Development of Fluorescent FRET Probes for “Off-On” Detection of L-Cysteine Based on Gold Nanoparticles and Porous Silicon Nanoparticles in Ethanol Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Instruments
2.3. Sythesis of Citrate-Stabilized AuNPs
2.4. Sythesis of Amino-Capped SiNPs
2.5. Procedures for Fluorescence Detection of L-Cys
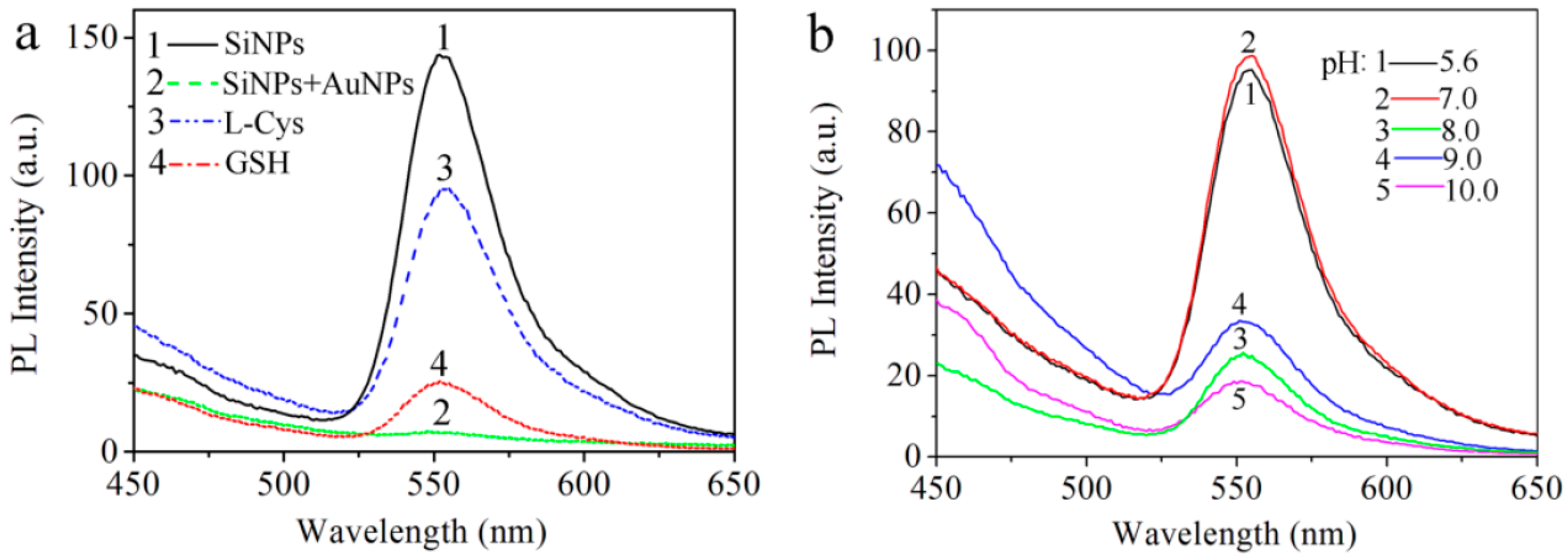
3. Results and Discussion
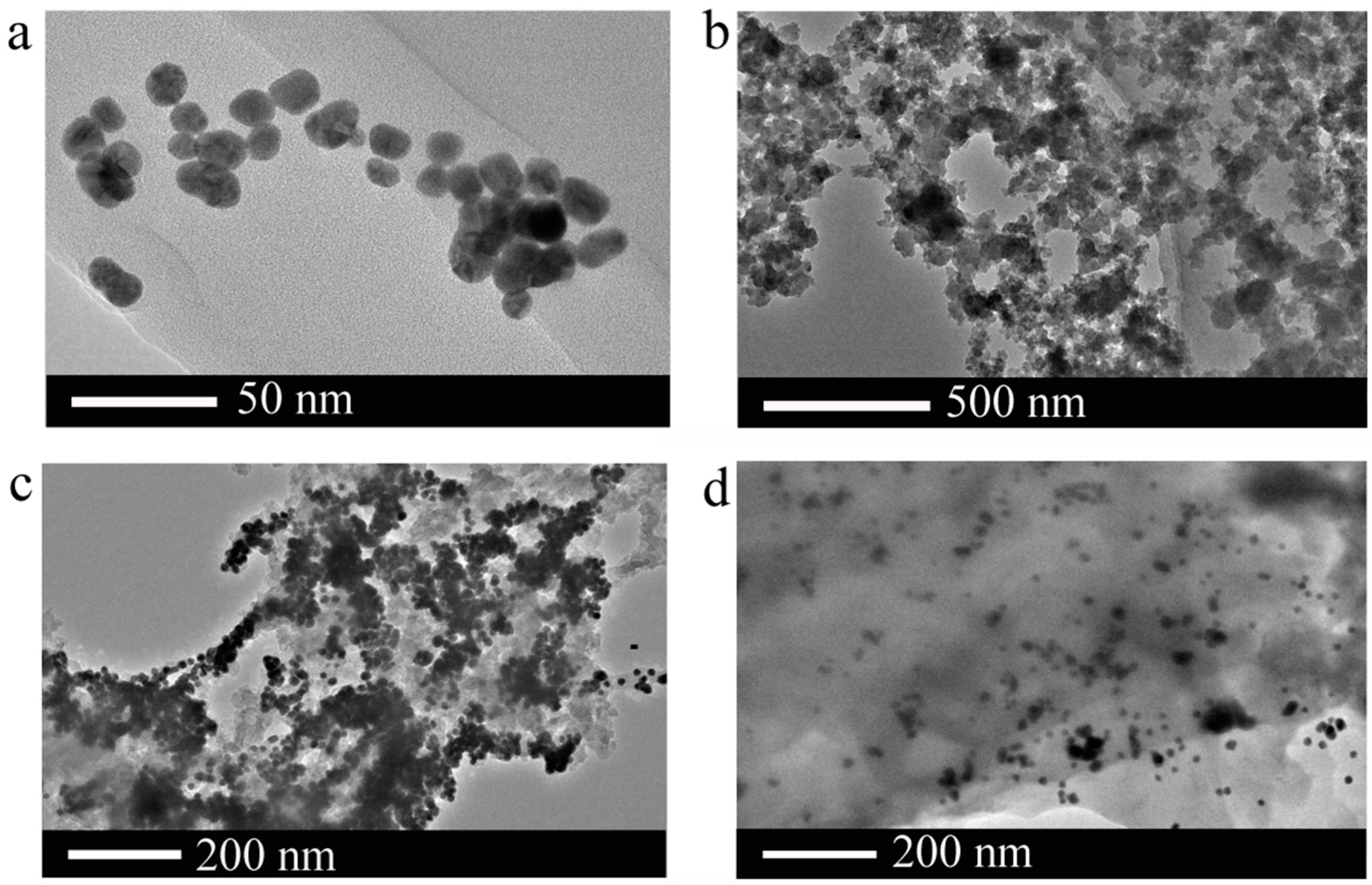
3.1. Characterization of AuNPs and SiNPs
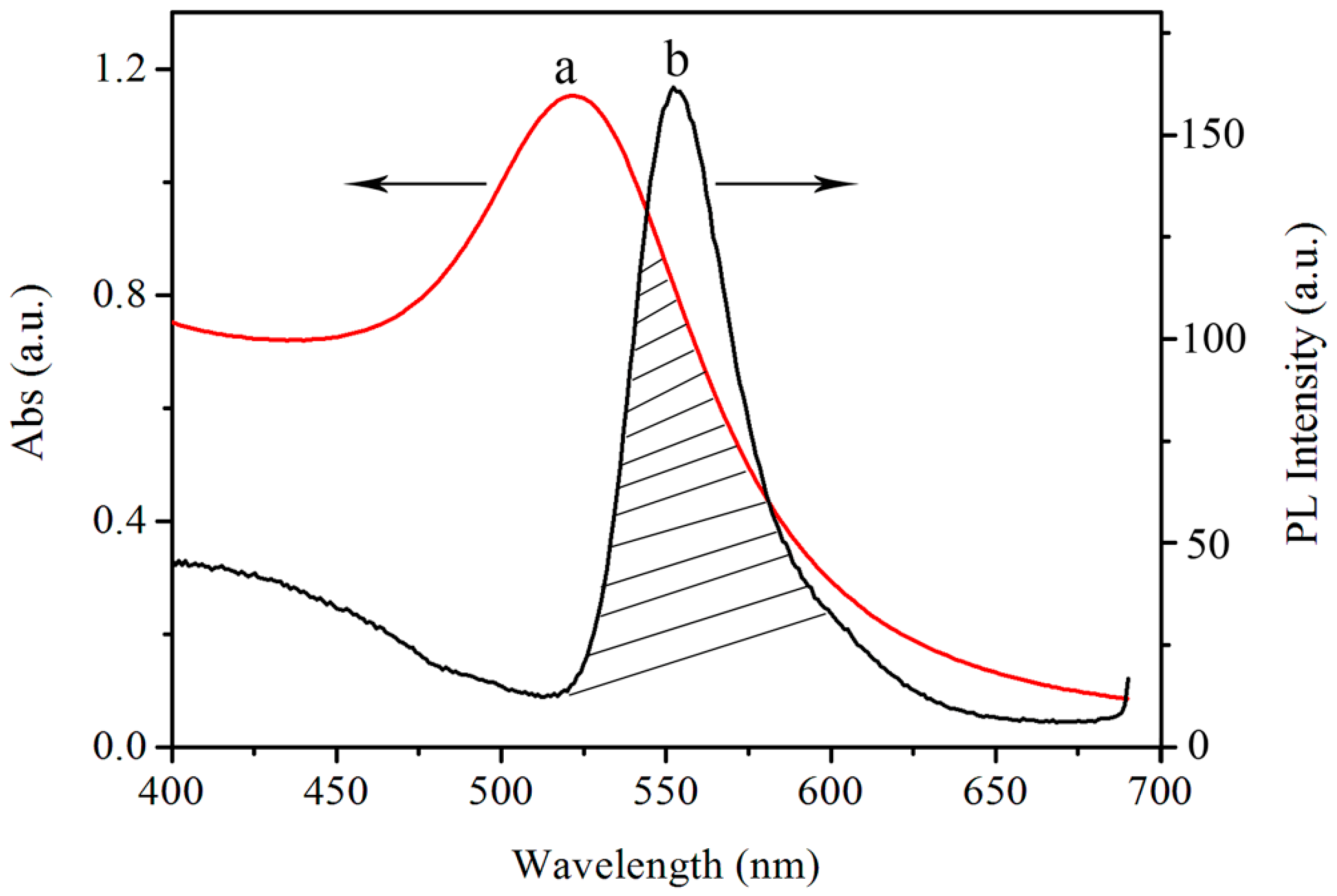
3.2. Optical Characteristics of AuNPs and SiNPs-Ethanol
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kawano, Y.; Ohtsu, I.; Tamakoshi, A.; Shiroyama, M.; Tsuruoka, A.; Saiki, K.; Takumi, K.; Nonaka, G.; Nakanishi, T.; Hishiki, T.; et al. Involvement of the yciW gene in L-cysteine and L-methionine metabolism in Escherichia coli. J. Biosci. Bioeng. 2015, 119, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Ghazaryan, V.V.; Minkov, V.S.; Boldyreva, E.V.; Petrosyan, A.M. L-Cysteine halogenides: A new family of salts with an L-cysteine/Lcysteinium dimeric cation. J. Mol. Struct. 2016, 1121, 60–69. [Google Scholar] [CrossRef]
- Falkowski, M.; Rebis, T.; Kryjewski, M.; Popenda, L.; Lijewski, S.; Jurga, S.; Mielcarek, J.; Milczarek, G.; Goslinski, T. An enhanced electrochemical nanohybrid sensing platform consisting of reduced graphene oxide and sulfanyl metalloporphyrazines for sensitive determination of hydrogen peroxide and L-cysteine. Dyes Pigments 2017, 138, 190–203. [Google Scholar] [CrossRef]
- Yang, S.; Li, G.; Liu, L.; Wang, G.; Wang, D.; Qu, L. Preparation of nickel oxide nanoparticles on N-doped reduced graphene oxide: A two-dimensional hybrid for electrocatalytic sensing of L-cysteine. J. Alloys Compd. 2017, 691, 834–840. [Google Scholar] [CrossRef]
- Aswini, K.K.; Mohan, A.M.V.; Biju, V.M. Molecularly imprinted polymer based electrochemical detection of L-cysteine at carbon paste electrode. Mater. Sci. Eng. C 2014, 37, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Luo, J.; Guo, Y.; Kou, J.; Li, B.; Zhang, Z. Nanoparticle coated paper-based chemiluminescence device for the determination of L-cysteine. Talanta 2014, 120, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tricard, S.; Yue, P.; Zhao, J.; Fang, J.; Shen, W. Polypyrrole and graphene quantum dots @ Prussian blue hybrid film on graphite felt electrodes: Application for amperometric determination of L-cysteine. Biosens. Bioelectron. 2016, 77, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, H.; Pei, M.; Zhang, G.; Hu, L.; Han, J. A new fluorescence “off–on” chemodosimeter for L-cysteine based on water-soluble polythiophene. Talanta 2013, 115, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Zong, J.; Yang, X.; Trinchi, A.; Hardin, S.; Cole, I.; Zhu, Y.; Li, C.; Muster, T.; Wei, G. Carbon dots as fluorescent probes for “off-on” detection of Cu2+ and L-cysteine in aqueous solution. Biosens. Bioelectron. 2014, 51, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Huo, F.; Lia, J.; Chao, J.; Zhang, Y.; Yin, C. An off-on fluorescent probe for specifically detecting cysteine and its application in bioimaging. Sens. Actuators B Chem. 2016, 237, 127–132. [Google Scholar] [CrossRef]
- Liu, X.; Luo, L.; Ding, Y.; Kang, Z.; Ye, D. Simultaneous determination of L-cysteine and L-tyrosine using Au-nanoparticles/poly-eriochrome black T film modified glassy carbon electrode. Bioelectrochemistry 2012, 86, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chan, C.; Pang, Y.; Ye, W.; Tian, F.; Lyu, J.; Zhang, Y.; Yang, M. A fluorescence resonance energy transfer (FRET) biosensor based on graphene quantumdots (GQDs) and gold nanoparticles (AuNPs) for the detection of mecA gene sequence of Staphylococcus aureus. Biosens. Bioelectron. 2015, 67, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Bu, D.; Zhuang, H.; Yang, G.; Ping, X. An immunosensor designed for polybrominated biphenyl detection based on fluorescence resonance energy transfer (FRET) between carbon dots and gold nanoparticles. Sens. Actuators B Chem. 2014, 195, 540–548. [Google Scholar] [CrossRef]
- Ahn, K.S.; Lim, K.R.; Jeong, D.; Lee, B.Y.; Kim, K.S.; Lee, W.Y. Fluorescence energy transfer inhibition bioassay for cholera toxin based on galactose-stabilized gold nanoparticles and amine-terminated quantum dots. Microchem. J. 2016, 124, 9–14. [Google Scholar] [CrossRef]
- Xu, X.; Qiao, J.; Li, N.; Qi, L.; Zhang, S. Fluorescent probe for turn-on sensing of L-cysteine by ensemble of AuNCs and polymer protected AuNPs. Anal. Chim. Acta 2015, 879, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.Y.; Deng, N.; Liang, J.J.; Zhou, K.N.; Fu, Q.Q.; Tang, Y. A fluorescent polymer dots positive readout fluorescent quenching lateral flow sensor for ractopamine rapid detection. Anal. Chim. Acta 2015, 854, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Murugavelu, M.; Karthikeyan, B. Study of Ag-Pd bimetallic nanoparticles modified glassy carbon electrode for detection of L-cysteine. Superlattices Microstruct. 2014, 75, 916–926. [Google Scholar] [CrossRef]
- Shankar, S.; John, S.A. 4-Amino-6-hydroxy-2-mercaptopyrimidine capped gold nanoparticles as fluorophore for the ultrasensitive and selective determination of L-cysteine. Sens. Actuators B Chem. 2015, 221, 1202–1208. [Google Scholar] [CrossRef]
- Pandey, P.C.; Pandey, A.K.; Chauhan, D.S. Nanocomposite of Prussian blue based sensor for L-cysteine: Synergetic effect of nanostructured gold and palladium on electrocatalysis. Electrochim. Acta 2012, 74, 23–31. [Google Scholar] [CrossRef]
- Saha, J.; Dey, D.; Roy, A.D.; Bhattacharjee, D.; Hussain, S.A. Multi step FRET among three laser dyes Pyrene, Acriflavine and Rhodamine B. J. Lumin. 2016, 172, 168–174. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, Y.; Luo, Y.; Shen, F.; Sun, C. Efficient fluorescence resonance energy transfer between oppositely charged CdTe quantum dots and gold nanoparticles for turn-on fluorescence detection of glyphosate. Talanta 2014, 125, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, Q.; Liu, Z.; Wang, X.; Su, X. A novel enzyme-mimic nanosensor based on quantum dot-Au nanoparticle@silica mesoporous microsphere for the detection of glucose. Anal. Chim. Acta 2014, 840, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Peng, X.; Li, H.; Zhang, Y.; Yao, S. On-off-on fluorescent silicon nanoparticles for recognition of chromium(VI) and hydrogen sulfide based on the inner filter effect. Sens. Actuators B Chem. 2017, 238, 196–203. [Google Scholar] [CrossRef]
- Baig, M.I.; Ingole, P.G.; Choi, W.K.; Jeon, J.; Jang, B.; Moon, J.H.; Lee, H.K. Synthesis and characterization of thin film nanocomposite membranes incorporated with surface functionalized Silicon nanoparticles for improved water vapor permeation performance. Chem. Eng. J. 2017, 308, 27–39. [Google Scholar] [CrossRef]
- Huang, S.; Xiao, Q.; Li, R.; Guan, H.L.; Liu, J.; Liu, X.R.; He, Z.K.; Liu, Y. A simple and sensitive method for L-cysteine detection based on the fluorescence intensity increment of quantum dots. Anal. Chim. Acta 2009, 645, 73–78. [Google Scholar] [CrossRef] [PubMed]


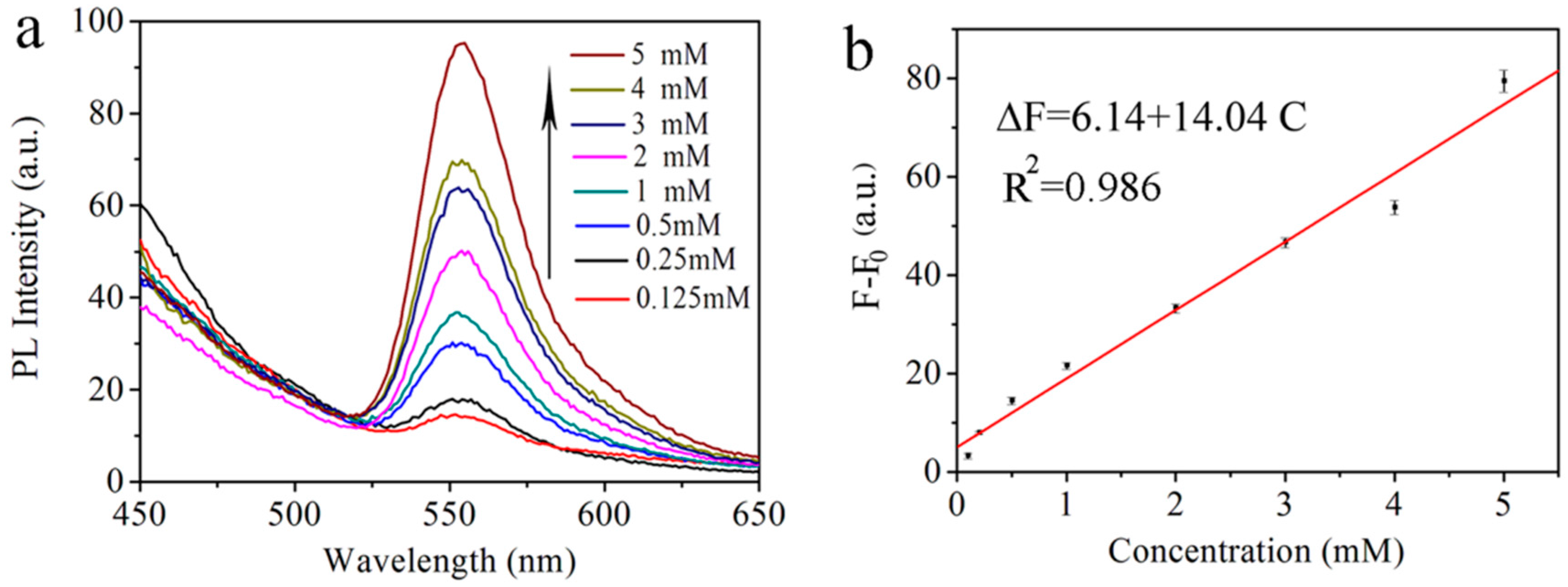
| Concentrations | ∆F (a.u.) | (a.u.) | s (a.u.) | RSD | ± s (a.u.) | ||
|---|---|---|---|---|---|---|---|
| ∆F1 | ∆F2 | ∆F3 | |||||
| L-Cys (0.125 mM) | 3.5 | 3.6 | 2.5 | 3.2 | 0.6 | 18.5% | 3.2 ± 0.6 |
| L-Cys (0.25 mM) | 7.6 | 8.0 | 8.0 | 7.9 | 0.3 | 3.8% | 7.9 ± 0.3 |
| L-Cys (0.5 mM) | 14.4 | 15.0 | 13.7 | 14.4 | 0.7 | 4.9% | 14.4 ± 0.7 |
| L-Cys (1.0 mM) | 21.2 | 21 | 22.4 | 21.5 | 0.7 | 3.2% | 21.5 ± 0.7 |
| L-Cys (2.0 mM) | 34.1 | 33.2 | 32.4 | 33.2 | 0.9 | 2.7% | 33.2 ± 0.9 |
| L-Cys (3.0 mM) | 46.4 | 47.7 | 45.6 | 46.6 | 1.0 | 2.1% | 46.6 ± 1.0 |
| L-Cys (4.0 mM) | 52.5 | 53.3 | 55.3 | 53.7 | 1.4 | 2.6% | 53.7 ± 1.4 |
| L-Cys (5.0 mM) | 78.2 | 77.9 | 82.1 | 79.4 | 2.3 | 2.9% | 79.4 ± 2.3 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Jia, Z. Development of Fluorescent FRET Probes for “Off-On” Detection of L-Cysteine Based on Gold Nanoparticles and Porous Silicon Nanoparticles in Ethanol Solution. Sensors 2017, 17, 520. https://doi.org/10.3390/s17030520
Zhang H, Jia Z. Development of Fluorescent FRET Probes for “Off-On” Detection of L-Cysteine Based on Gold Nanoparticles and Porous Silicon Nanoparticles in Ethanol Solution. Sensors. 2017; 17(3):520. https://doi.org/10.3390/s17030520
Chicago/Turabian StyleZhang, Hongyan, and Zhenhong Jia. 2017. "Development of Fluorescent FRET Probes for “Off-On” Detection of L-Cysteine Based on Gold Nanoparticles and Porous Silicon Nanoparticles in Ethanol Solution" Sensors 17, no. 3: 520. https://doi.org/10.3390/s17030520






