Humidity Sensing Properties of Paper Substrates and Their Passivation with ZnO Nanoparticles for Sensor Applications
Abstract
:1. Introduction
2. Materials and Methods
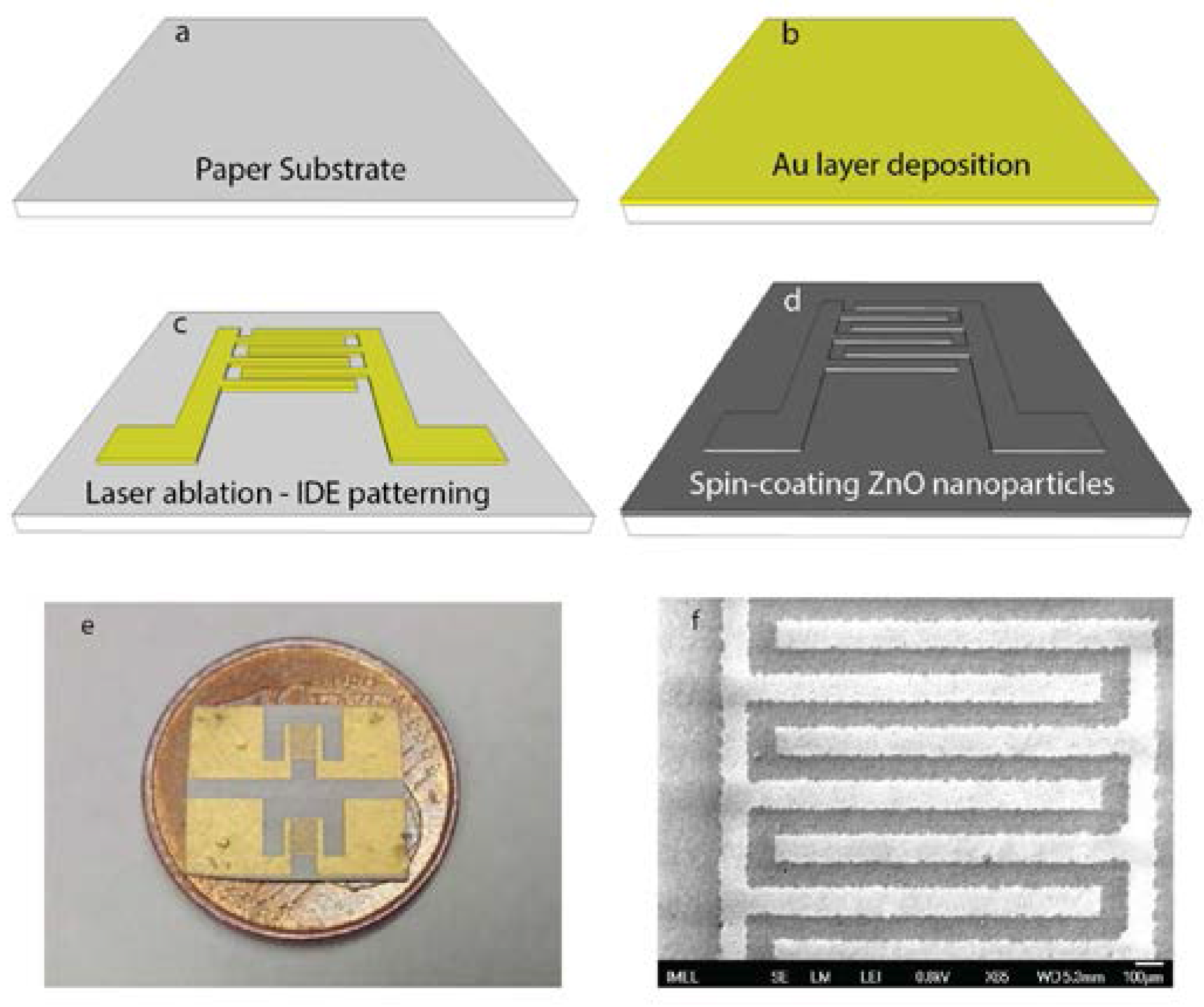
2.1. Fabrication of Paper-Based Devices
2.2. Preparation of ZnO Nanoparticles
2.3. Measurement Setup
3. Results and Discussion
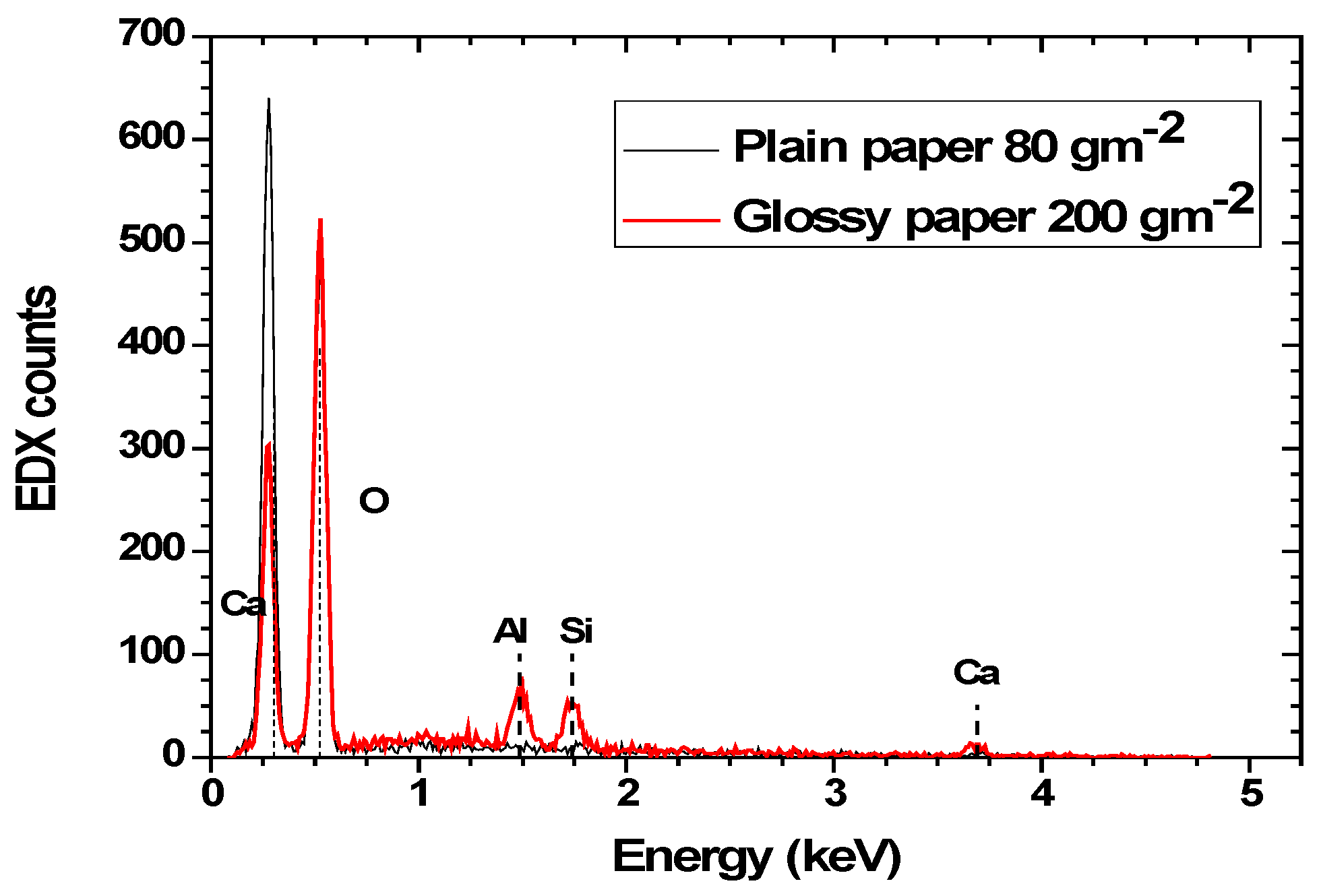
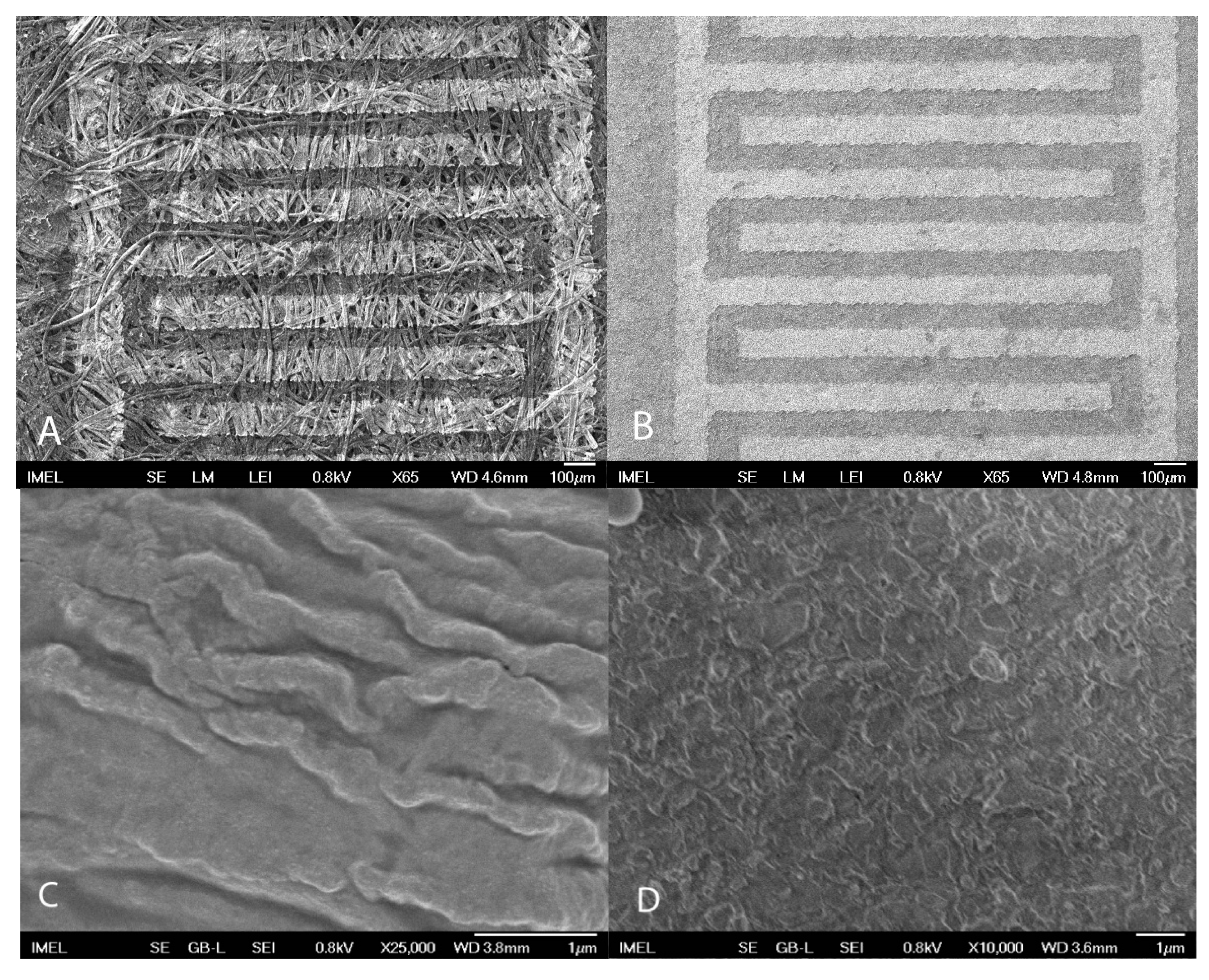
3.1. Structural Characteristics of Uncoated Devices
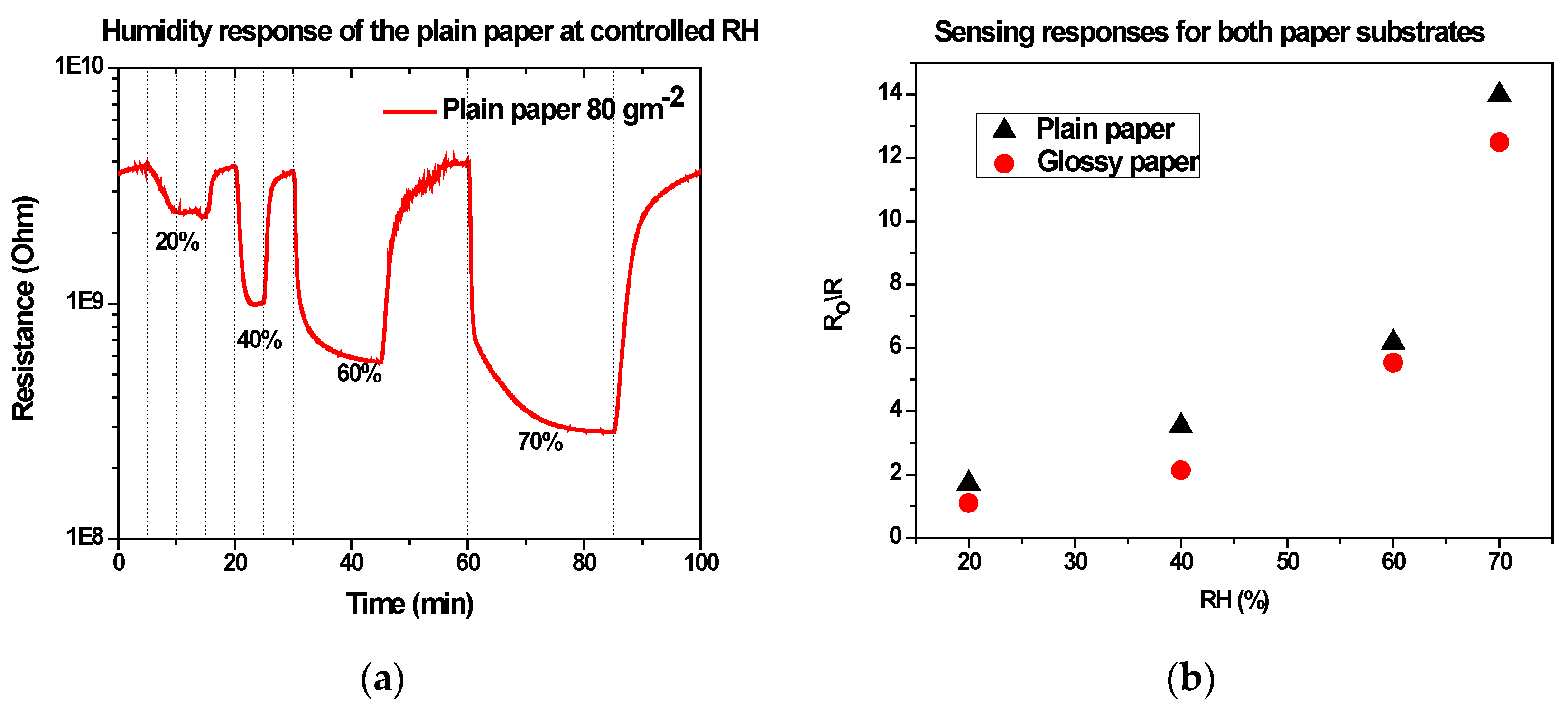
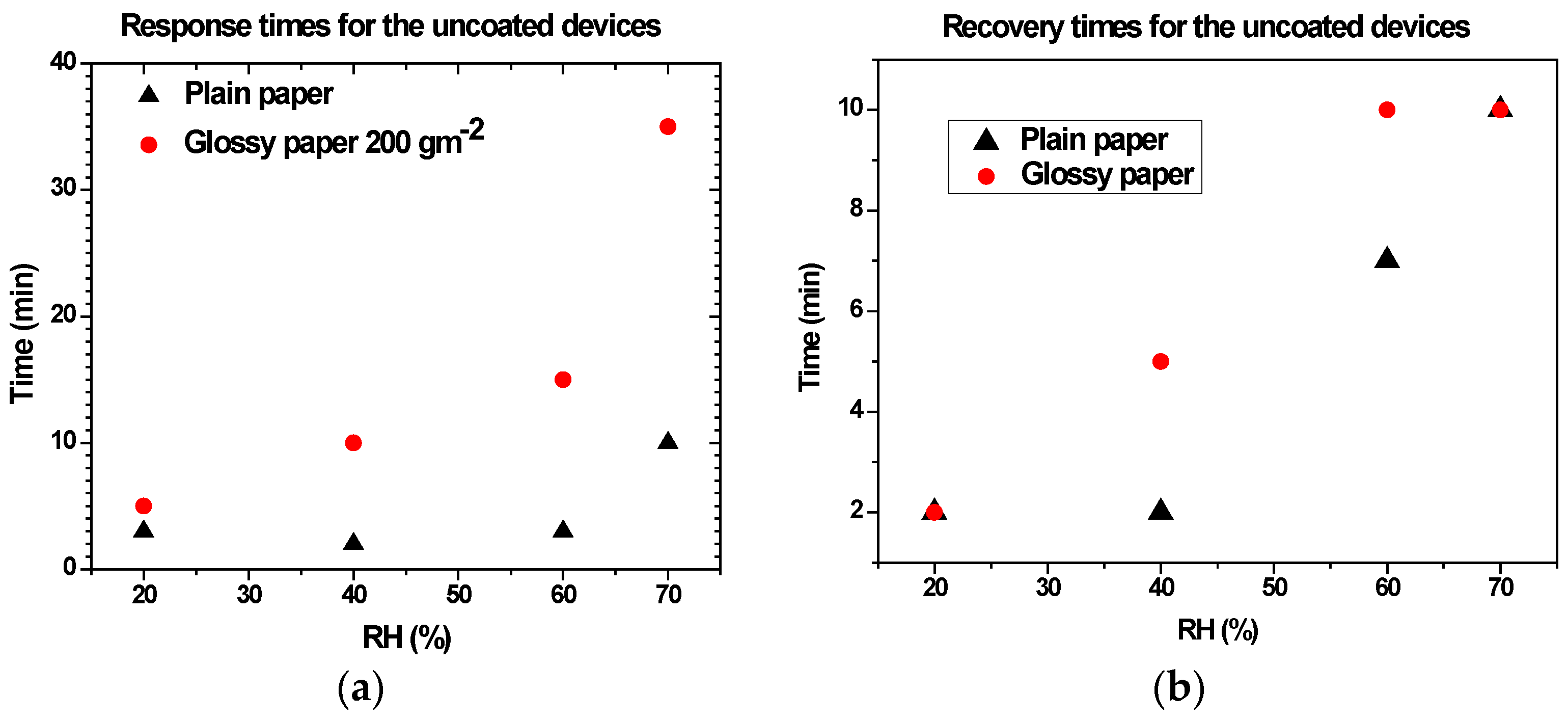
3.2. Humidity Characteristics of Uncoated Devices
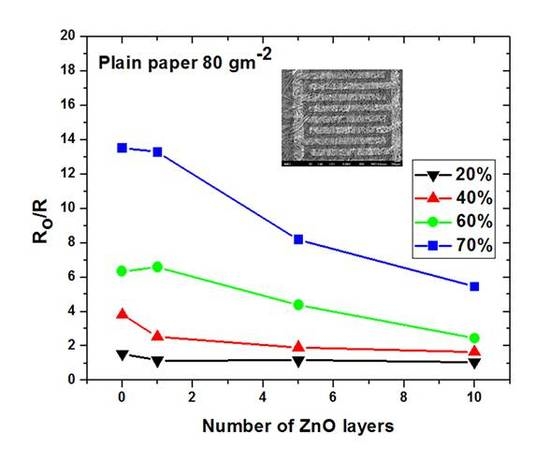
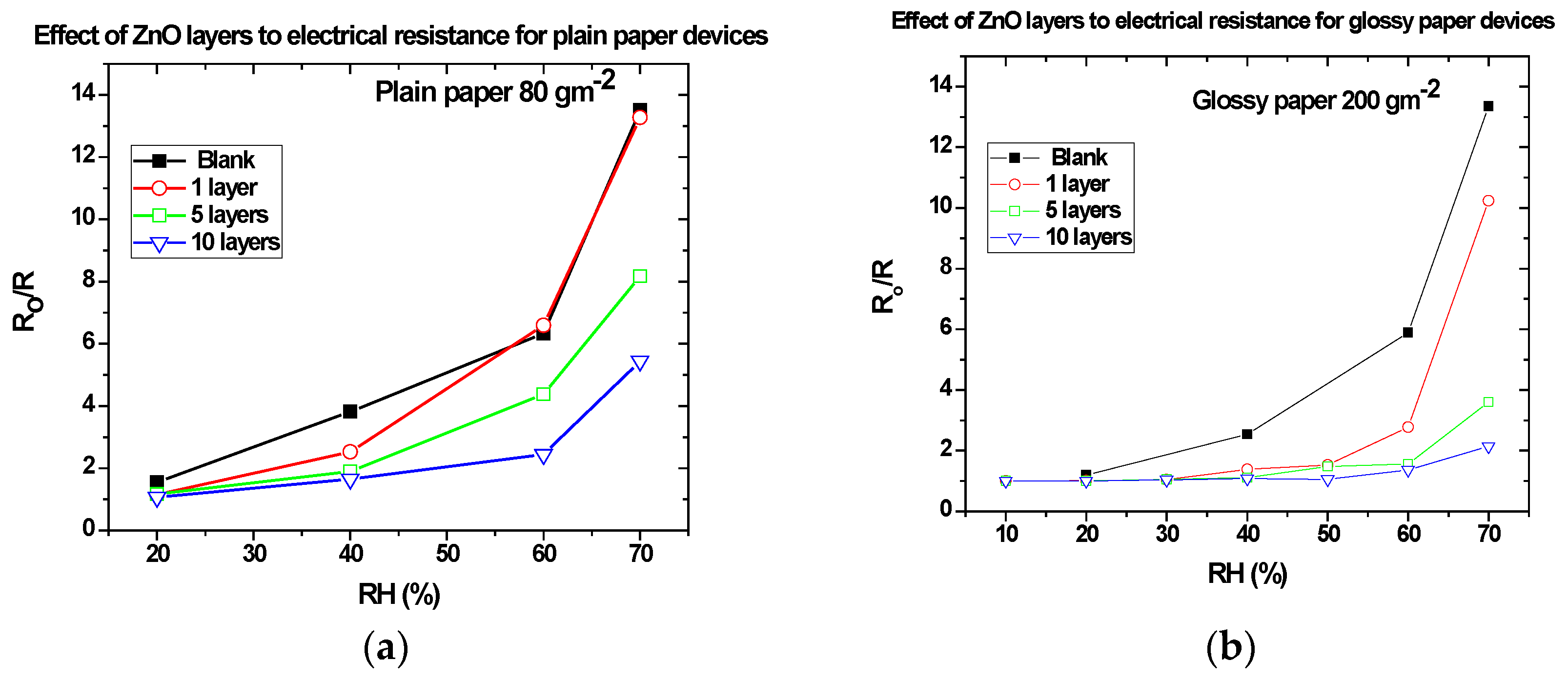
3.3. Effect of ZnO Nanoparticles on the Sensitivity of the Paper-Based Devices
4. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Huang, G.W.; Feng, Q.P.; Xiao, H.M.; Li, N.; Fu, S.Y. Rapid Laser Printing of Paper-based Multilayer Circuits. ACS Nano 2016, 10, 8895–8903. [Google Scholar] [CrossRef] [PubMed]
- Hübler, A.; Trnovec, B.; Zillger, T.; Ali, M.; Wetzold, N.; Mingebach, M.; Wagenpfahl, A.; Deibel, C.; Dyakono, V. Printed Paper Photovoltaic Cells. Adv. Mater. 2011, 1, 1018–1022. [Google Scholar] [CrossRef]
- Liana, D.D.; Raguse, B.; Gooding, J.J.; Chow, E. Recent advances in paper-based sensors. Sensors 2012, 12, 11505–11526. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Fraiwan, A.; Choi, S. Paper-based batteries: A review. Biosens. Bioelectron. 2014, 54, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Cui, Y.; Karabulut, E.; Wågberg, L.; Zhu, H.; Hu, L. Nanostructured paper for flexible energy and electronic devices. MRS Bull. 2013, 38, 320–325. [Google Scholar] [CrossRef]
- Sarfraz, J.; Ihalainen, P.; Määttänen, A.; Peltonen, J.; Lindén, M. Printed hydrogen sulfide gas sensor on paper substrate based on polyaniline composite. Thin Solid Films 2013, 534, 621–628. [Google Scholar] [CrossRef]
- Hardman, D.J.; Slater, J.H.; Reid, A.G.; Lang, W.K.; Jackson, J.R. Biochemical and Immunochemical Assay Device. U.S. Patent 6,573,108, 3 June 2003. [Google Scholar]
- Hossain, S.M.; Brennan, J.D. β-Galactosidase-based colorimetric paper sensor for determination of heavy metals. Anal. Chem. 2011, 83, 8772–8778. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Vak, D.; Clark, N.; Subbiah, J.; Wong, W.W.H.; Jones, D.J.; Watkins, S.E.; Wilson, G. Organic photovoltaic modules fabricated by an industrial gravure printing proofer. Sol. Energy Mater. Sol. C 2013, 109, 47–55. [Google Scholar] [CrossRef]
- Arena, A.; Donato, N.; Saitta, G.; Bonavita, A.; Rizzo, G.; Neri, G. Flexible ethanol sensors on glossy paper substrates operating at room temperature. Sens. Actuators B Chem. 2010, 145, 488–494. [Google Scholar] [CrossRef]
- Sarfraz, J.; Määttänen, A.; Ihalainen, P.; Keppeler, M.; Lindén, M.; Peltonen, J. Printed copper acetate based H2S sensor on paper substrate. Sens. Actuators B Chem. 2012, 173, 868–873. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Voznyy, O.; Hu, L.; Fu, Q.; Zhou, D. Physically flexible, rapid-response gas sensor based on colloidal quantum dot solids. Adv. Mater. 2014, 26, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Comini, E.; Sberveglieri, G. Metal oxide nanowires as chemical sensors. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Segev-Bar, M.; Haick, H. Flexible Sensors Based on Nanoparticles. ACS Nano 2013, 7, 8366–8378. [Google Scholar] [CrossRef] [PubMed]
- Bearzotti, A.; Macagnano, A.; Pantalei, S.; Zampetti, E.; Venditti, I.; Fratoddi, I.; Russo, M.V. Alcohol vapor sensory properties of nanostructured conjugated polymers. J. Phys. Condes. Matter 2008, 20, 474207–474212. [Google Scholar] [CrossRef]
- Fratoddi, I.; Bearzotti, A.; Venditti, I.; Cametti, C.; Russo, M.V. Role of nanostructured polymers on the improvement of electrical response-based relative humidity sensors. Sens. Actuators B Chem. 2016, 225, 96–108. [Google Scholar] [CrossRef]
- Mraović, M.; Muck, T.; Pivar, M.; Trontelj, J.; Pleteršek, A. Humidity Sensors Printed on Recycled Paper and Cardboard. Sensors 2014, 14, 13628–13643. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Xie, L.; Chen, Q.; Zheng, L.R. Low-cost printed chipless RFID humidity sensor tag for intelligent packaging. IEEE Sens. J. 2015, 15, 3201–3208. [Google Scholar] [CrossRef]
- Chitnis, G.; Ziaie, B. Waterproof Active Paper via Laser Surface Micropatterning of Magnetic Nanoparticles. ACS Appl. Mater. Interfaces 2012, 4, 4435–4439. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Tang, J.; Li, J.; Xiao, H. Plasma-induced polymerization for enhancing paper hydrophobicity. Carnohydr. Polym. 2013, 92, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Ogihara, H.; Xie, J.; Okagaki, J.; Saji, T. Simple method for preparing superhydrophobic paper: spray-deposited hydrophobic silica nanoparticle coatings exhibit high water-repellency and transparency. Langmuir 2012, 28, 4605–4608. [Google Scholar] [CrossRef] [PubMed]
- Stanssens, D.; Van den Abbeele, H.; Vonck, L.; Schoukens, G.; Deconinck, M.; Samyn, P. Creating water-repellent and super-hydrophobic cellulose substrates by deposition of organic nanoparticle. Mater. Lett. 2011, 65, 1781–1784. [Google Scholar] [CrossRef]
- Bollstrom, R.; Pettersson, F.; Dolietis, P.; Preston, J.; Osterbacka, R.; Toivakka, M. Impact of humidity on functionality of on-paper printed electronics. Nanotechnology 2014, 25, 094003. [Google Scholar] [CrossRef] [PubMed]
- Carmona-Ribeiro, A.M. Biomimetic nanoparticles: Preparation, characterization and biomedical applications. Int. J. Nanomed. 2010, 5, 249–259. [Google Scholar] [CrossRef]
- Koutzagioti, C.; Ntalos, G.; Tsamis, C.; Makarona, E. Low-cost multi-functional bioinspired wood coatings based on ZnO nanostructures. In Proceedings of the E-MRS 2015 Spring Meeting, Lille, France, 11–15 May 2015.
- Makarona, E.; Skoulikidou, M.C.; Kyrasta, T.; Smyrnakis, A.; Zeniou, A.; Gogolides, E.; Tsamis, C. Controllable fabrication of bioinspired three-dimensional ZnO/Si nanoarchitectures. Mater. Lett. 2015, 142, 211–216. [Google Scholar] [CrossRef]
- Niarchos, G. Design, Fabrication and Characterization of a Vibrational-Driven Piezoelectric Microgenerator. Ph.D. Thesis, National and Kapodistrian University of Athens, Athens, Greece, 2013. [Google Scholar]
- Gurav, K.V.; Gang, M.G.; Shin, S.W.; Patil, U.M.; Desmukh, P.R.; Agawane, G.L.; Suryawashi, M.P.; Pawar, S.M.; Patil, P.S.; Lokhande, C.D.; et al. Gas sensing properties of hydrothermally grown ZnOnanorods with different aspect ratios. Sens. Actuators B Chem. 2014, 190, 439–445. [Google Scholar] [CrossRef]
- Venditti, I.; Barbero, N.; Russo, M.V.; Di Carlo, A.; Decker, F.; Fratoddi, I.; Barolo, C.; Dini, D. Electrodeposited ZnO with squaraine sensitizers as photoactive anode of DSCs. Mater. Res. Express 2014, 1, 015040. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Yacoubi-Ouslim, A.; Wang, G.Y. Structure study of electrodeposited ZnO nanowires. Microelectron. J. 2005, 36, 625–628. [Google Scholar] [CrossRef]
- Guo, M.; Diao, P.; Wang, X.; Cai, S. The effect of hydrothermal growth temperature on preparation and photoelectrochemical performance of ZnO nanorod array films. J. Solid State Chem. 2005, 178, 3210–3215. [Google Scholar] [CrossRef]
- Oskam, G. Metal oxide nanoparticles: Synthesis, characterization and application. J. Sol-Gel Sci. Technol. 2006, 37, 161–164. [Google Scholar] [CrossRef]
- Hosono, E.; Fujihara, S.; Honma, I.; Zhou, H. The fabrication of an upright-standing zinc oxide nanosheet for use in dye-sensitized solar cells. Adv. Mater. 2005, 17, 2091–2094. [Google Scholar] [CrossRef]
- Saito, M.; Fujihara, S. Large photocurrent generation in dye-sensitized ZnO solar cells. Energy Environ. Sci. 2008, 1, 280–283. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Yun, S.; Kim, J. Flexible humidity and temperature sensor based on cellulose–polypyrrolenanocomposite. Sens. Actuators A Phys. 2011, 165, 194–199. [Google Scholar] [CrossRef]
- Han, J.W.; Kim, B.; Li, J.; Meyyappan, M. Carbon Nanotube Based Humidity Sensor on Cellulose Paper. J. Phys. Chem. C 2012, 116, 22094–22097. [Google Scholar] [CrossRef]
- Balde, M.; Vena, A.; Sorli, B. Fabrication of porous anodic aluminium oxide layers on paper for humidity sensors. Sens. Actuators B Chem. 2015, 220, 829–839. [Google Scholar] [CrossRef]
- Amin, E.M.; Bhuiyan, M.S.; Karmakar, N.C. Development of a low cost printable chipless RFID humidity sensor. IEEE Sens. J. 2014, 14, 140–149. [Google Scholar] [CrossRef]

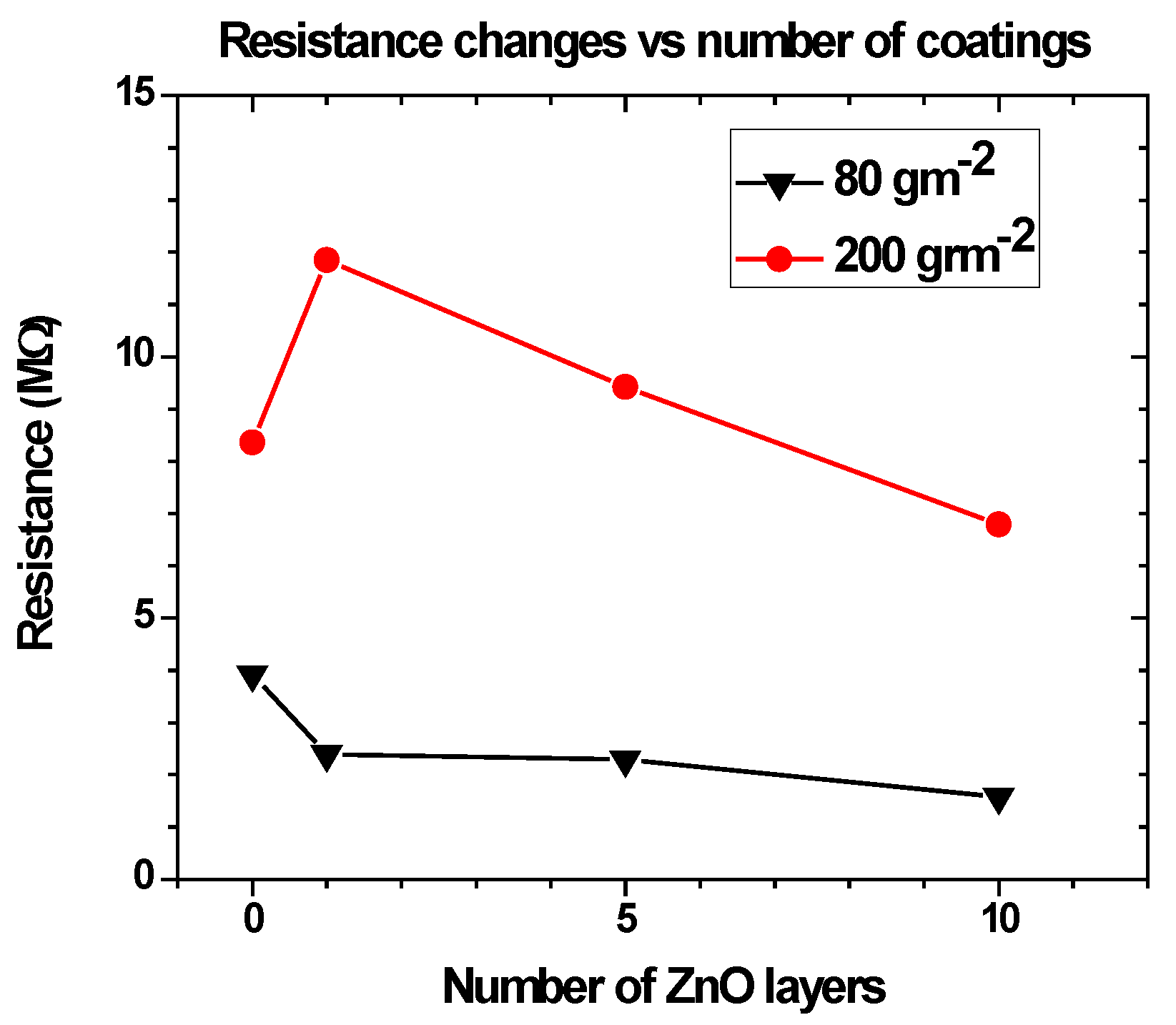
| Reference | Year | Sensing Material | Minimum RH (%) | Response/Recovery time |
|---|---|---|---|---|
| [35] | 2011 | Polypyrrole | 40 | 418 s/418 s |
| [36] | 2012 | Carbonnanotube | 20 | 6 s/120 s |
| [37] | 2014 | Aluminumoxide | 25 | / |
| [38] | 2015 | PVA | 50 | / |
| [18] | 2015 | Paper | 30 | 4 min/6 min |
| This work | 2016 | Porouspaper | 20 | 1 min/2–10 min |
| This work | 2016 | Smooth paper | 40 | 10 min/8 min |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niarchos, G.; Dubourg, G.; Afroudakis, G.; Georgopoulos, M.; Tsouti, V.; Makarona, E.; Crnojevic-Bengin, V.; Tsamis, C. Humidity Sensing Properties of Paper Substrates and Their Passivation with ZnO Nanoparticles for Sensor Applications. Sensors 2017, 17, 516. https://doi.org/10.3390/s17030516
Niarchos G, Dubourg G, Afroudakis G, Georgopoulos M, Tsouti V, Makarona E, Crnojevic-Bengin V, Tsamis C. Humidity Sensing Properties of Paper Substrates and Their Passivation with ZnO Nanoparticles for Sensor Applications. Sensors. 2017; 17(3):516. https://doi.org/10.3390/s17030516
Chicago/Turabian StyleNiarchos, Georgios, Georges Dubourg, Georgios Afroudakis, Markos Georgopoulos, Vasiliki Tsouti, Eleni Makarona, Vesna Crnojevic-Bengin, and Christos Tsamis. 2017. "Humidity Sensing Properties of Paper Substrates and Their Passivation with ZnO Nanoparticles for Sensor Applications" Sensors 17, no. 3: 516. https://doi.org/10.3390/s17030516