing Material. Sensors 2017, 17, 320. https://doi.org/10.3390/s17020320
Bannov AG, Prášek J, Jašek O, Zajíčková L. Investigation of Pristine Graphite Oxide as Room-Temperature Chemiresistive Ammonia Gas Sensing Material. Sensors. 2017; 17(2):320. https://doi.org/10.3390/s17020320
Chicago/Turabian StyleBannov, Alexander G., Jan Prášek, Ondřej Jašek, and Lenka Zajíčková. 2017. "Investigation of Pristine Graphite Oxide as Room-Temperature Chemiresistive Ammonia Gas Sensing Material" Sensors 17, no. 2: 320. https://doi.org/10.3390/s17020320







