Long Term Amperometric Recordings in the Brain Extracellular Fluid of Freely Moving Immunocompromised NOD SCID Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Solutions
2.2. In Vivo Compounds
2.3. Amperometric Sensor Recordings
2.4. In Vivo Implantation and Surgery Protocol
2.5. Animal Experimental Conditions
2.6. Instrumentation, Software and Data Analysis
3. Results and Discussion
3.1. Effect of Control Saline Injections on NO and O2 Currents in the Striatum of Freely Moving NOD SCID Mice
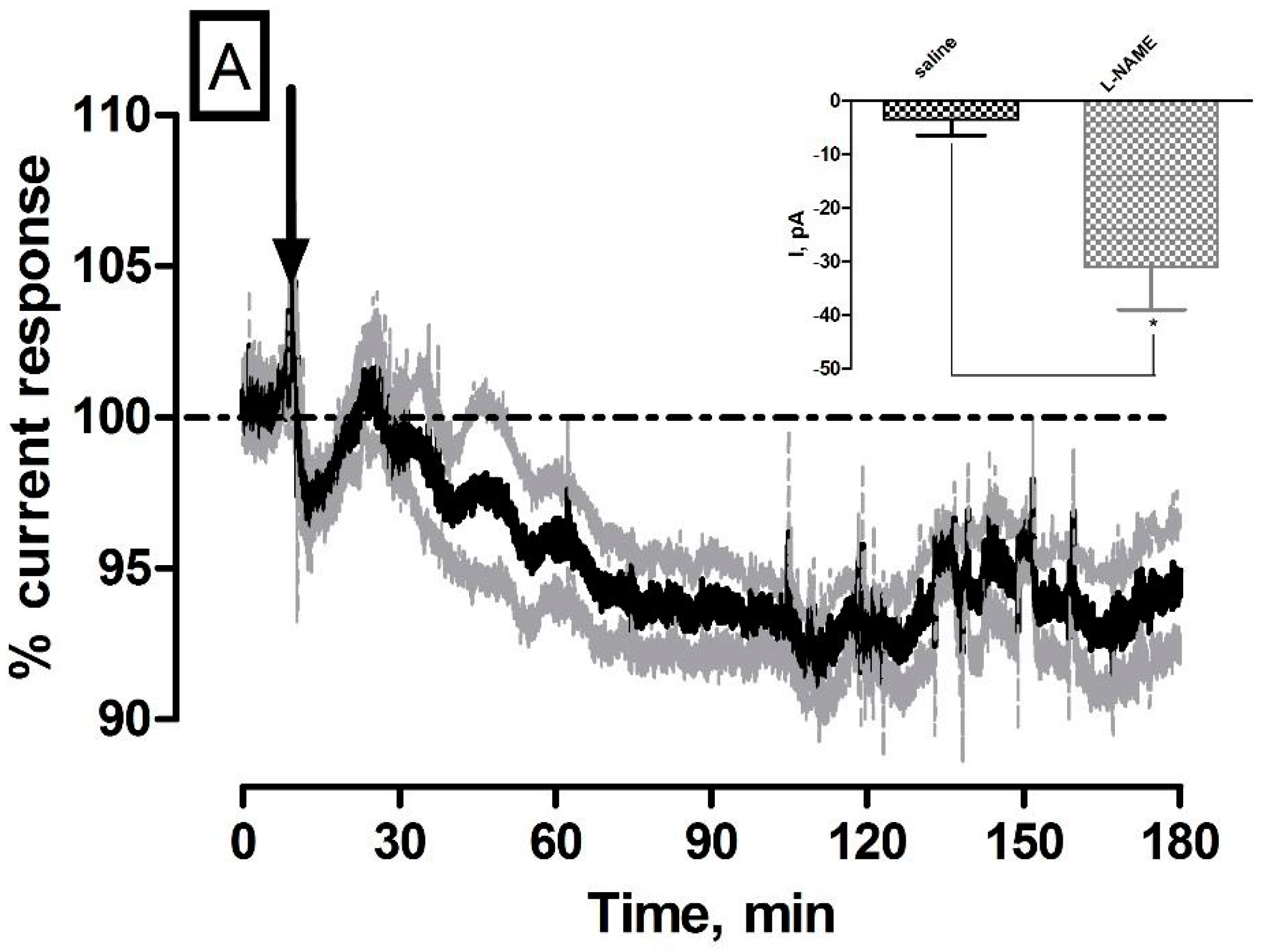
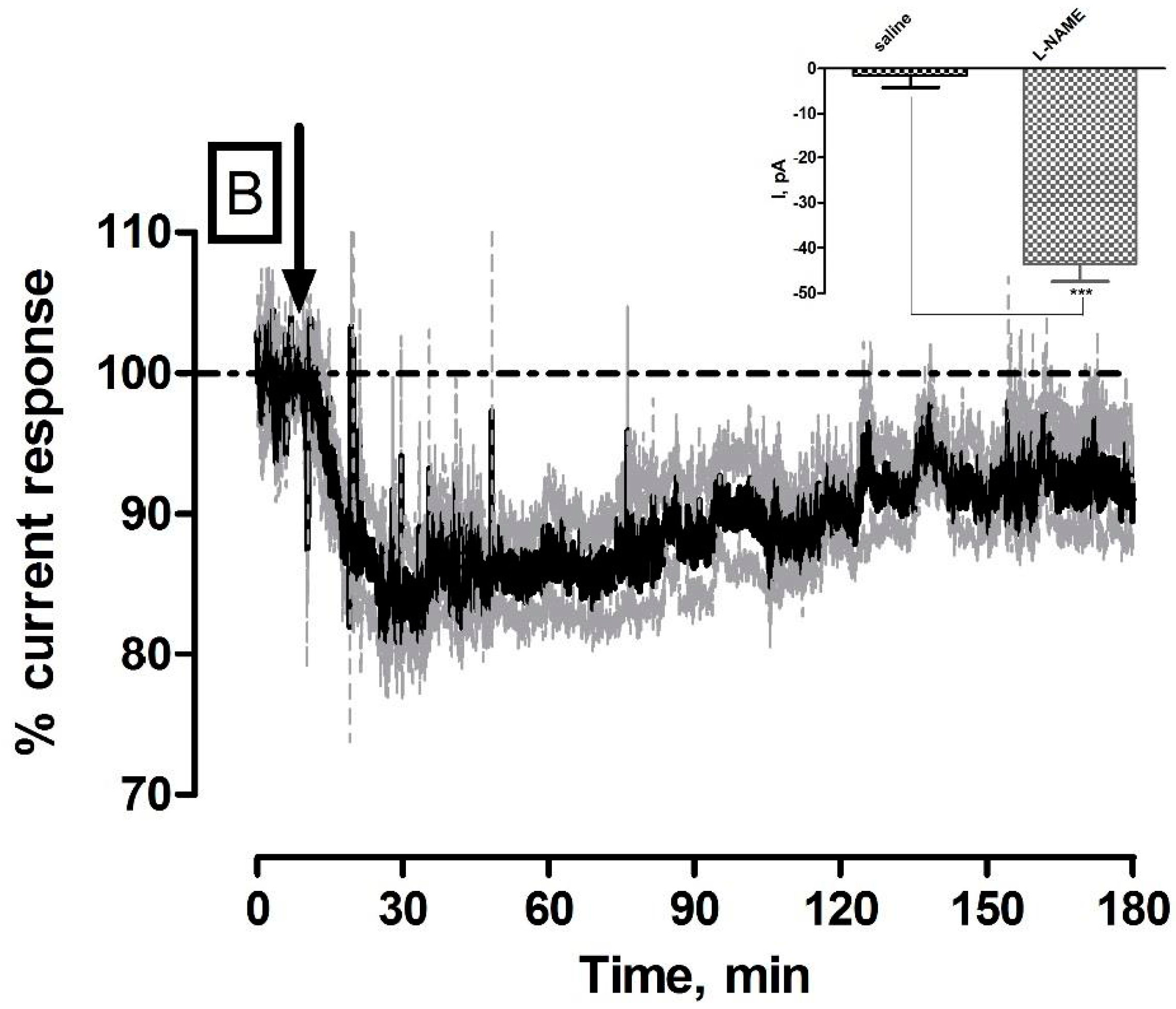
3.2. Effect of Systemic Administration of the NOS Inhibitor l-NAME on NO and O2 Currents in the Striatum of Freely Moving NOD SCID Mice
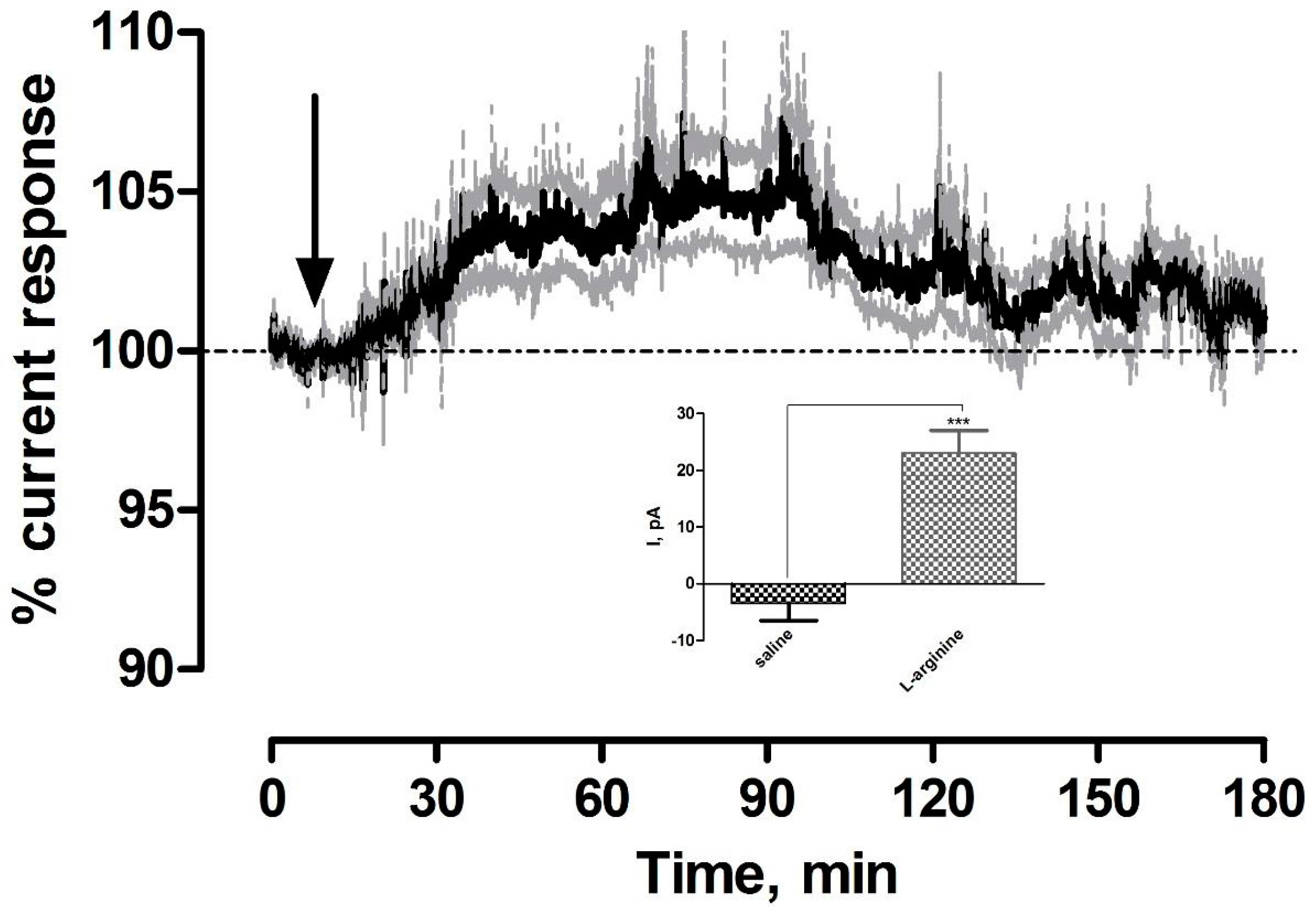
3.3. Effect of Systemic Administration of the NO Precursor l-arginine on NO Currents in the Striatum of Freely Moving NOD SCID Mice
3.4. Effect of Systemic Administration of Chloral Hydrate Anaesthetic on O2 Currents in the Striatum of Freely Moving NOD SCID Mice
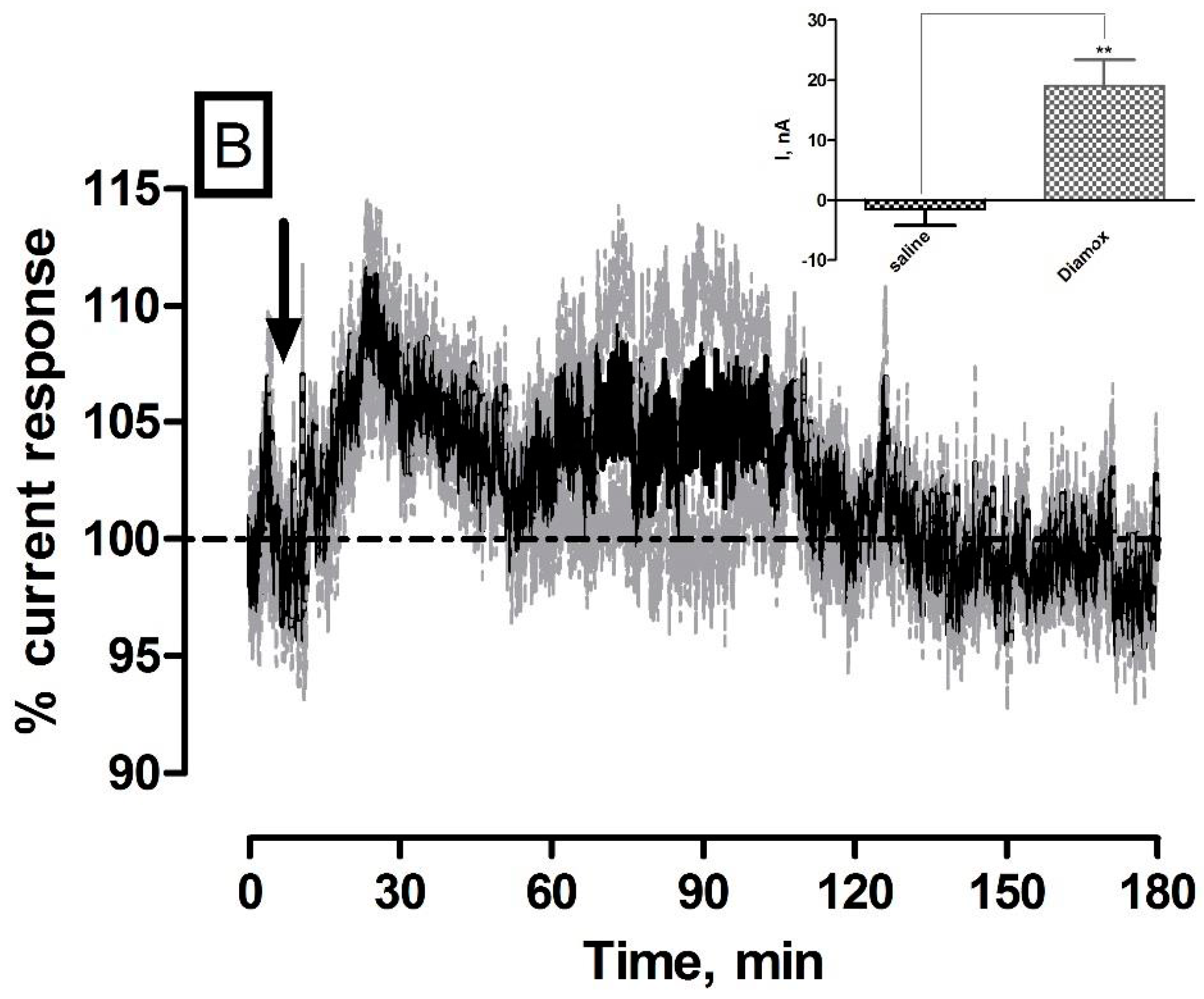
3.5. Effect of Systemic Administration of the Carbonic Anhydrase Inhibitor Diamox on O2 Currents in the Striatum of Freely Moving NOD SCID Mice
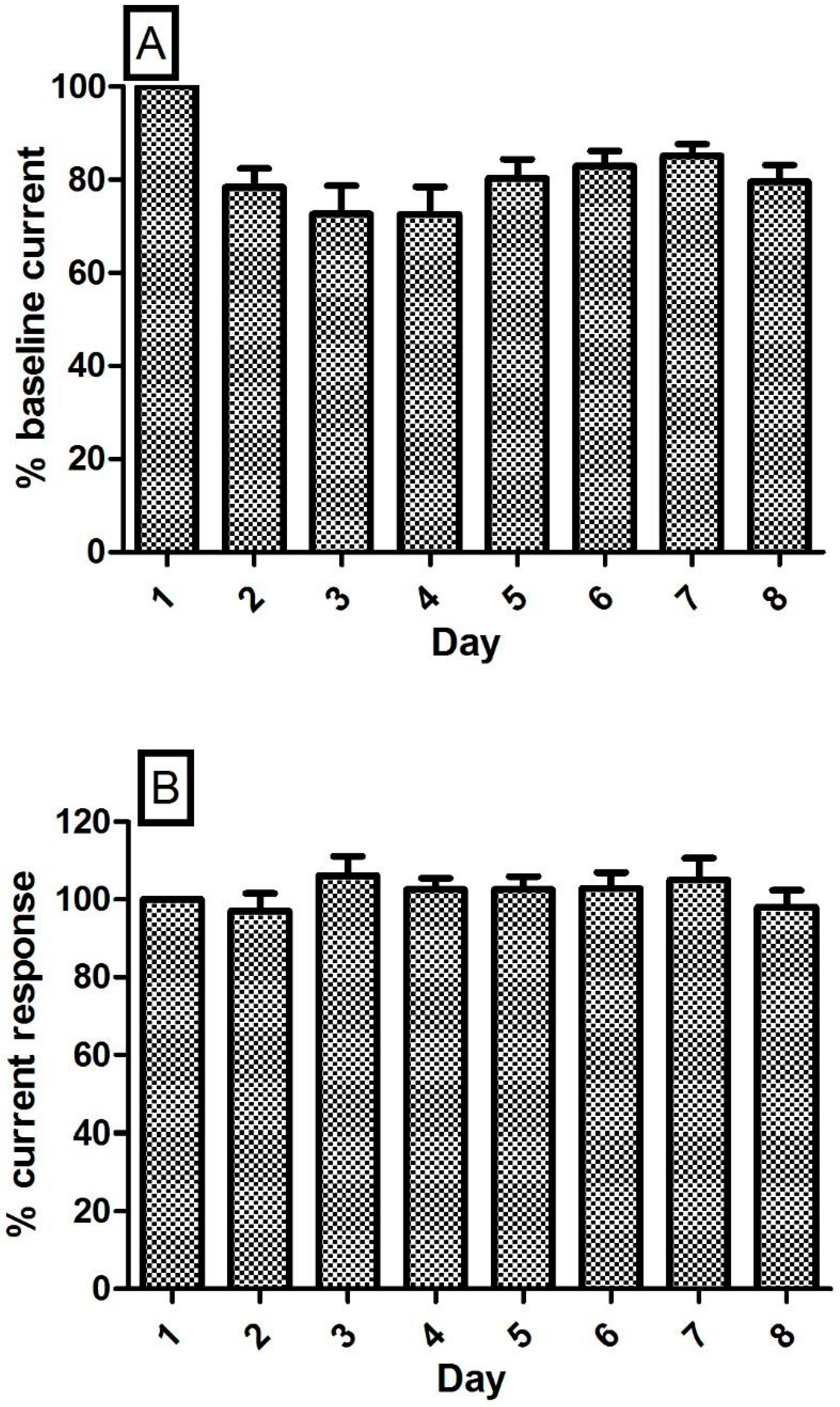
3.6. Stability of the NO and O2 Currents in the Striatum of Freely Moving NOD SCID Mice
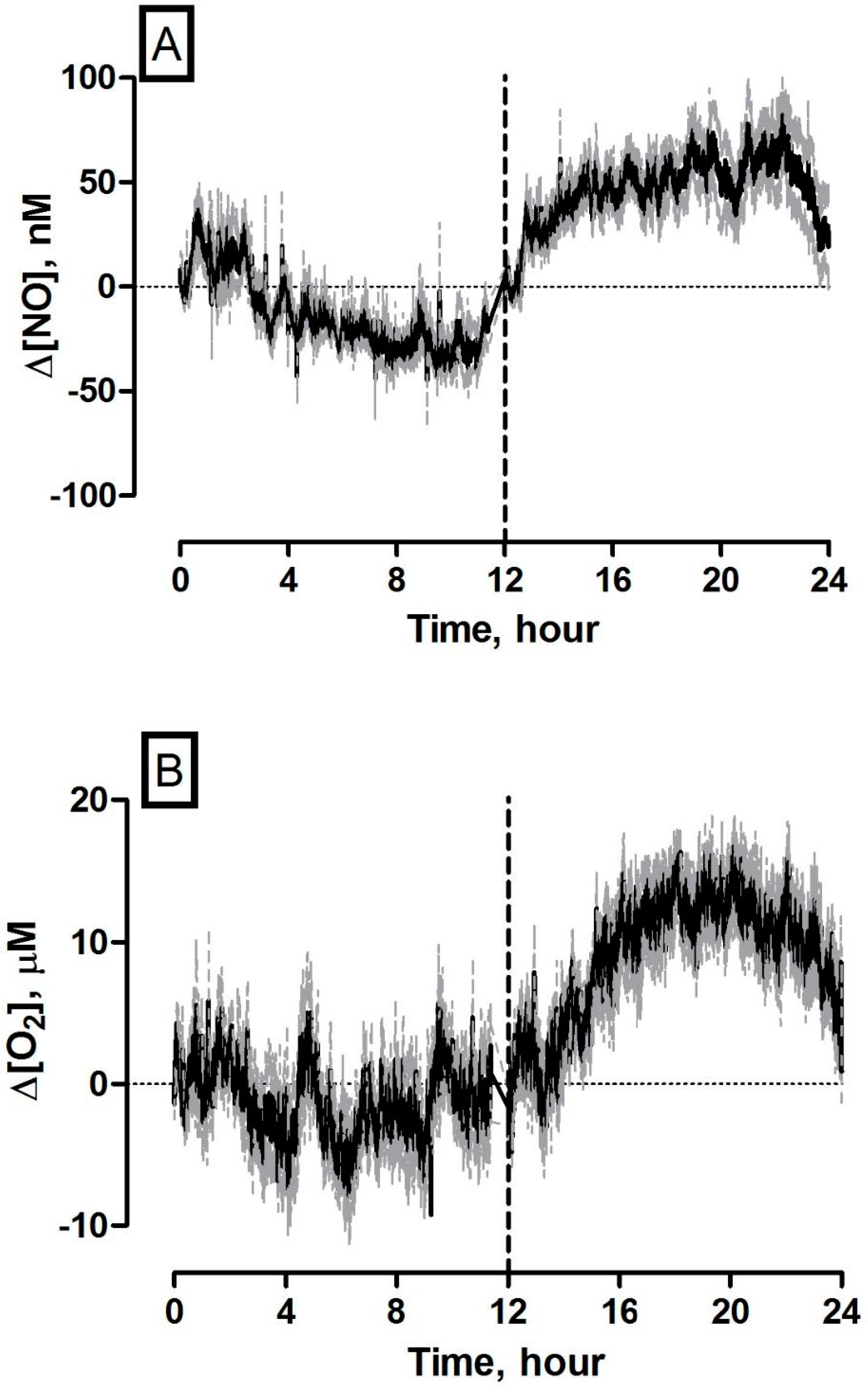
3.7. 24 Hours Recording of NO and O2 Concentration Dynamics in the Striatum of Freely Moving NOD SCID Mice
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ito, R.; Takahashi, T.; Katano, I.; Ito, M. Current advances in humanized mouse models. Cell. Mol. Immunol. 2012, 9, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Bosma, G.C.; Custer, R.P.; Bosma, M.J. A severe combined immunodeficiency mutation in the mouse. Nature 1983, 301, 527–530. [Google Scholar] [CrossRef] [PubMed]
- Ninomiya, M.; Kiyoi, H.; Ito, M.; Hirose, Y.; Ito, M.; Naoe, T. Retinoic acid syndrome in NOD/scid mice induced by injecting an acute promyelocytic leukemia cell line. Leukemia 2004, 18, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Shiokawa, M.; Takahashi, T.; Murakami, A.; Kita, S.; Ito, M.; Sugamura, K.; Ishii, N. In vivo assay of human NK-dependent ADCC using NOD/SCID/gamma c(null) (NOG) mice. Biochem. Biophys. Res. Commun. 2010, 399, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.G.; Meissner, E.; Chen, J.Z.; Su, L.S. Current humanized mouse models for studying human immunology and HIV-1 immuno-pathogenesis. Sci. China-Life Sci. 2010, 53, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Namikawa, R.; Kaneshima, H.; Lieberman, M.; Weissman, I.L.; McCune, J.M. Infection of the SCID-hu mouse by HIV-1. Science 1988, 242, 1684–1686. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Kawai, K.; Mitsui, T.; Taniguchi, K.; Monnai, M.; Wakui, M.; Ito, M.; Suematsu, M.; Peltz, G.; Nakamura, M.; et al. The reconstituted ‘humanized liver’ in TK-NOG mice is mature and functional. Biochem. Biophys. Res. Commun. 2011, 405, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.F.; Schiller, D.E.; Elliott, J.F.; Douglas, D.N.; Hao, C.H.; Rinfret, A.; Addison, W.R.; Fischer, K.P.; Churchill, T.A.; Lakey, J.R.T.; et al. Hepatitis C virus replication in mice with chimeric human livers. Nat. Med. 2001, 7, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Hargus, G.; Ehrlich, M.; Arauzo-Bravo, M.J.; Hemmer, K.; Hallmann, A.L.; Reinhardt, P.; Kim, K.P.; Adachi, K.; Santourlidis, S.; Ghanjati, F.; et al. Origin-Dependent Neural Cell Identities in Differentiated Human iPSCs In Vitro and after Transplantation into the Mouse Brain. Cell Rep. 2014, 8, 1697–1703. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, K.; Zhang, M.Y.; van Wullen, T.; Sakalem, M.; Tapia, N.; Baumuratov, A.; Kaltschmidt, C.; Kaltschmidt, B.; Scholer, H.R.; Zhang, W.Q.; et al. Induced Neural Stem Cells Achieve Long-Term Survival and Functional Integration in the Adult Mouse Brain. Stem Cell Rep. 2014, 3, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, P.; Glatza, M.; Hemmer, K.; Tsytsyura, Y.; Thiel, C.S.; Hoing, S.; Moritz, S.; Parga, J.A.; Wagner, L.; Bruder, J.M.; et al. Derivation and Expansion Using Only Small Molecules of Human Neural Progenitors for Neurodegenerative Disease Modeling. PLoS ONE 2013, 8, e59252. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef] [PubMed]
- Shibasaki, H. Human brain mapping: Hemodynamic response and electrophysiology. Clin. Neurophysiol. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Risterucci, C.; Jeanneau, K.; Schoppenthau, S.; Bielser, T.; Kunnecke, B.; von Kienlin, M.; Moreau, J.L. Functional magnetic resonance imaging reveals similar brain activity changes in two different animal models of schizophrenia. Psychopharmacology 2005, 180, 724–734. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, N.J.; Bolger, F.B.; Palsson, E.; Lowry, J.P. An Investigation of Hypofrontality in an Animal Model of Schizophrenia Using Real-Time Microelectrochemical Sensors for Glucose, Oxygen, and Nitric Oxide. ACS Chem. Neurosci. 2013, 4, 825–831. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, N.J.; O’Riordan, S.L.; Klamer, D.; Lowry, J.P.; Palsson, E. Increased brain nitric oxide levels following ethanol administration. Nitric Oxide 2015, 47, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Finnerty, N.J.; O’Riordan, S.L.; Lowry, J.P.; Cloutier, M.; Wellstead, P. Continuous Real-Time in vivo Measurement of Cerebral Nitric Oxide Supports Theoretical Predictions of an Irreversible Switching in Cerebral ROS after Sufficient Exposure to External Toxins. J. Parkinsons Dis. 2013, 3, 351–362. [Google Scholar] [PubMed]
- Bolger, F.B.; Lowry, J.P. Brain tissue oxygen: In vivo monitoring with carbon paste electrodes. Sensors 2005, 5, 473–487. [Google Scholar] [CrossRef]
- Finnerty, N.J.; O’Riordan, S.L.; Brown, F.O.; Serra, P.A.; O’Neill, R.D.; Lowry, J.P. In vivo characterisation of a Nafion (R)-modified Pt electrode for real-time nitric oxide monitoring in brain extracellular fluid. Anal. Methods 2012, 4, 550–557. [Google Scholar] [CrossRef]
- Finnerty, N.J.; O’Riordan, S.L.; Palsson, E.; Lowry, J.P. Brain nitric oxide: Regional characterisation of a real-time microelectrochemical sensor. J. Neurosci. Methods 2012, 209, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.O.; Finnerty, N.J.; Bolger, F.B.; Millar, J.; Lowry, J.P. Calibration of NO sensors for in vivo voltammetry: Laboratory synthesis of NO and the use of UV–visible spectroscopy for determining stock concentrations. Anal. Bioanal. Chem. 2005, 381, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Brown, F.O.; Finnerty, N.J.; Lowry, J.P. Nitric oxide monitoring in brain extracellular fluid: Characterisation of Nafion (R)-modified Pt electrodes in vitro and in vivo. Analyst 2009, 134, 2012–2020. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Zhu, Z.; Zhang, M.; Ye, Z. Reaction-based turn-on electrochemiluminescent sensor with a ruthenium(II) complex for selective detection of extracellular hydrogen sulfide in rat brain. Anal. Chem. 2015, 87, 1839–1845. [Google Scholar] [CrossRef] [PubMed]
- Park, S.S.; Hong, M.; Ha, Y.J.; Sim, J.; Jhon, G.J.; Lee, Y.; Suh, M. The real-time in vivo electrochemical measurement of nitric oxide and carbon monoxide release upon direct epidural electrical stimulation of the rat neocortex. Analyst 2015, 140, 3415–3421. [Google Scholar] [CrossRef] [PubMed]
- Garguilo, M.G.; Michael, A.C. Quantitation of choline in the extracellular fluid of brain tissue with amperometric microsensors. Anal. Chem. 1994, 66, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.B.; Mitchell, K.M.; Albahadily, F.N.; Michaelis, E.K.; Wilson, G.S. Direct measurement of glutamate release in the brain using a dual enzyme-based electrochemical sensor. Brain Res. 1994, 659, 117–125. [Google Scholar] [CrossRef]
- Bolger, F.B.; McHugh, S.B.; Bennett, R.; Li, J.; Ishiwari, K.; Francois, J.; Conway, M.W.; Gilmour, G.; Bannerman, D.M.; Fillenz, M.; et al. Characterisation of carbon paste electrodes for real-time amperometric monitoring of brain tissue oxygen. J. Neurosci. Methods 2011, 195, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Fillenz, M.; O’Neill, R.D. Effects of light reversal on the circadian pattern of motor activity and voltammetric signals recorded in rat forebrain. J. Physiol.-Lond. 1986, 374, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.A.; O’Neill, R.D. Major differences in the behaviour of carbon paste and carbon fibre electrodes in a protein-lipid matrix: Implications for voltammetry in vivo. Analyst 1998, 123, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Alderton, W.K.; Cooper, C.E.; Knowles, R.G. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 2001, 357, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Kovach, A.G.B.; Szabo, C.; Benyo, Z.; Csaki, C.; Greenberg, J.H.; Reivich, M. Effects of NG-Nitro-l-arginine on regional cerebral blood flow in the cat. J. Physiol.-Lond. 1992, 449, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Lassen, L.H.; Sperling, B.; Andersen, A.R.; Olesen, J. The effect of i.v. L-N-G methylarginine hydrochloride (L-NMMA: 546C88) on basal and acetazolamide (Diamox (R)) induced changes of blood velocity in cerebral arteries and regional cerebral blood flow in man. Cephalalgia 2005, 25, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, E.; Moskowitz, M.A.; Huang, Z.H.; Yoshida, T.; Irikura, K.; Dalkara, T. l-arginine infusion promotes nitric oxide-dependent vasodilation, increases cerebral blood flow, and reduces infarction volume in the rat. Stroke 1994, 25, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Garthwaite, J. Concepts of neural nitric oxide-mediated transmission. Eur. J. Neurosci. 2008, 27, 2783–2802. [Google Scholar] [CrossRef] [PubMed]
- Bruckdorfer, R. The basics about nitric oxide. Mol. Aspects Med. 2005, 26, 3–31. [Google Scholar] [CrossRef] [PubMed]
- Hojerpedersen, E. Effect of acetazolamide on cerebral blood-flow in subacute and chronic cerebrovascular-disease. Stroke 1987, 18, 887–891. [Google Scholar] [CrossRef]
- Okazawa, H.; Yamauchi, H.; Sugimoto, K.; Toyoda, H.; Kishibe, Y.; Takahashi, M. Effects of acetazolamide on cerebral blood flow, blood volume, and oxygen metabolism: A positron emission tomography study with healthy volunteers. J. Cereb. Blood Flow Metab. 2001, 21, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Mulla, I.A.L.; Lowry, J.P.; Serra, P.A.; O’Neill, R.D. Development of a voltammetric technique for monitoring brain dopamine metabolism: Compensation for interference caused by DOPAC electrogenerated during homovanillic acid detection. Analyst 2009, 134, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, M.; Xiao, T.; Hao, J.; Li, R.; Mao, L. Protein pretreatment of microelectrodes enables in vivo electrochemical measurements with easy precalibration and interference-free from proteins. Anal. Chem. 2016, 88, 7238–7244. [Google Scholar] [CrossRef] [PubMed]
- Endo, Y.; Jinnai, K.; Endo, M.; Fujita, K.; Kimura, F. Diurnal Variation of Cerebral Blood Flow in Rat Hippocampus. Stroke 1990, 21, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Kocharyan, A.; Schechter, A.N.; Silva, A.C. The role of nitrite in neurovascular coupling. Brain Res. 2011, 1407, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; MacVicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Kostin, A.; McGinty, A.; Szymusiak, R.; Alam, N.M. Sleep-wake and diurnal modulation of nitric oxide in the perifornical-lateral hypothalamic area: Real-time detection in freely behaving rats. Neuroscience 2013, 254, 275–284. [Google Scholar] [CrossRef] [PubMed]


| Administration | ∆ I | ∆ Conc | vs. Baseline | vs. Saline |
|---|---|---|---|---|
| Saline (NO) | −4 ± 3 pA | 4 ± 3 nM | p = 0.30 | - |
| Saline (O2) | −2 ± 3 nA | 1 ± 2 µM | p = 0.64 | - |
| l-NAME (NO) | −31 ± 8 pA | 30 ± 8 nM | ** p < 0.01 | * p < 0.05 |
| l-NAME (O2) | −44 ± 4 nA | 26 ± 2 µM | *** p < 0.001 | *** p < 0.001 |
| l-arginine (NO) | 23 ± 4 pA | 22 ± 4 nM | *** p < 0.001 | *** p < 0.001 |
| Chloral hydrate (O2) | 87 ± 22 nA | 51 ± 13 µM | * p < 0.05 | ** p < 0.01 |
| Diamox (O2) | 19 ± 4 nA | 11 ± 2 µM | * p < 0.05 | ** p < 0.01 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reid, C.H.; Finnerty, N.J. Long Term Amperometric Recordings in the Brain Extracellular Fluid of Freely Moving Immunocompromised NOD SCID Mice. Sensors 2017, 17, 419. https://doi.org/10.3390/s17020419
Reid CH, Finnerty NJ. Long Term Amperometric Recordings in the Brain Extracellular Fluid of Freely Moving Immunocompromised NOD SCID Mice. Sensors. 2017; 17(2):419. https://doi.org/10.3390/s17020419
Chicago/Turabian StyleReid, Caroline H., and Niall J. Finnerty. 2017. "Long Term Amperometric Recordings in the Brain Extracellular Fluid of Freely Moving Immunocompromised NOD SCID Mice" Sensors 17, no. 2: 419. https://doi.org/10.3390/s17020419





