A Colorimetric Sensor for the Highly Selective Detection of Sulfide and 1,4-Dithiothreitol Based on the In Situ Formation of Silver Nanoparticles Using Dopamine
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Apparatus
2.3. Recommended Analytical Procedure
2.4. Colorimetric Sensing of S2− in Fetal Bovine Serum
3. Results and Discussion
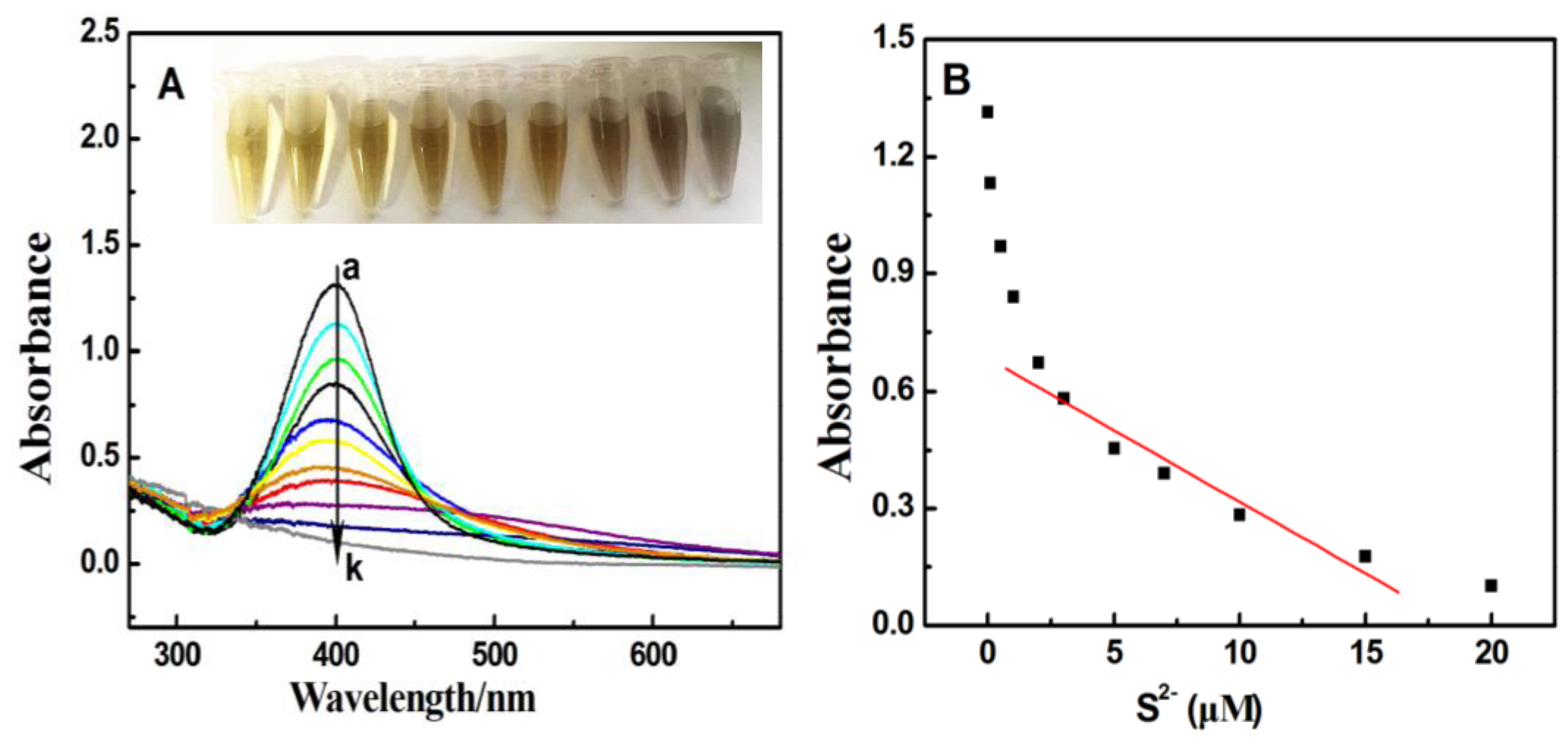
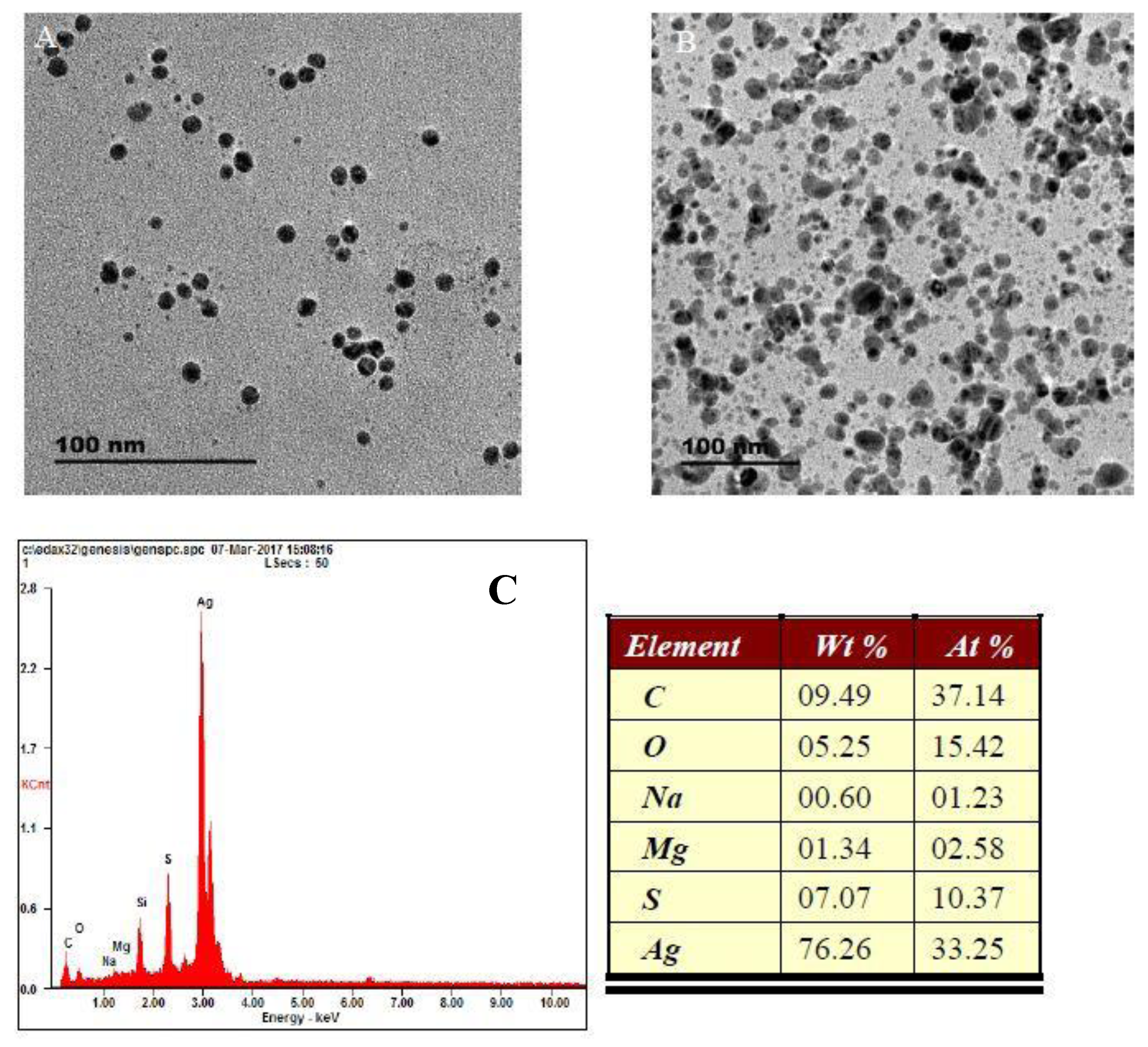
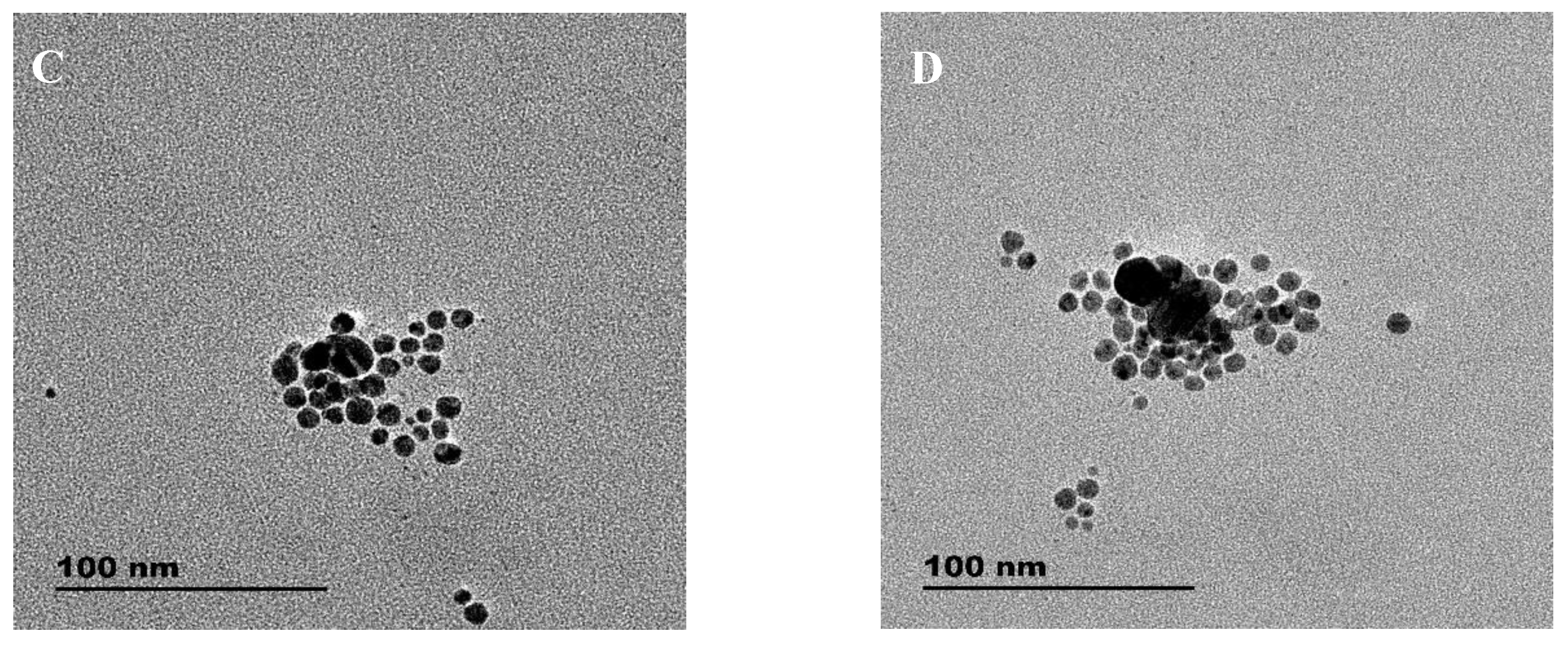
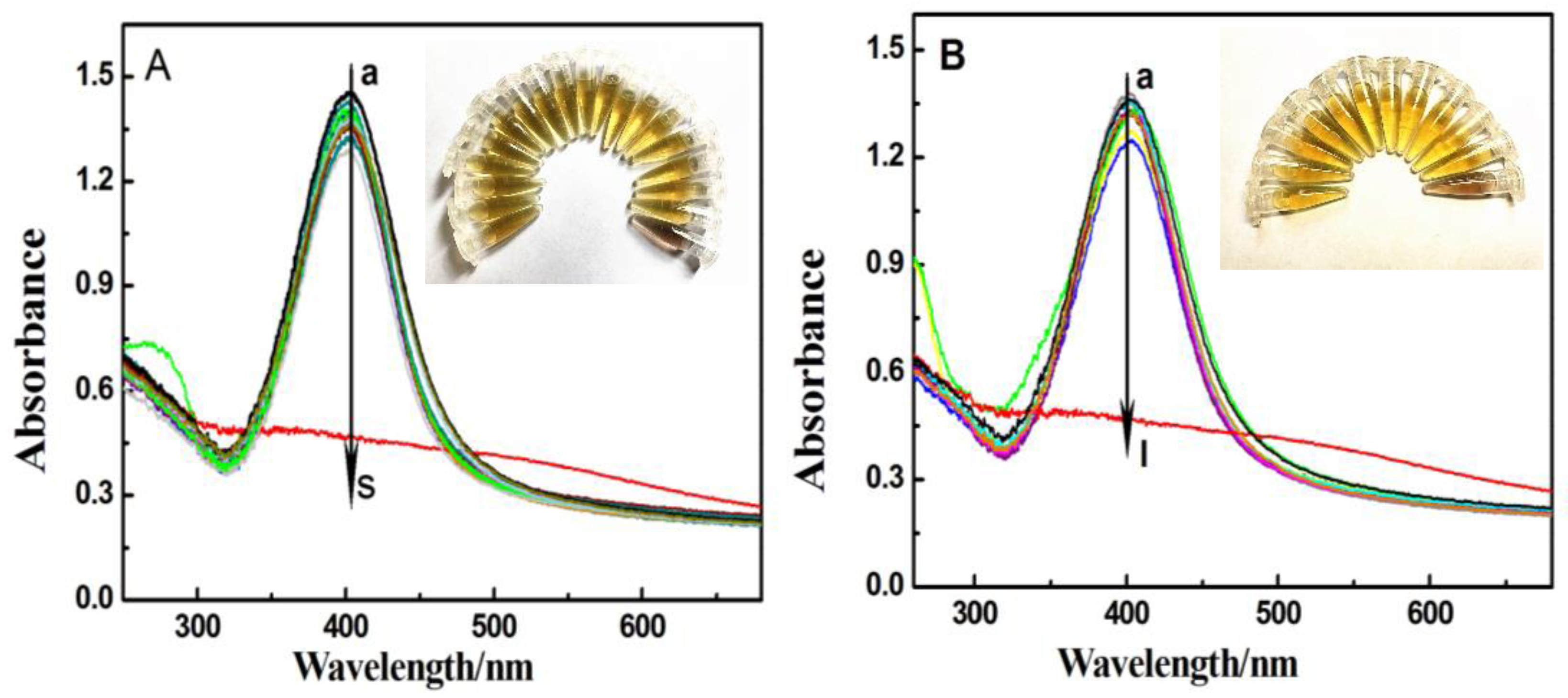
3.1. Absorption and TEM Studies of Silver/Dopamine Nanoparticles after Incubation with S2−
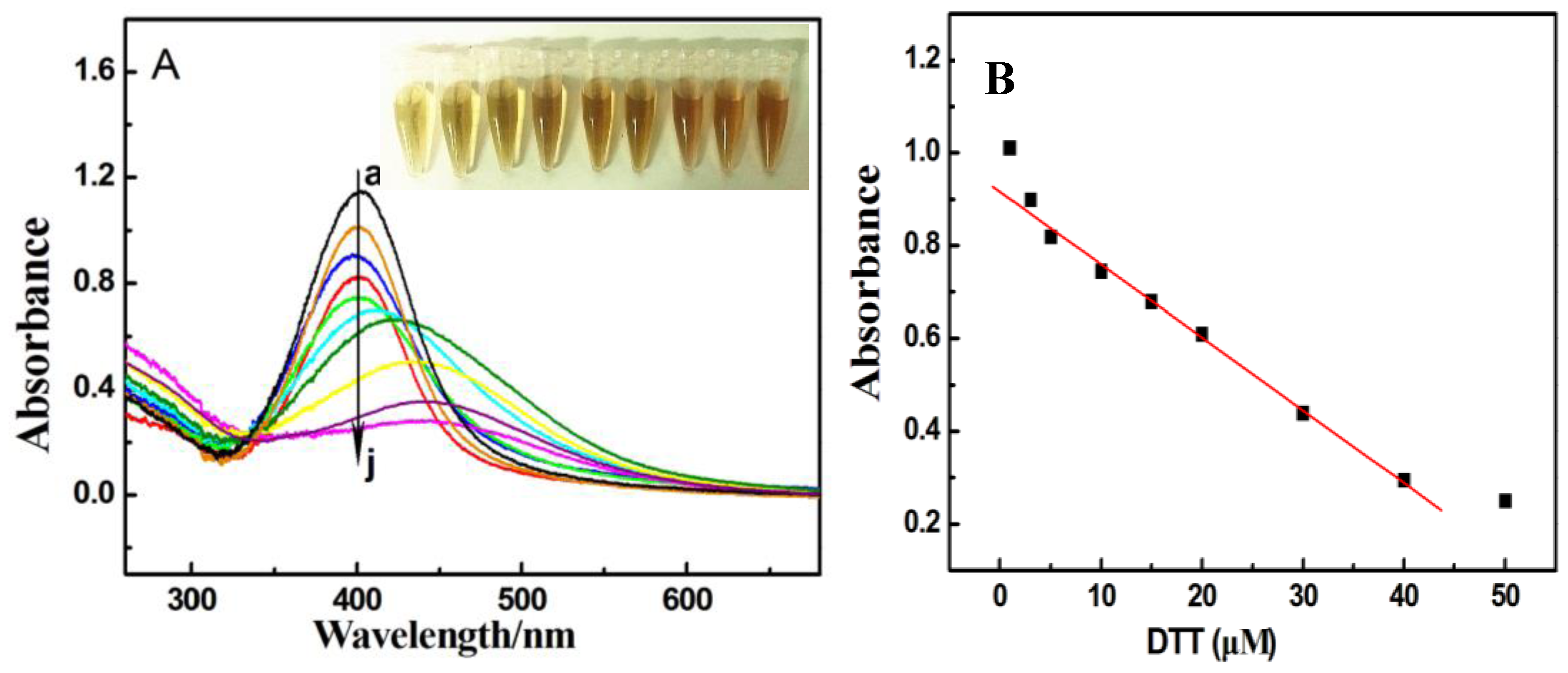
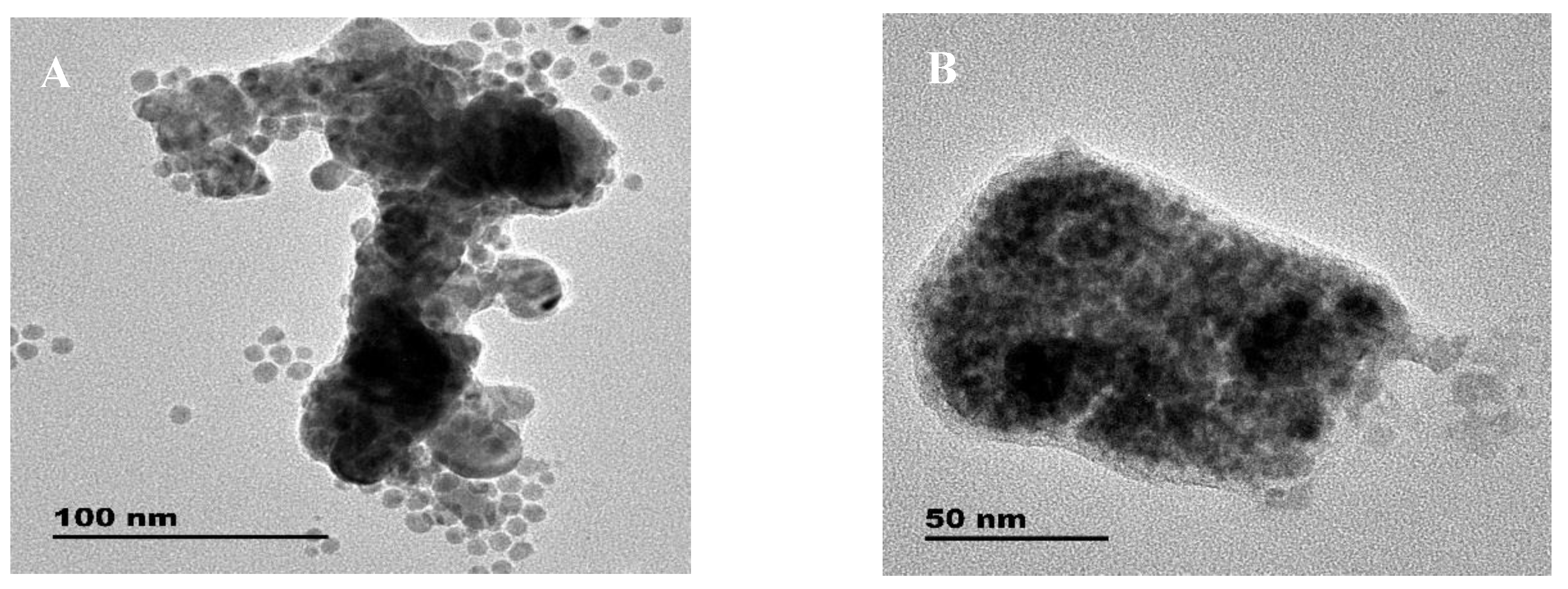
3.2. Absorption and TEM Studies of Silver/Dopamine Nanoparticles after Incubation with Thiol Compounds
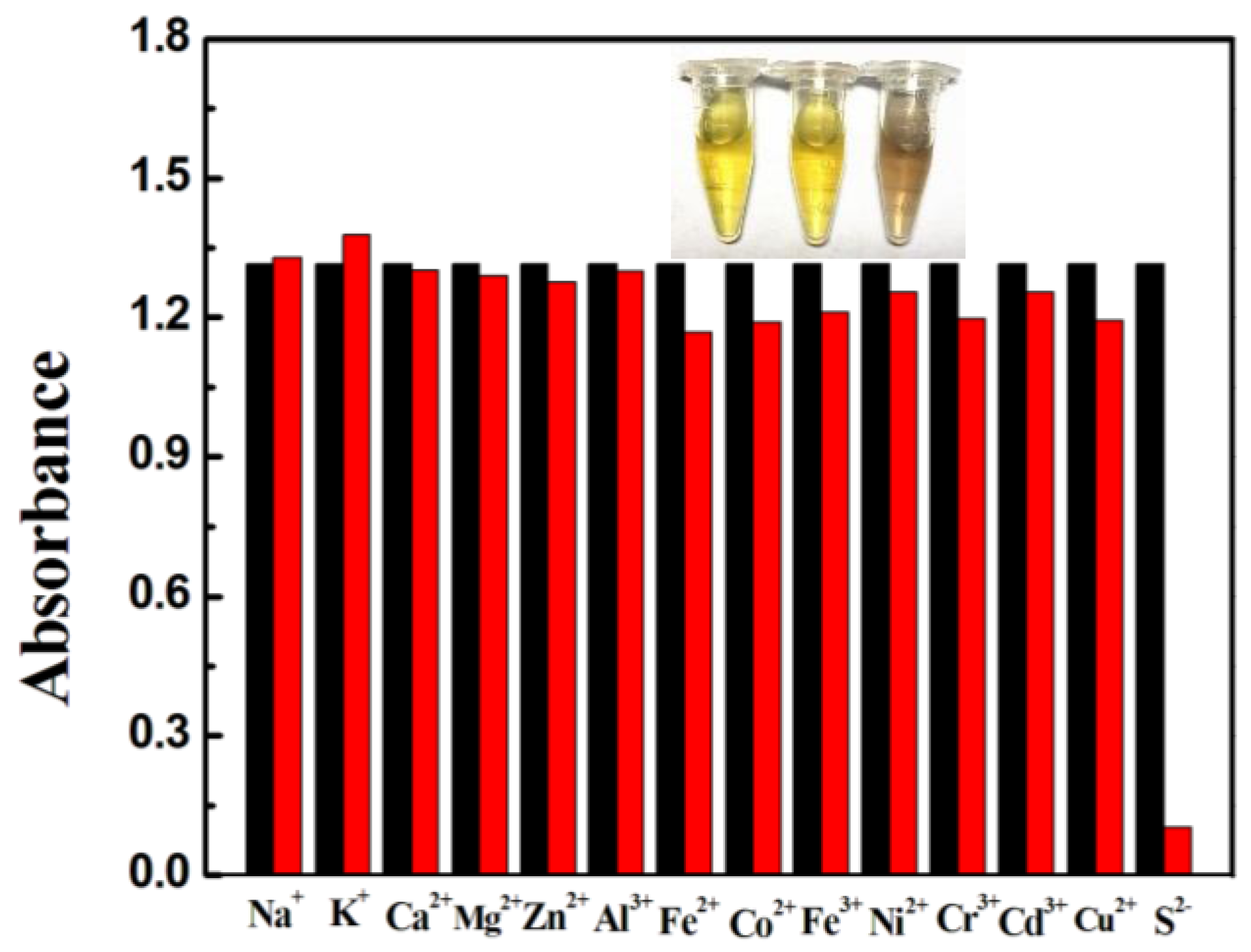
3.3. Interference Study
3.4. The Determination of S2− in Fetal Bovine Serum
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Doujaiji, B.; Al-Tawfiq, J.A. Hydrogen sulfide exposure in an adult male. Ann. Saudi Med. 2010, 30, 76–80. [Google Scholar] [PubMed]
- Bagarinao, T. Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquat. Toxicol. 1992, 24, 21–62. [Google Scholar] [CrossRef]
- Reiffenstein, R.J.; Hulbert, W.C.; Roth, S.H. Toxicology of Hydrogen Sulfide. Ann. Rev. Pharmacol. 1992, 32, 109–134. [Google Scholar] [CrossRef] [PubMed]
- Wallace, J.L. Hydrogen Sulfide: A Rescue Molecule for Mucosal Defence and Repair. Dig. Dis. Sci. 2012, 57, 1432. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.A. Hydrogen sulfide: A new gaseous signal molecule and blood pressure regulator. J. Nephrol. 2009, 22, 173–176. [Google Scholar] [PubMed]
- Wei, H.J.; Li, X.; Tang, X.-Q. Therapeutic benefits of H2S in Alzheimer’s disease. J. Clin. Neurosci. 2014, 21, 1665–1669. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.Y. A new hope for neurodegeneration: possible role of hydrogen sulfide. J. Alzheimers Dis. 2011, 24, 173–182. [Google Scholar]
- Ling, L.; Mohamed, M.S.B.; Moore, P.K. Multiple Roles of H2S in Inflammation: A New Class of Therapeutics. Hydrog. Sulfide Its Ther. Appl. 2013. [Google Scholar] [CrossRef]
- Tan, G.; Pan, S.H.; Li, J.; Dong, X.S.; Kang, K.; Zhao, M.Y.; Jiang, X.; Kanwar, J.R.; Qiao, H.Q.; Jiang, H.C.; et al. Hydrogen sulfide attenuates carbon tetrachloride-induced hepatotoxicity, liver cirrhosis and portal hypertension in rats. PLoS ONE 2011, 6, e25943. [Google Scholar] [CrossRef] [PubMed]
- Fiorucci, S.; Antonelli, E.; Mencarelli, A.; Orlandi, S.; Renga, B.; Rizzo, G.; Distrutti, E.; Shah, V.; Morelli, A. The third gas: H2S regulates perfusion pressure in both the isolated and perfused normal rat liver and in cirrhosis. Hepatology 2005, 42, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Kamoun, P.; Belardinelli, M.C.; Chabli, A.; Lallouchi, K.; Chadefaux-Vekemans, B. Endogenous hydrogen sulfide overproduction in Down syndrome. Am. J. Med. Genet. Part A 2003, 116, 310. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Tashkhourian, J.; Sharghi, H. Development of Sulfide-Selective Optode Membranes Based on Immobilization of Methylene Blue on Optically Transparent Triacetylcellulose Film. Instrum. Sci. Technol. 2005, 33, 703–714. [Google Scholar] [CrossRef]
- Dilgin, Y.; Canarslan, S.; Ayyildiz, O.; Ertek, B.; Nisli, G. Flow injection analysis of sulphide based on its photoelectrocatalytic oxidation at poly-methylene blue modified glassy carbon electrode. Electrochim. Acta 2012, 66, 173–179. [Google Scholar] [CrossRef]
- Colon, M.; Todolí, J.L.; Hidalgo, M.; Iglesias, M. Development of novel and sensitive methods for the determination of sulfide in aqueous samples by hydrogen sulfide generation-inductively coupled plasma-atomic emission spectroscopy. Anal. Chim. Acta 2008, 609, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Cmelík, J.; Machátb, J.; Otruba, V.; Kanicky, V. Contribution to vapor generation-inductively coupled plasma spectrometric techniques for determination of sulfide in water samples. Talanta 2010, 80, 1777–1781. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.J.; Liu, X.; Jin, X.J.; Wu, J.; Xie, Y.J.; Liu, W.S.; Yao, X.J.; Tang, Y. A retrievable and highly selective fluorescent sensor for detecting copper and sulfide. Sens. Actuators B Chem. 2013, 185, 125–131. [Google Scholar] [CrossRef]
- Lawrence, N.S.; Davis, J.; Compton, R.G. Analytical strategies for the detection of sulfide: A review. Talanta 2000, 52, 771. [Google Scholar] [CrossRef]
- Chen, W.Y.; Lan, G.Y.; Chang, H.T. Use of fluorescent DNA-templated gold/silver nanoclusters for the detection of sulfide ions. Anal. Chem. 2011, 83, 9450–9455. [Google Scholar] [CrossRef] [PubMed]
- Ardelean, M.; Manea, F.; Vaszilcsina, N.; Podea, R. Electrochemical detection of sulphide in water/seawater using nanostructured carbon-epoxy composite electrodes. Anal. Methods 2014, 6, 4775–4782. [Google Scholar] [CrossRef]
- Bond, A.M.; Heritage, I.D.; Wallace, G.G.; McCormick, M.J. Simultaneous determination of free sulfide and cyanide by ion chromatography with electrochemical detection. Anal. Chem. 1982, 54, 582–585. [Google Scholar] [CrossRef]
- Kaushik, R.; Kumar, P.; Ghosh, A.; Gupta, N.; Kaur, D.; Arora, S.; Amilan, D.J. Alizarin red S-Zinc(II) fluorescent ensemble for selective detection of hydrogen sulphide and assay with an H2S donor. Rsc. Adv. 2015, 5, 79309–79316. [Google Scholar] [CrossRef]
- Liang, Z.H.; Tsoi, T.H.; Chan, C.F.; Dai, L.X.; Wu, Y.D.; Du, G.Y.; Zhu, L.Z.; Lee, C.S.; Wong, W.T.; Law, G.L.; et al. A smart“off-on” gate for the in situ detection of hydrogen sulphide with Cu(II)-assisted europium emission. Chem. Sci. 2015, 7, 2151–2156. [Google Scholar] [CrossRef]
- Meng, Q.T.; Zhang, R.; Jia, H.M.; Gao, X. A reversible fluorescence chemosensor for sequentially quantitative monitoring copper and sulfide in living cells. Talanta 2015, 143, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.L.; Liu, J.M.; Wang, X.X.; Lin, L.P.; Jiao, L.; Zheng, Z.Y.; Zhang, L.H.; Jiang, S.L. A promising gold nanocluster fluorescent sensor for the highly sensitive and selective detection of S2−. Sens. Actuators B Chem. 2013, 188, 53–58. [Google Scholar] [CrossRef]
- Yu, C.; Leng, M.; Liu, M.z.; Yu, Y.; Liu, D.; Wang, C. Synthesis of normal and flattened rhombic dodecahedral Ag2S particles. Crystengcomm 2012, 14, 3772–3777. [Google Scholar] [CrossRef]
- Ke, J.; Li, X.Y.; Shi, Y.; Zhao, Q.D.; Jiang, X.C. A facile and highly sensitive probe for Hg(II) based on metal-induced aggregation of ZnSe/ZnS quantum dots. Nanoscale 2012, 4, 4996–5001. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Yu, P.; Yang, L.F.; Mao, L.Q. Competitive coordination of Cu2+ between cysteine and pyrophosphate ion: Toward sensitive and selective sensing of pyrophosphate ion in synovial fluid of arthritis patients. Anal. Chem. 2013, 85, 2516–2522. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.M.; Rameshkumar, P.; Huang, N.M.; Wei, L.S. Visual and spectrophotometric determination of mercury(II) using silver nanoparticles modified with graphene oxide. Microchim. Acta 2016, 183, 597–603. [Google Scholar] [CrossRef]
- Rastegarzadeh, S.; Hashemi, F. A surface plasmon resonance sensing method for determining captopril based on in situ formation of silver nanoparticles using ascorbic acid. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 122, 536–541. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.J.; Jiang, Q.; Wang, Y.X.; Yu, P.; Yang, L.F.; Mao, L.Q. Real-Time colorimetric assay of inorganic pyrophosphatase activity based on reversibly competitive coordination of Cu2+ between cysteine and pyrophosphate ion. Anal. Chem. 2013, 85, 9409–9415. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Xu, X.H.; Liu, X.; Li, H.; Nie, Z.; Qing, M.; Huang, Y.; Yao, S.Z. A gold nanoparticles colorimetric assay for label-free detection of protein kinase activity based on phosphorylation protection against exopeptidase cleavage. Biosens. Bioelectron. 2014, 53, 295. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.P.; Li, L. Colorimetric thrombin assay using aptamer-functionalized gold nanoparticles acting as a peroxidase mimetic. Microchim. Acta 2016, 183, 485–490. [Google Scholar] [CrossRef]
- Pelle, F.D.; González, M.C.; Sergi, M.; Carlo, M.D.; Compagnone, D.; Escarpa, A. Gold Nanoparticles-based Extraction-Free Colorimetric Assay in Organic Media: An Optical Index for Determination of Total Polyphenols in Fat-Rich Samples. Anal. Chem. 2015, 87, 6905–6911. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Ulmann, P.A.; Han, M.S.; Mirkin, C.A. A DNA-gold nanoparticle-based colorimetric competition assay for the detection of cysteine. NANO Lett. 2008, 8, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Vilela, D.; González, M.C.; Escarpa, A. Sensing colorimetric approaches based on gold and silver nanoparticles aggregation: Chemical creativity behind the assay. A review. Anal. Chim. Acta 2012, 751, 24–43. [Google Scholar] [CrossRef] [PubMed]
- Miao, P.; Liu, T.; Li, X.X.; Ning, L.M.; Yin, J.; Han, K. Highly sensitive, label-free colorimetric assay of trypsin using silver nanoparticles. Biosens. Bioelectron. 2013, 49C, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.C.; Xie, Y.; Lu, J.; Zhu, L.Y.; He, W.; Qian, Y.T. Preparation, Characterization, and Catalytic Effect of CS2-Stabilized Silver Nanoparticles in Aqueous Solution. Langmuir 2001, 17, 3795–3799. [Google Scholar] [CrossRef]
- Ma, Y.R.; Niu, H.Y.; Zhang, X.L.; Cai, Y.Q. Colorimetric detection of copper ions in tap water during the synthesis of silver/dopamine nanoparticles. Chem. Commun. 2011, 47, 12643–12645. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Y. Responsive Lanthanide Coordination Polymer for Hydrogen Sulfide. Anal. Chem. 2013, 85, 11020–11025. [Google Scholar] [CrossRef] [PubMed]
- Hyšpler, R.; Tichá, A.; Indrová, M.; Zadák, Z.; Hyšplerová, L.; Gasparič, J.; Churáček, J. A simple, optimized method for the determination of sulphide in whole blood by GC-MS as a marker of bowel fermentation processes. J. Chromatogr. B 2002, 770, 255–259. [Google Scholar] [CrossRef]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Zhao, L.; Miao, Y.; Liu, C.; Zhang, C. A Colorimetric Sensor for the Highly Selective Detection of Sulfide and 1,4-Dithiothreitol Based on the In Situ Formation of Silver Nanoparticles Using Dopamine. Sensors 2017, 17, 626. https://doi.org/10.3390/s17030626
Zhao L, Zhao L, Miao Y, Liu C, Zhang C. A Colorimetric Sensor for the Highly Selective Detection of Sulfide and 1,4-Dithiothreitol Based on the In Situ Formation of Silver Nanoparticles Using Dopamine. Sensors. 2017; 17(3):626. https://doi.org/10.3390/s17030626
Chicago/Turabian StyleZhao, Lingzhi, Liu Zhao, Yanqing Miao, Chunye Liu, and Chenxiao Zhang. 2017. "A Colorimetric Sensor for the Highly Selective Detection of Sulfide and 1,4-Dithiothreitol Based on the In Situ Formation of Silver Nanoparticles Using Dopamine" Sensors 17, no. 3: 626. https://doi.org/10.3390/s17030626




