Alternative cDEP Design to Facilitate Cell Isolation for Identification by Raman Spectroscopy
Abstract
:1. Introduction
2. Materials and Methods
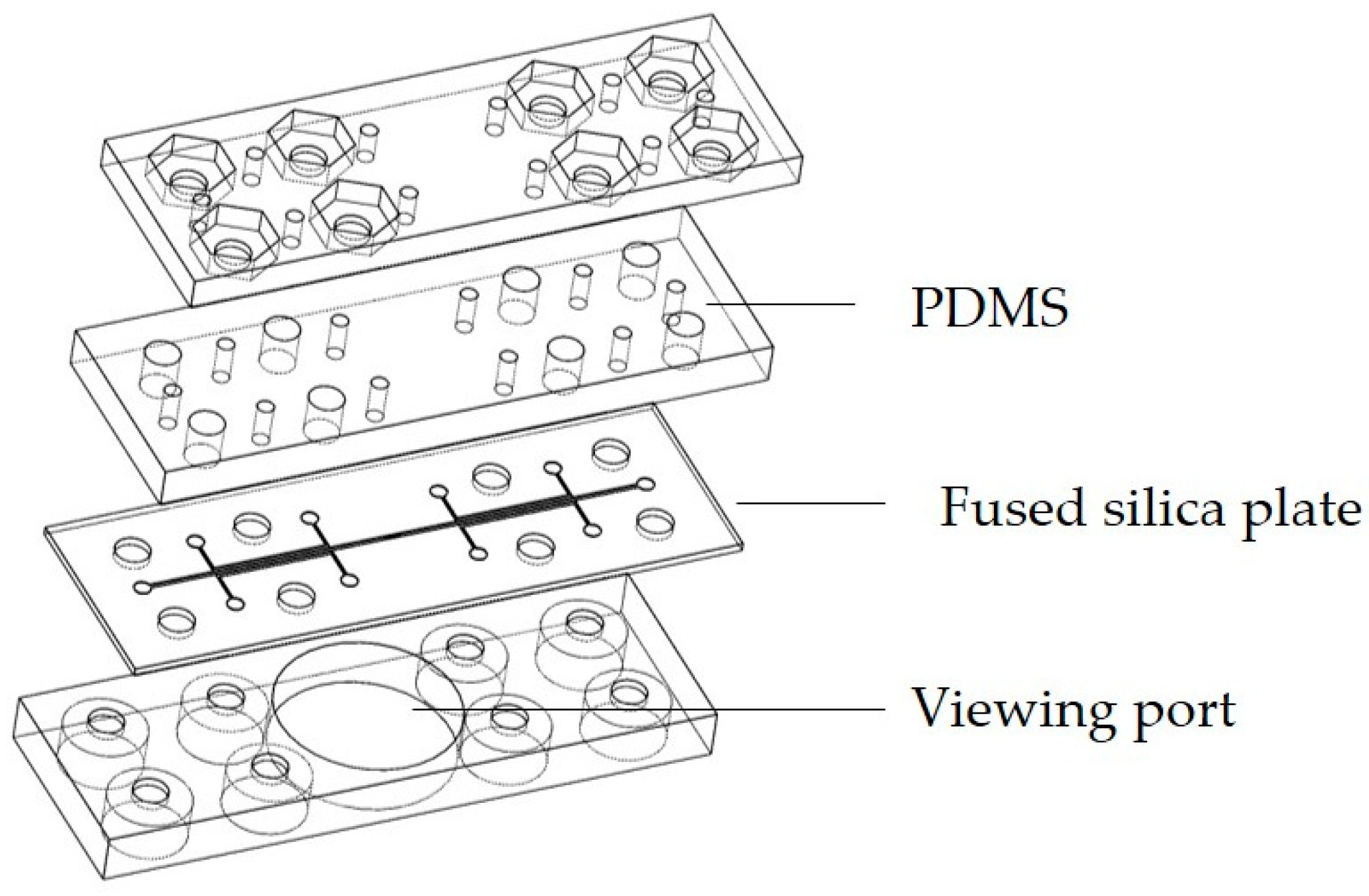
2.1. Device Fabrication
2.2. Sample Preparation
2.3. Experimental Setup
3. Results
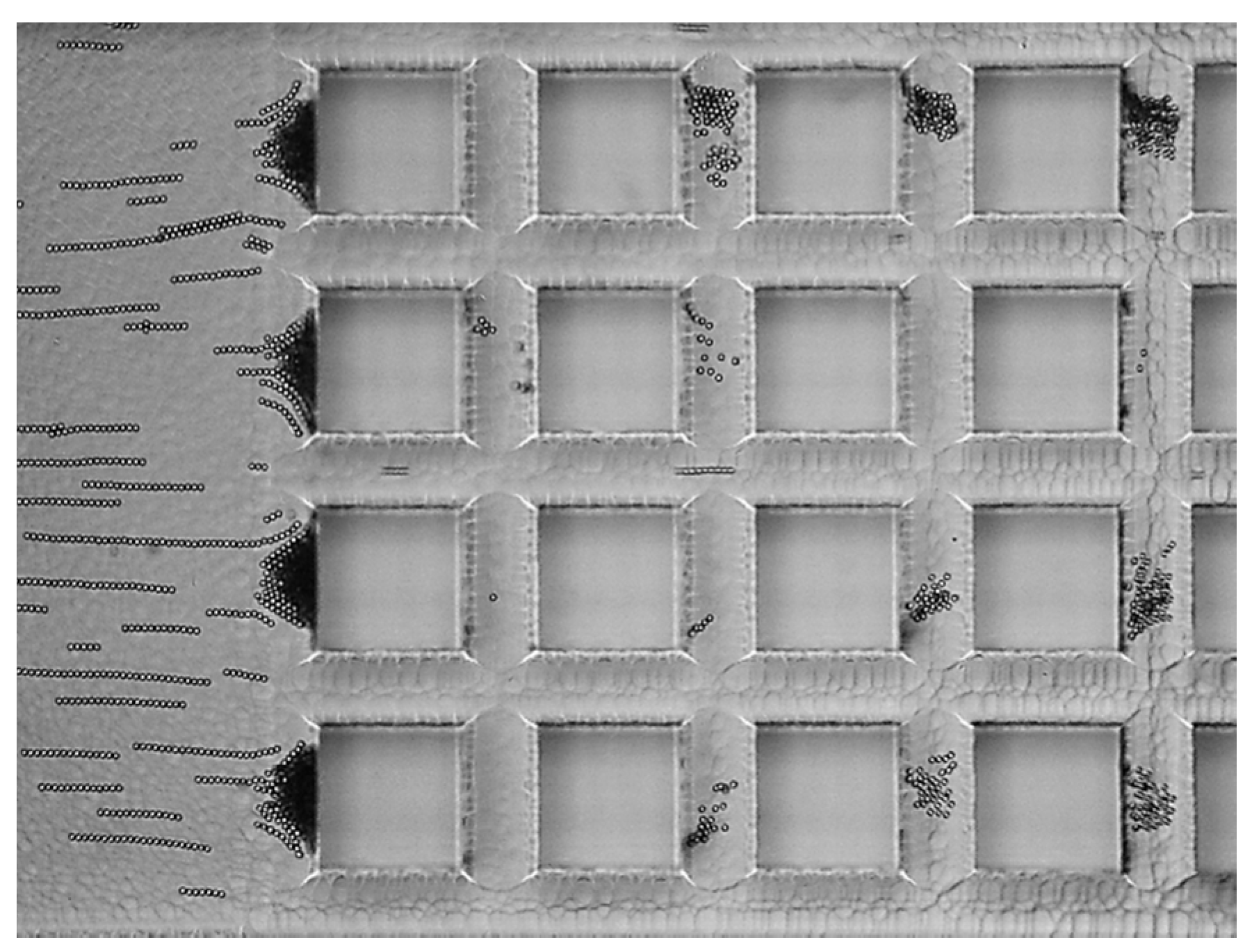
3.1. Contactless Dielectrophoresis
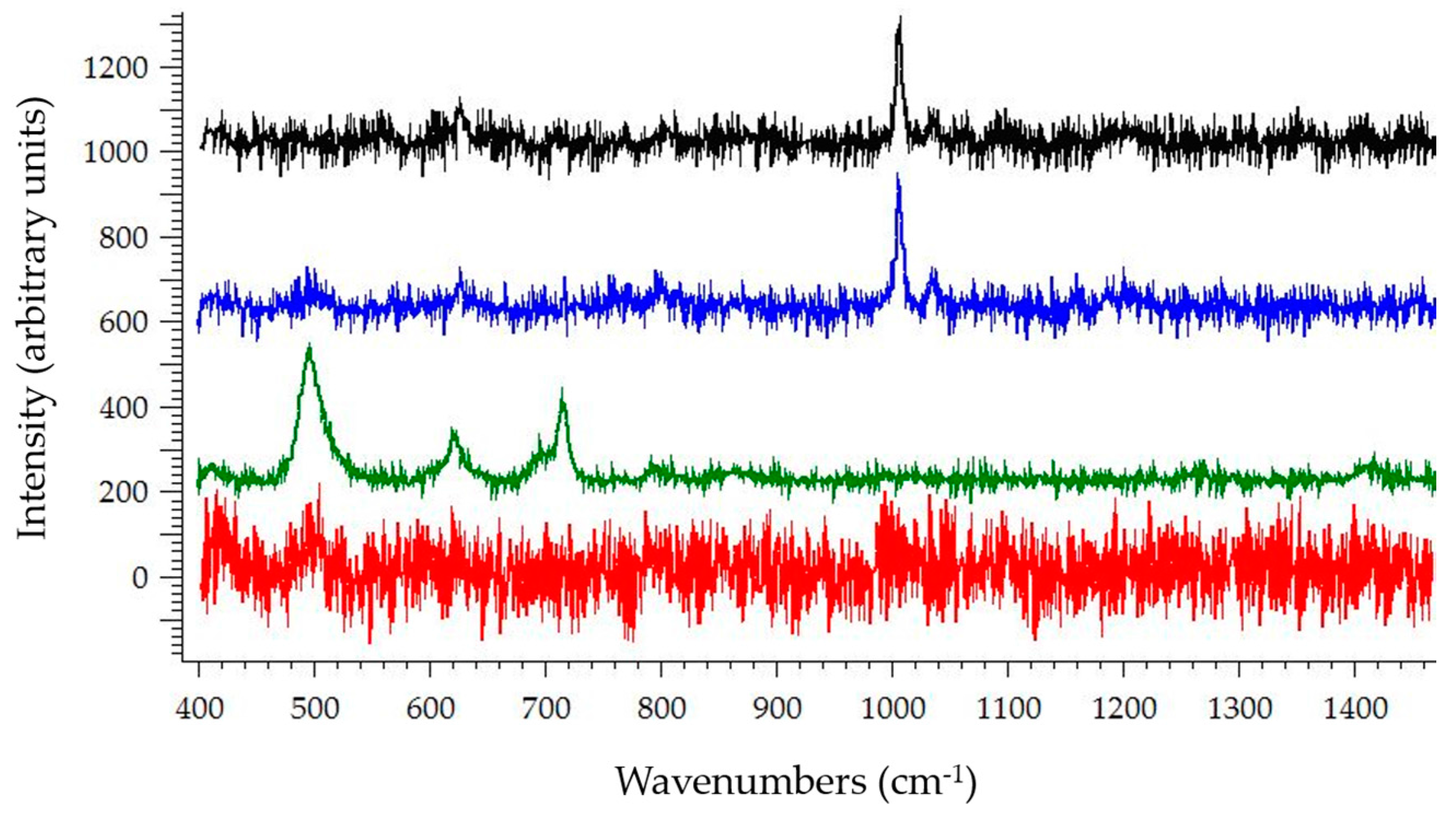
3.2. Raman Spectroscopy
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Srivastava, S.K.; Gencoglu, A.; Minerick, A.R. DC insulator dielectrophoretic applications in microdevice technology: A review. Anal. Bioanal. Chem. 2011, 399, 301–321. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.B. Electromechanics of Particles; Cambridge University Press: New York, NY, USA, 1995. [Google Scholar]
- Martinez-Duarte, R. Microfabrication technologies in dielectrophoresis applications—A review. Electrophoresis 2012, 33, 3110–3132. [Google Scholar] [CrossRef] [PubMed]
- Pethig, R. Review article—Dielectrophoresis: Status of the theory, technology, and applications. Biomicrofluidics 2010, 4, 022811. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, Z.R. Cellular dielectrophoresis: Applications to the characterization, manipulation, separation and patterning of cells. Electrophoresis 2011, 32, 2466–2487. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, H.; Caldwell, J.L.; Sano, M.B.; Davalos, R.V. Contactless dielectrophoresis: A new technique for cell manipulation. Biomed. Microdevices 2009, 11, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.P. Strategies for dielectrophoretic separation in laboratory-on-a-chip systems. Electrophoresis 2002, 23, 2569–2582. [Google Scholar] [CrossRef]
- Lau, A.Y.; Lee, L.P.; Chan, J.W. An integrated optofluidic platform for Raman-activated cell sorting. Lab Chip 2008, 8, 1116–1120. [Google Scholar] [CrossRef] [PubMed]
- Casabella, S.; Scully, P.; Goddard, N.; Gardner, P. Automated analysis of single cells using Laser Tweezers Raman Spectroscopy. Analyst 2016, 141, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Dochow, S.; Krafft, C.; Neugebauer, U.; Bocklitz, T.; Henkel, T.; Mayer, G.; Albert, J.; Popp, J. Tumour cell identification by means of Raman spectroscopy in combination with optical traps and microfluidic environments. Lab Chip 2011, 11, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U.-C.; Ramoji, A.; Glaser, U.; Sachse, S.; Leiterer, C.; Csaki, A.; Hübner, U.; Fritzsche, W.; Pfister, W.; Bauer, M.; et al. Combined Dielectrophoresis–Raman Setup for the Classification of Pathogens Recovered from the Urinary Tract. Anal. Chem. 2013, 85, 10717–10724. [Google Scholar] [CrossRef] [PubMed]
- Schröder, U.-C.; Beleites, C.; Assmann, C.; Glaser, U.; Huebner, U.; Pfister, W.; Fritzsche, W.; Popp, J.; Neugebauer, U. Detection of vancomycin resistances in enterococci within 3 1/2 hours. Sci. Rep. 2015, 5, 8217. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.-F.; Chen, T.-Y.; Lu, R.-J.; Wu, H.-W. Rapid identification of bacteria utilizing amplified dielectrophoretic force-assisted nanoparticle-induced surface-enhanced Raman spectroscopy. Nanoscale Res. Lett. 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.-Y.; Huang, C.-H.; Hsieh, W.-H.; Liu, L.-H.; Lin, Y.-C.; Chu, C.-C.; Wang, S.-T.; Kuo, I.-T.; Chau, L.-K.; Yang, C.-Y. On-line SERS Detection of Single Bacterium Using Novel SERS Nanoprobes and a Microfluidic Dielectrophoresis Device. Small 2014, 10, 4700–4710. [Google Scholar] [CrossRef] [PubMed]
- Madiyar, F.R.; Bhana, S.; Swisher, L.Z.; Culbertson, C.T.; Huang, X.; Li, J. Integration of a nanostructured dielectrophoretic device and a surface-enhanced Raman probe for highly sensitive rapid bacteria detection. Nanoscale 2015, 7, 3726–3736. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.-F.; Lin, C.-C.; Lin, D.-Y.; Chang, H.-C. A dielectrophoretic chip with a roughened metal surface for on-chip surface-enhanced Raman scattering analysis of bacteria. Biomicrofluidics 2010, 4, 034104. [Google Scholar] [CrossRef] [PubMed]
- Cheng, I.-F.; Chang, H.-C.; Chen, T.-Y.; Hu, C.; Yang, F.-L. Rapid (<5 min) Identification of Pathogen in Human Blood by Electrokinetic Concentration and Surface-Enhanced Raman Spectroscopy. Sci. Rep. 2013, 3, 2365. [Google Scholar]
- Hanson, C.; Israelsen, N.D.; Sieverts, M.; Vargis, E. Fabricating a UV-Vis and Raman Spectroscopy Immunoassay Platform. J. Vis. Exp. 2016. [Google Scholar] [CrossRef] [PubMed]
- Israelsen, N.D.; Wooley, D.; Hanson, C.; Vargis, E. Rational design of Raman-labeled nanoparticles for a dual-modality, light scattering immunoassay on a polystyrene substrate. J. Biol. Eng. 2016. [Google Scholar] [CrossRef] [PubMed]
- Förster-Zuegel, F.; Grotepaß, T.; Schlaak, H.F. Characterization of the dielectric breakdown field strength of PDMS thin films: Thickness dependence and electrode shape. SPIE Proc. 2015, 9430. [Google Scholar] [CrossRef]
- Gerratt, A.P.; Bergbreiter, S. Dielectric breakdown of PDMS thin films. J. Micromech. Microeng. 2013, 23, 067001. [Google Scholar] [CrossRef]
- Sano, M.B.; Salmanzadeh, A.; Davalos, R.V. Multilayer contactless dielectrophoresis: Theoretical considerations. Electrophoresis 2012, 33, 1938–1946. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, H.; Caldwell, J.L.; Davalos, R.V. A microfluidic system for biological particle enrichment using contactless dielectrophoresis. JALA 2010, 15, 224–232. [Google Scholar] [CrossRef]
- Čemažar, J.; Douglas, T.A.; Schmelz, E.M.; Davalos, R.V. Enhanced contactless dielectrophoresis enrichment and isolation platform via cell-scale microstructures. Biomicrofluidics 2016, 10, 014109. [Google Scholar] [CrossRef] [PubMed]
- Farmehini, V.; Rohani, A.; Su, Y.-H.; Swami, N.S. A wide-bandwidth power amplifier for frequency-selective insulator-based dielectrophoresis. Lab Chip 2014, 14, 4183–4187. [Google Scholar] [CrossRef] [PubMed]
- Klein, N.; Gafni, H. The maximum dielectric strength of thin silicon oxide films. IEEE Trans. Electron Devices 1966, ED-13, 281–289. [Google Scholar] [CrossRef]
- Sano, M.B.; Caldwell, J.L.; Davalos, R.V. Modeling and development of a low frequency contactless dielectrophoresis (cDEP) platform to sort cancer cells from dilute whole blood samples. Biosens. Bioelectron. 2011, 30, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Elvington, E.S.; Salmanzadeh, A.; Stremler, M.A.; Davalos, R.V. Label-free isolation and enrichment of cells through contactless dielectrophoresis. J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Salmanzadeh, A.; Elvington, E.S.; Roberts, P.C.; Schmelz, E.M.; Davalos, R.V. Sphingolipid metabolites modulate dielectric characteristics of cells in a mouse ovarian cancer progression model. Integr. Biol. 2013, 5, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.B.; Gallo-Villanueva, R.C.; Lapizco-Encinas, B.H.; Davalos, R.V. Simultaneous electrokinetic flow and dielectrophoretic trapping using perpendicular static and dynamic electric fields. Microfluid. Nanofluid. 2013, 15, 599–609. [Google Scholar] [CrossRef]
- Henslee, E.A.; Sano, M.B.; Rojas, A.D.; Schmelz, E.M.; Davalos, R.V. Selective concentration of human cancer cells using contactless dielectrophoresis. Electrophoresis 2011, 32, 2523–2529. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, H.; Sano, M.B.; Henslee, E.A.; Caldwell, J.L.; Davalos, R.V. Selective isolation of live/dead cells using contactless dielectrophoresis (cDEP). Lab Chip 2010, 10, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Sano, M.B.; Henslee, E.A.; Schmelz, E.M.; Davalos, R.V. Contactless dielectrophoretic spectroscopy: Examination of the dielectric properties of cells found in blood. Electrophoresis 2011, 32, 3164–3171. [Google Scholar] [CrossRef] [PubMed]
- Salmanzadeh, A.; Kittur, H.; Sano, M.B.; Roberts, P.C.; Schmelz, E.M.; Davalos, R.V. Dielectrophoretic differentiation of mouse ovarian surface epithelial cells, macrophages, and fibroblasts using contactless dielectrophoresis. Biomicrofluidics 2012, 6, 24104. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.V.; Hayes, M.A. Development of the resolution theory for gradient insulator-based dielectrophoresis. Electrophoresis 2015, 36, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.V.; DeMichele, A.F.; Kemp, L.; Hayes, M.A. Differentiation of Escherichia coli serotypes using DC gradient insulator dielectrophoresis. Anal. Bioanal. Chem. 2014, 406, 183–192. [Google Scholar] [CrossRef] [PubMed]


| Source | Voltage (VRMS) | Frequency (kHz) | Flow Rate (μL/h) |
|---|---|---|---|
| [28] | 200 | 5–50 | 5 |
| [29] | 200 | 5–70 | 5 |
| [30] | 250 | 500 | Not reported 1 |
| [22] | 250 | 600 | 1000 |
| [27] | 227–250 | 50–100 | 10 |
| [31] | 20–50 | 120–320 | 20 |
| [23] | 70–190 | 300 | 20 |
| [32] | 20–150 | 140–500 | 20 |
| [6] | 250 | 85 | 10–15 |
| [33] | 200–300 | 10–70 | 5 |
| [34] | 50–200 | 200–600 | 20 |
| [24] | 250–350 | 30 | 1200–2160 |
| Current article | 350 | 0.1 | 5 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanson, C.; Vargis, E. Alternative cDEP Design to Facilitate Cell Isolation for Identification by Raman Spectroscopy. Sensors 2017, 17, 327. https://doi.org/10.3390/s17020327
Hanson C, Vargis E. Alternative cDEP Design to Facilitate Cell Isolation for Identification by Raman Spectroscopy. Sensors. 2017; 17(2):327. https://doi.org/10.3390/s17020327
Chicago/Turabian StyleHanson, Cynthia, and Elizabeth Vargis. 2017. "Alternative cDEP Design to Facilitate Cell Isolation for Identification by Raman Spectroscopy" Sensors 17, no. 2: 327. https://doi.org/10.3390/s17020327






