Reconstruction of Undersampled Big Dynamic MRI Data Using Non-Convex Low-Rank and Sparsity Constraints
Abstract
:1. Introduction
2. Background
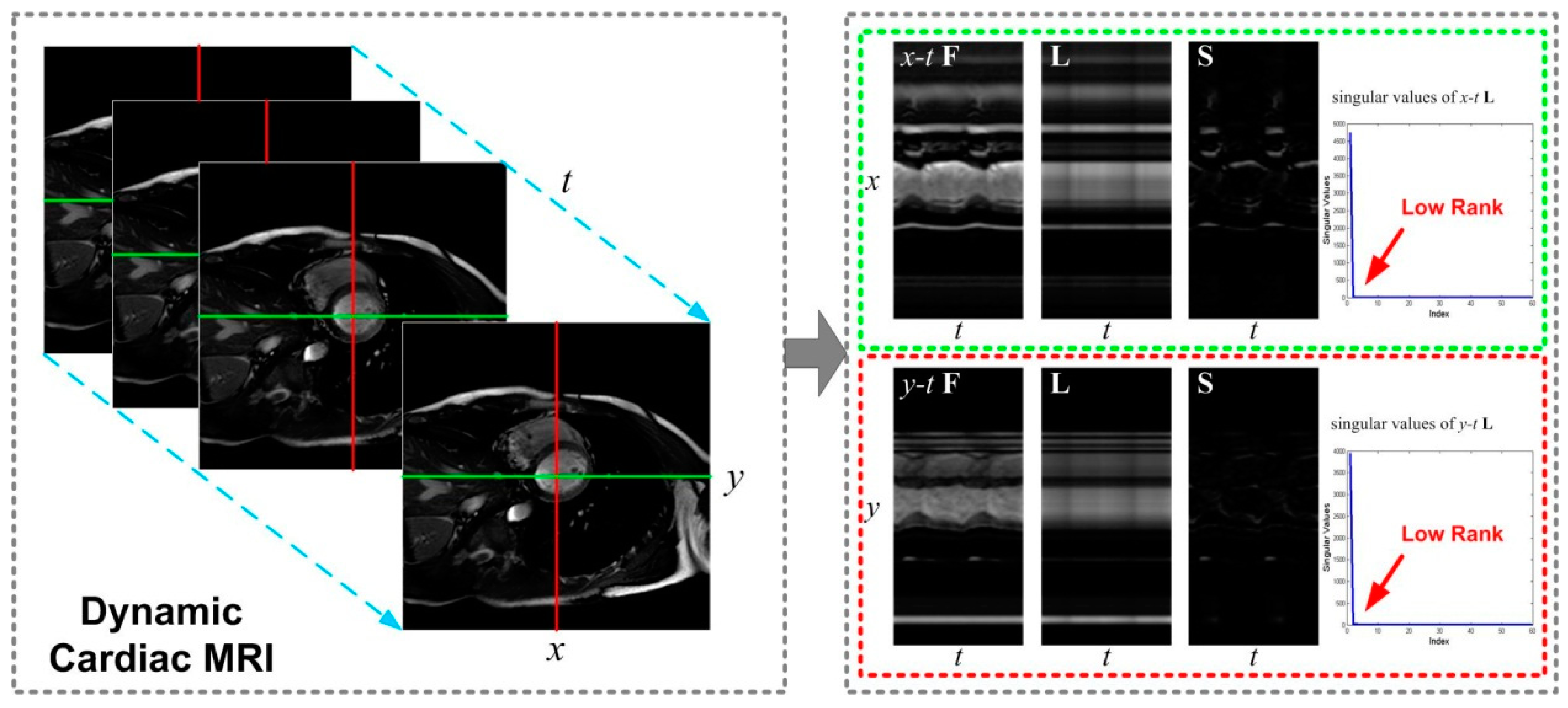
2.1. Dynamic MRI from Partial Measurements
2.2. Robust Principal Component Analysis
| Algorithm 1. RPCA |
| 1 Input: Casorati matrix , decomposition parameter |
| 2 Initialize: , k |
| 3 while stopping criterion is not satisfied do |
| 4 |
| 5 |
| 6 |
| 7 end while |
| 8 Output: |
3. k-t NCRPCA: Formulation
3.1. Joint Non-Convex Low-Rank and Sparsity Constraints
3.2. Numerical Optimization Algorithm
| Algorithm 2. k-t NCRPCA |
| 1 Input: Fourier transform ,-space data and parameters . |
| 2 Initialize: , . |
| 3 while stopping criterion is not satisfied do |
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
| 10 |
| 11 and |
| 12 end while |
| 13 Output: |
4. Experimental Results and Discussion
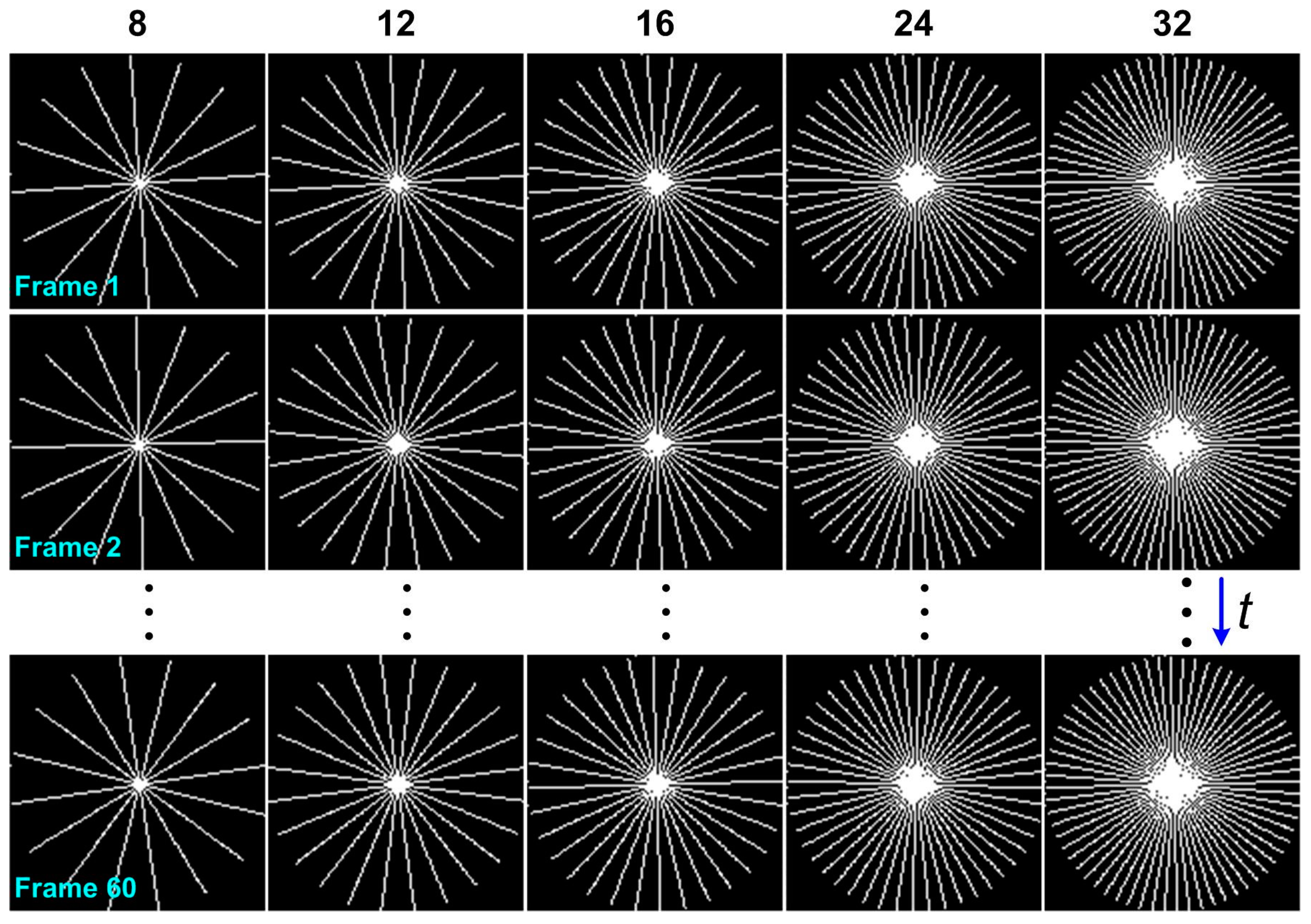
4.1. Acquired Datasets
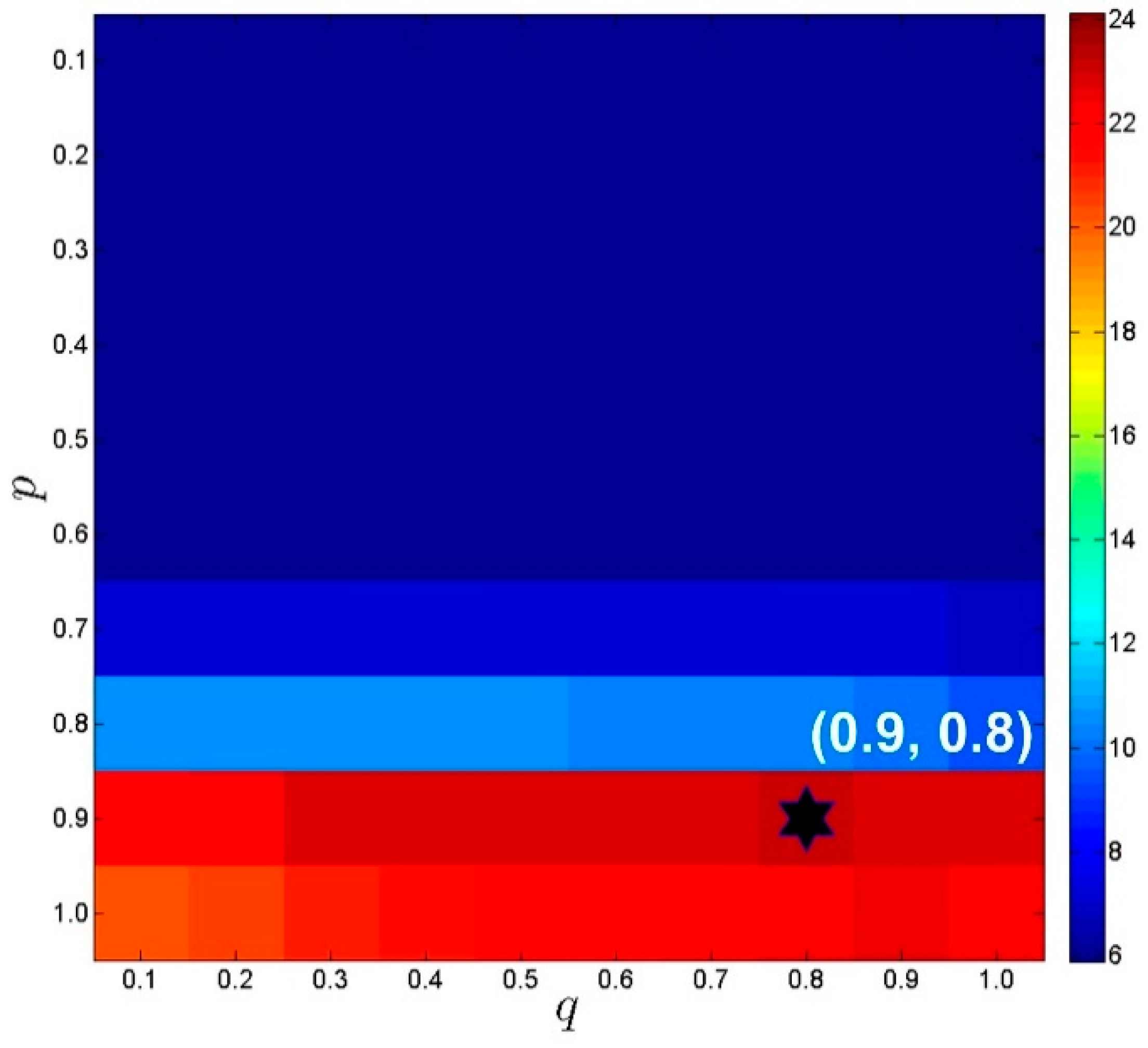
4.2. Parameters Settings
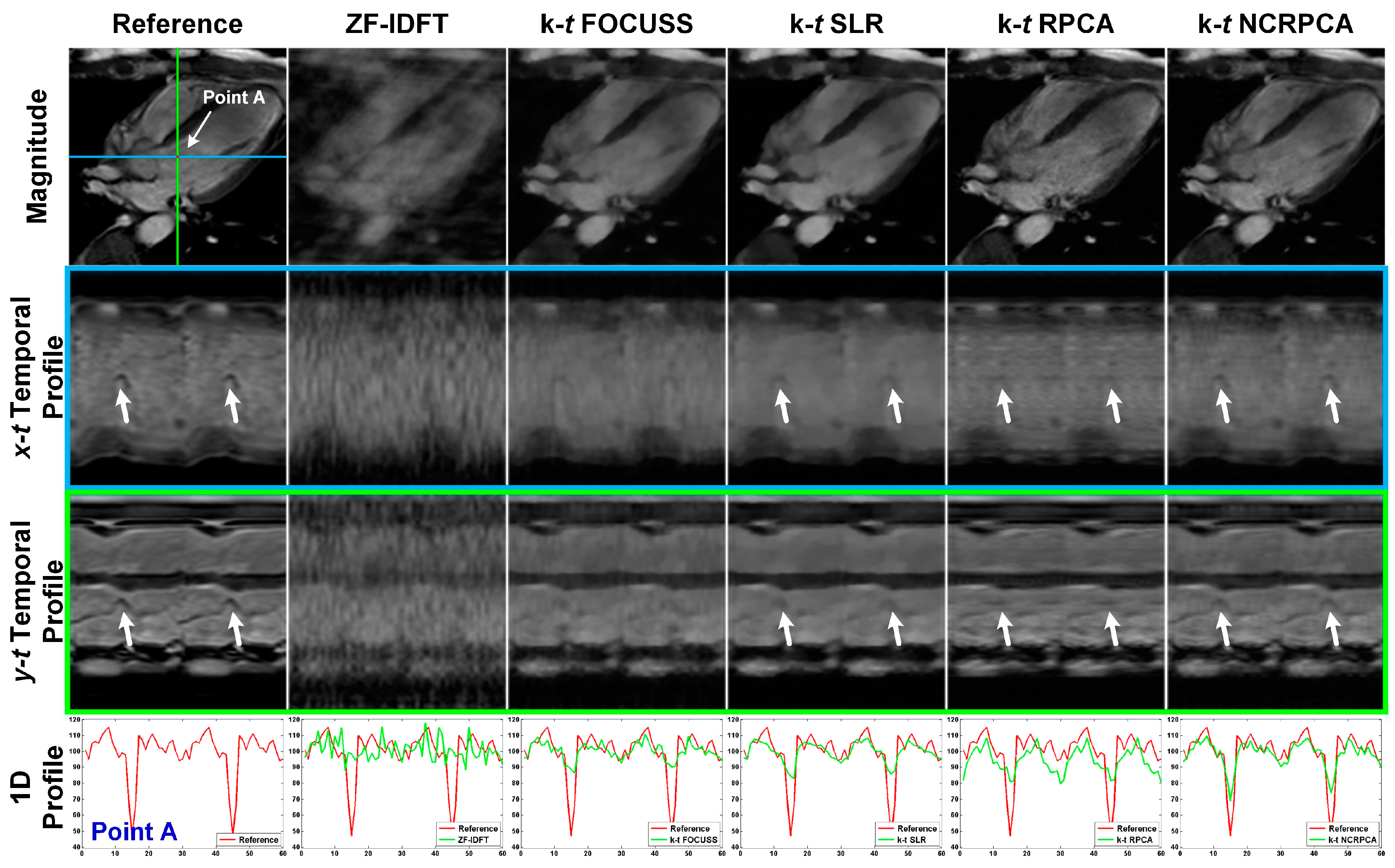
4.3. Comparisons on In Vivo Axial Cardiac Dataset
4.4. Comparisons on In Vivo Coronal Cardiac Dataset
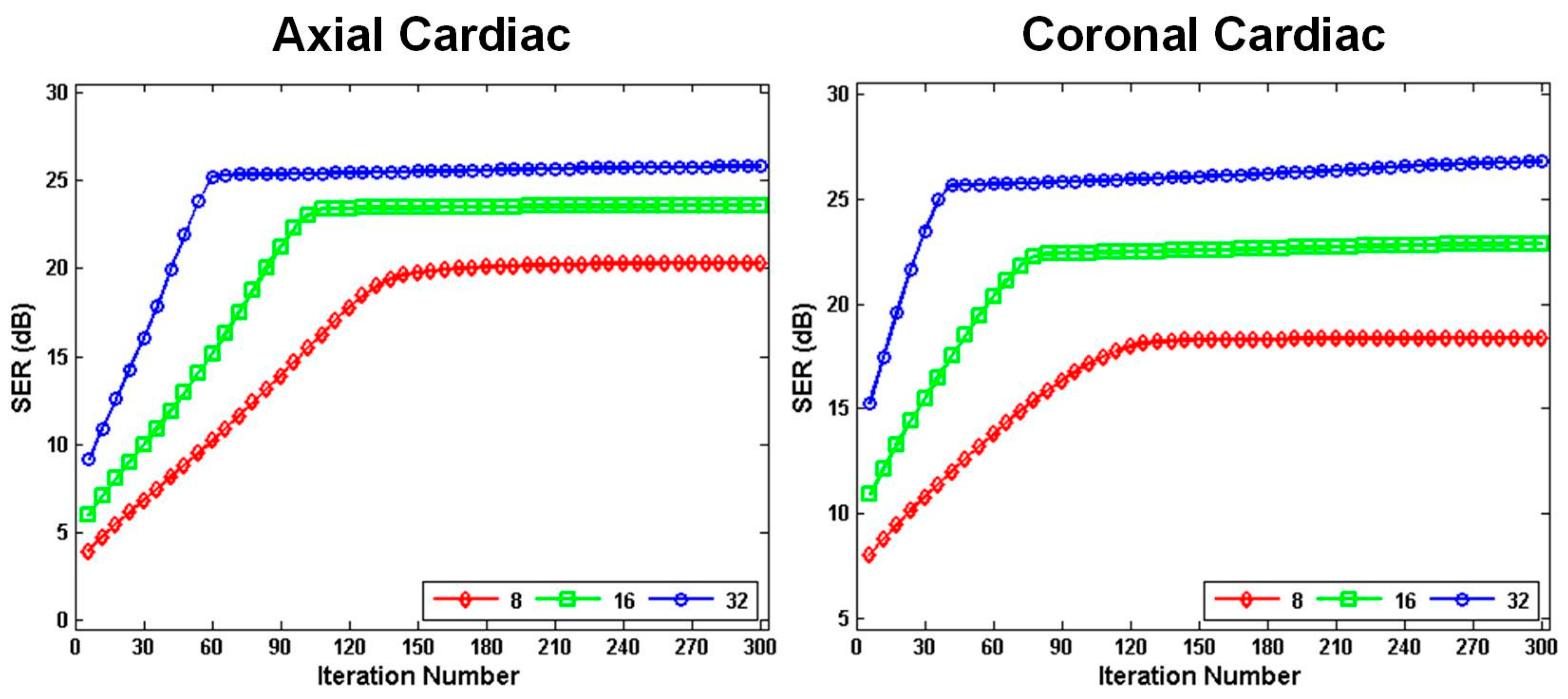
4.5. Algorithm Convergence and Robustness
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Edelman, R.R.; Warach, S. Magnetic resonance imaging. N. Engl. J. Med. 1993, 328, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Vasilakos, A.V.; Yang, L.T.; Song, L.; Pan, Y.; Kannan, R.; Li, Y. Comparative analysis of quality of service and memory usage for adaptive failure detectors in healthcare systems. IEEE J. Sel. Areas Commun. 2009, 27, 495–509. [Google Scholar] [CrossRef]
- O'connor, J.P.; Jackson, A.; Parker, G.J.; Roberts, C.; Jayson, G.C. Dynamic contrast-enhanced MRI in clinical trials of antivascular therapies. Nat. Rev. Clin. Oncol. 2012, 9, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Xiong, N.; Jia, X.; Yang, L.T.; Vasilakos, A.V.; Li, Y.; Pan, Y. A distributed efficient flow control scheme for multirate multicast networks. IEEE Trans. Parallel Distrib. Syst. 2010, 21, 1254–1266. [Google Scholar] [CrossRef]
- Harada, T.; Tsuji, Y.; Mikami, Y.; Hatta, Y.; Sakamoto, A.; Ikeda, T.; Kubo, T. The clinical usefulness of preoperative dynamic MRI to select decompression levels for cervical spondylotic myelopathy. Magn. Reson. Imaging 2010, 28, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Leach, M.O.; Morgan, B.; Tofts, P.S.; Buckley, D.L.; Huang, W.; Horsfield, M.A.; Whitcher, B. Imaging vascular function for early stage clinical trials using dynamic contrast-enhanced magnetic resonance imaging. Eur. Radiol. 2012, 22, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Xiong, N.N.; Vasilakos, A.V.; Sun, X. EPCBIR: An efficient and privacy-preserving content-based image retrieval scheme in cloud computing. Inf. Sci. 2016, 387, 195–204. [Google Scholar] [CrossRef]
- Lingala, S.G.; DiBella, E.; Adluru, G.; McGann, C.; Jacob, M. Accelerating free breathing myocardial perfusion MRI using multi coil radial k-t SLR. Phys. Med. Biol. 2013, 58, 7309–7327. [Google Scholar] [CrossRef] [PubMed]
- Denney, T.R.; Prince, J.L. Reconstruction of 3-D left ventricular motion from planar tagged cardiac MR images: An estimation theoretic approach. IEEE Trans. Med. Imaging 1995, 14, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Thesen, S.; Heid, O.; Mueller, E.; Schad, L.R. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn. Reson. Med. 2000, 44, 457–465. [Google Scholar] [CrossRef]
- Mahapatra, D.; Buhmann, J.M. Prostate MRI segmentation using learned semantic knowledge and graph cuts. IEEE Trans. Biomed. Eng. 2014, 61, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Wang, X.; Sun, X.; Liu, Q.; Xiong, N. Steganalysis of LSB matching using differences between nonadjacent pixels. Multimed. Tools Appl. 2016, 75, 1947–1962. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Zheng, Y.; Jeon, B.; Wu, Q.J. An improved anisotropic hierarchical fuzzy c-means method based on multivariate student t-distribution for brain MRI segmentation. Pattern Recognit. 2016, 60, 778–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Z.; Wu, L.; Wang, S. A hybrid method for MRI brain image classification. Expert Syst. Appl. 2011, 38, 10049–10053. [Google Scholar] [CrossRef]
- Wen, X.; Shao, L.; Xue, Y.; Fang, W. A rapid learning algorithm for vehicle classification. Inf. Sci. 2015, 295, 395–406. [Google Scholar] [CrossRef]
- Li, Z.; Lai, Z.; Xu, Y.; Yang, J.; Zhang, D. A locality-constrained and label embedding dictionary learning algorithm for image classification. IEEE Trans. Neural Netw. Learn. Syst. 2017, 28, 278–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.W.; Shi, L.; Yu, S.C.; Wang, D. A two-step optimization approach for nonlocal total variation-based Rician noise reduction in magnetic resonance images. Med. Phys. 2015, 42, 5167–5187. [Google Scholar] [CrossRef] [PubMed]
- Baselice, F.; Ferraioli, G.; Pascazio, V.; Sorriso, A. Bayesian MRI denoising in complex domain. Magn. Reson. Imaging 2017, 38, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Pizzolato, M.; Boutelier, T.; Deriche, R. Perfusion deconvolution in DSC-MRI with dispersion-compliant bases. Med. Image Anal. 2017, 36, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.S.; Cheong, D.L.H.; Hou, Z. Issues of discontinuity in the impulse residue function for deconvolution analysis of dynamic contrast-enhanced MRI data. Magn. Reson. Med. 2011, 66, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Paulsen, J.L.; Zhu, G.; Song, Y.; Chun, S.; Cho, G.; Ackerstaff, E.; Koutcher, J.A.; Cho, H. Temporal/spatial resolution improvement of in vivo DCE-MRI with compressed sensing-optimized FLASH. Magn. Reson. Imaging 2012, 30, 741–752. [Google Scholar] [CrossRef] [PubMed]
- Pain, F.; Besret, L.; Vaufrey, F.; Grégoire, M.C.; Pinot, L.; Gervais, P.; Ploux, L.; Bloch, G.; Mastrippolito, R.; Lanièce, P.; et al. In vivo quantification of localized neuronal activation and inhibition in the rat brain using a dedicated high temporal-resolution β+-sensitive microprobe. Proc. Natl. Acad. Sci. USA 2002, 99, 10807–10812. [Google Scholar] [CrossRef] [PubMed]
- Boschetto, D.; Di Prima, P.; Castellaro, M.; Bertoldo, A.; Grisan, E. Baseline constrained reconstruction of DSC-MRI tracer kinetics from sparse fourier data. In Proceedings of the IEEE International Symposium on Biomedical Imaging, Beijing, China, 29 April–2 May 2014; pp. 321–324.
- Candes, E.J.; Romberg, J.; Tao, T. Robust uncertainty principles: Exact signal reconstruction from highly incomplete frequency information. IEEE Trans. Inf. Theory 2006, 52, 489–509. [Google Scholar] [CrossRef]
- Donoho, D.L. Compressed sensing. IEEE Trans. Inf. Theory 2006, 52, 1289–1306. [Google Scholar] [CrossRef]
- Lustig, M.; Donoho, D.; Pauly, J.M. Sparse MRI: The application of compressed sensing for rapid MR imaging. Magn. Reson. Med. 2007, 58, 1182–1195. [Google Scholar] [CrossRef] [PubMed]
- Lustig, M.; Donoho, D.L.; Santos, J.M.; Pauly, J.M. Compressed sensing MRI. IEEE Signal Process. Mag. 2008, 25, 72–82. [Google Scholar] [CrossRef]
- Zhang, Y.; Peterson, B.S.; Ji, G.; Dong, Z. Energy preserved sampling for compressed sensing MRI. Comput. Math. Method Med. 2014, 2014, 546814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dong, Z.; Phillips, P.; Wang, S.; Ji, G.; Yang, J. Exponential wavelet iterative shrinkage thresholding algorithm for compressed sensing magnetic resonance imaging. Inf. Sci. 2015, 322, 115–132. [Google Scholar] [CrossRef]
- Cai, N.; Wang, S.; Zhu, S.; Liang, D. Accelerating dynamic cardiac MR imaging using structured sparse representation. Comput. Math. Methods Med. 2013, 2013, 160139. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Sung, K.; Nayak, K.S.; Kim, E.Y.; Ye, J.C. k-t FOCUSS: A general compressed sensing framework for high resolution dynamic MRI. Magn. Reson. Med. 2009, 61, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Ye, J.C.; Kim, E.Y. Improved k-t BLAST and k-t SENSE using FOCUSS. Phys. Med. Biol. 2007, 52, 3201–3226. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Srichai, M.B.; Lim, R.P.; Harrison, A.; King, W.; Adluru, G.; Dibella, E.V.; Sodickson, D.K.; Otazo, R.; Kim, D. Highly accelerated real-time cardiac cine MRI using k-t SPARSE-SENSE. Magn. Reson. Med. 2013, 70, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Candes, E.J.; Recht, B. Exact matrix completion via convex optimization. Found. Comput. Math. 2009, 9, 717–772. [Google Scholar] [CrossRef]
- Otazo, R.; Candes, E.; Sodickson, D.K. Low-rank plus sparse matrix decomposition for accelerated dynamic MRI with separation of background and dynamic components. Magn. Reson. Med. 2015, 73, 1125–1136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lai, Z.; Zhou, Z.; Kuang, F.; Jin, Z. A truncated nuclear norm regularization method based on weighted residual error for matrix completion. IEEE Trans. Image Process. 2016, 25, 316–330. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xu, Y.; Li, X.; Lai, Z.; Wong, W.K. Robust semi-supervised subspace clustering via non-negative low-rank representation. IEEE Trans. Cybern. 2016, 46, 1828–1838. [Google Scholar] [CrossRef] [PubMed]
- Lingala, S.G.; Hu, Y.; DiBella, E.; Jacob, M. Accelerated dynamic MRI exploiting sparsity and low-rank structure: k-t SLR. IEEE Trans. Med. Imaging 2011, 30, 1042–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Haldar, J.P.; Christodoulou, A.G.; Liang, Z.P. Image reconstruction from highly undersampled (k,t)-space data with joint partial separability and sparsity constraints. IEEE Trans. Med. Imaging 2012, 31, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Candes, E.J.; Li, X.D.; Ma, Y.; Wright, J. Robust principal component analysis? J. ACM 2011, 58, 1–37. [Google Scholar] [CrossRef]
- Yuan, X.; Yang, J. Sparse and low-rank matrix decomposition via alternating direction methods. Pac. J. Optim. 2013, 9, 167–180. [Google Scholar]
- Tao, M.; Yuan, X.M. Recovering low-rank and sparse components of matrices from incomplete and noisy observations. SIAM J. Optim. 2011, 21, 57–81. [Google Scholar] [CrossRef]
- Wright, J.; Ganesh, A.; Min, K.R.; Ma, Y. Compressive principal component pursuit. Inf. Inference 2013, 2, 32–68. [Google Scholar] [CrossRef]
- Trémoulhéac, B.; Dikaios, N.; Atkinson, D.; Arridge, S.R. Dynamic MR image reconstruction-separation from undersampled (k,t)-space via low-rank plus sparse prior. IEEE Trans. Med. Imaging 2014, 33, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A.; Ward, R.K. An algorithm for sparse MRI reconstruction by Schatten p-norm minimization. Magn. Reson. Imaging 2011, 29, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Trzasko, J.; Manduca, A. Highly undersampled magnetic resonance image reconstruction via homotopic l0-minimization. IEEE Trans. Med. Imaging 2009, 28, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, A. Improved dynamic MRI reconstruction by exploiting sparsity and rank-deficiency. Magn. Reson. Imaging 2013, 31, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Sun, X.; Wang, B. Efficient algorithm for k-barrier coverage based on integer linear programming. China Commun. 2016, 13, 16–23. [Google Scholar] [CrossRef]
- Mehranian, A.; Reader, A. Non-convex joint-sparsity regularization for synergistic PET and SENSE MRI reconstruction. J. Nucl. Med. 2016, 57, 639. [Google Scholar]
- Ding, Y.; Selesnick, I.W. Artifact-free wavelet denoising: Non-convex sparse regularization, convex optimization. IEEE Signal Process. Lett. 2015, 22, 1364–1368. [Google Scholar] [CrossRef]
- Zhang, J.; Xiong, R.; Zhao, C.; Zhang, Y.; Ma, S.; Gao, W. CONCOLOR: Constrained non-convex low-rank model for image deblocking. IEEE Trans. Image Process. 2016, 25, 1246–1259. [Google Scholar] [CrossRef] [PubMed]
- Chartrand, R. Exact reconstruction of sparse signals via nonconvex minimization. IEEE Signal Process. Lett. 2007, 14, 707–710. [Google Scholar] [CrossRef]
- Chartrand, R.; Staneva, V. Restricted isometry properties and nonconvex compressive sensing. Inverse. Probl. 2008, 24, 035020:1–035020:14. [Google Scholar] [CrossRef]
- Yuan, C.; Sun, X.; Lv, R. Fingerprint liveness detection based on multi-scale LPQ and PCA. China Commun. 2016, 13, 60–65. [Google Scholar] [CrossRef]
- Gasso, G.; Rakotomamonjy, A.; Canu, S. Recovering sparse signals with a certain family of nonconvex penalties and DC programming. IEEE Trans. Signal Process. 2009, 57, 4686–4698. [Google Scholar] [CrossRef]
- Liu, R.W.; Shi, L.; Huang, W.; Xu, J.; Yu, S.C.H.; Wang, D. Generalized total variation-based MRI Rician denoising model with spatially adaptive regularization parameters. Magn. Reson. Imaging 2014, 32, 702–720. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.; Xu, Y.; Chen, Q.; Yang, J.; Zhang, D. Multilinear sparse principal component analysis. IEEE Trans. Neural Netw. Learn. Syst. 2014, 25, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, C.; Zhao, H.; Yu, W. Low-rank modeling and its applications in image analysis. ACM Comput. Surv. 2015, 47, 36. [Google Scholar] [CrossRef]
- Boyd, S.; Parikh, N.; Chu, E.; Peleato, B.; Eckstein, J. Distributed optimization and statistical learning via the alternating direction method of multipliers. Found. Trends Mach. Learn. 2011, 3, 1–122. [Google Scholar] [CrossRef]
- Cai, J.F.; Candes, E.J.; Shen, Z.W. A singular value thresholding algorithm for matrix completion. SIAM J. Optim. 2010, 20, 1956–1982. [Google Scholar] [CrossRef]
- Lu, Y.; Lai, Z.; Xu, Y.; Li, X.; Zhang, D.; Yuan, C. Low-rank preserving projections. IEEE Trans. Cybern. 2016, 46, 1900–1913. [Google Scholar] [CrossRef] [PubMed]
- Donoho, D.L. De-noising by soft-thresholding. IEEE Trans. Inf. Theory 1995, 41, 613–627. [Google Scholar] [CrossRef]
- Daubechies, I.; Defrise, M.; De Mol, C. An iterative thresholding algorithm for linear inverse problems with a sparsity constraint. Commun. Pure Appl. Math. 2004, 57, 1413–1457. [Google Scholar] [CrossRef]
- Zuo, W.M.; Meng, D.Y.; Zhang, L.; Feng, X.C.; Zhang, D. A generalized iterated shrinkage algorithm for non-convex sparse coding. In Proceedings of the IEEE International Conference on Computer Vision, Sydney, Australia, 1–8 December 2013; pp. 217–224.


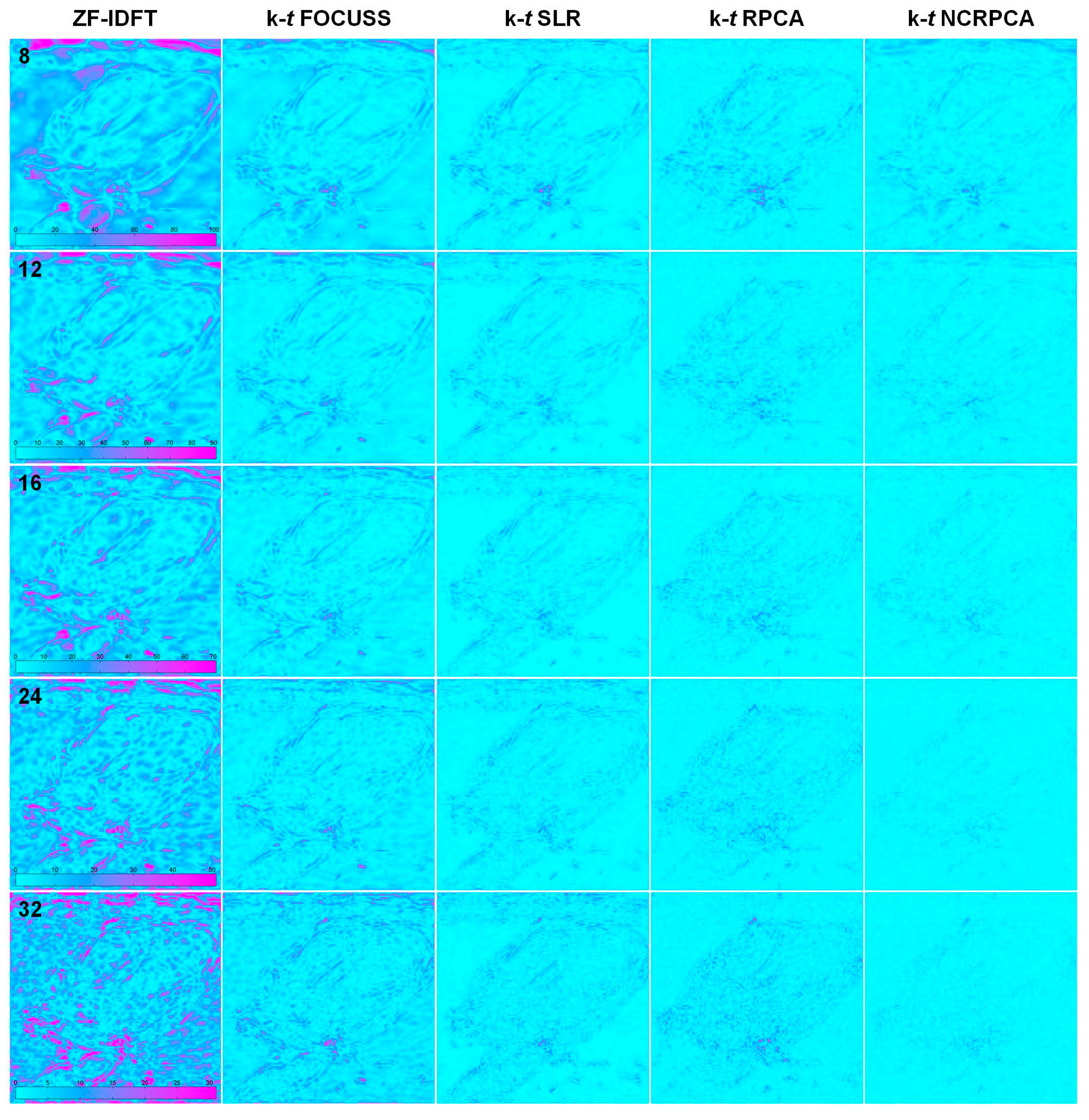
| Projections | ZF-IDFT | k-t FOCUSS | k-t SLR | k-t RPCA | k-t NCRPCA |
|---|---|---|---|---|---|
| 8 | 0.2555/3.4603 | 0.7376/11.2002 | 0.8802/12.6634 | 0.9476/19.6096 | 0.9503/20.1538 |
| 12 | 0.3088/4.4626 | 0.7969/12.9951 | 0.9257/15.6441 | 0.9760/22.0393 | 0.9784/22.9995 |
| 16 | 0.3608/5.3026 | 0.8518/15.2740 | 0.9518/17.7428 | 0.9769/22.9016 | 0.9789/23.7973 |
| 24 | 0.4408/6.7989 | 0.9004/17.0383 | 0.9671/20.3897 | 0.9828/24.0802 | 0.9942/24.9276 |
| 32 | 0.4981/8.1953 | 0.9257/20.4791 | 0.9831/22.8358 | 0.9870/25.1264 | 0.9956/25.8280 |
| Projections | ZF-IDFT | k-t FOCUSS | k-t SLR | k-t RPCA | k-t NCRPCA |
|---|---|---|---|---|---|
| 8 | 0.3281/7.3112 | 0.7041/16.1893 | 0.8328/16.9640 | 0.7948/17.5719 | 0.8268/17.8084 |
| 12 | 0.4306/8.7548 | 0.8099/17.9173 | 0.8894/18.8529 | 0.8682/20.2187 | 0.8937/20.6879 |
| 16 | 0.4974/9.9446 | 0.8618/19.6515 | 0.9300/20.8879 | 0.9134/21.9271 | 0.9384/22.6999 |
| 24 | 0.5929/11.9903 | 0.9064/21.9681 | 0.9546/23.1806 | 0.9383/23.5353 | 0.9768/25.1972 |
| 32 | 0.6753/13.9492 | 0.9489/24.2931 | 0.9757/25.7613 | 0.9674/25.4710 | 0.9887/27.2034 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.W.; Shi, L.; Yu, S.C.H.; Xiong, N.; Wang, D. Reconstruction of Undersampled Big Dynamic MRI Data Using Non-Convex Low-Rank and Sparsity Constraints. Sensors 2017, 17, 509. https://doi.org/10.3390/s17030509
Liu RW, Shi L, Yu SCH, Xiong N, Wang D. Reconstruction of Undersampled Big Dynamic MRI Data Using Non-Convex Low-Rank and Sparsity Constraints. Sensors. 2017; 17(3):509. https://doi.org/10.3390/s17030509
Chicago/Turabian StyleLiu, Ryan Wen, Lin Shi, Simon Chun Ho Yu, Naixue Xiong, and Defeng Wang. 2017. "Reconstruction of Undersampled Big Dynamic MRI Data Using Non-Convex Low-Rank and Sparsity Constraints" Sensors 17, no. 3: 509. https://doi.org/10.3390/s17030509






