A Formaldehyde Sensor Based on Molecularly-Imprinted Polymer on a TiO2 Nanotube Array
Abstract
:1. Introduction
2. Materials and Methods
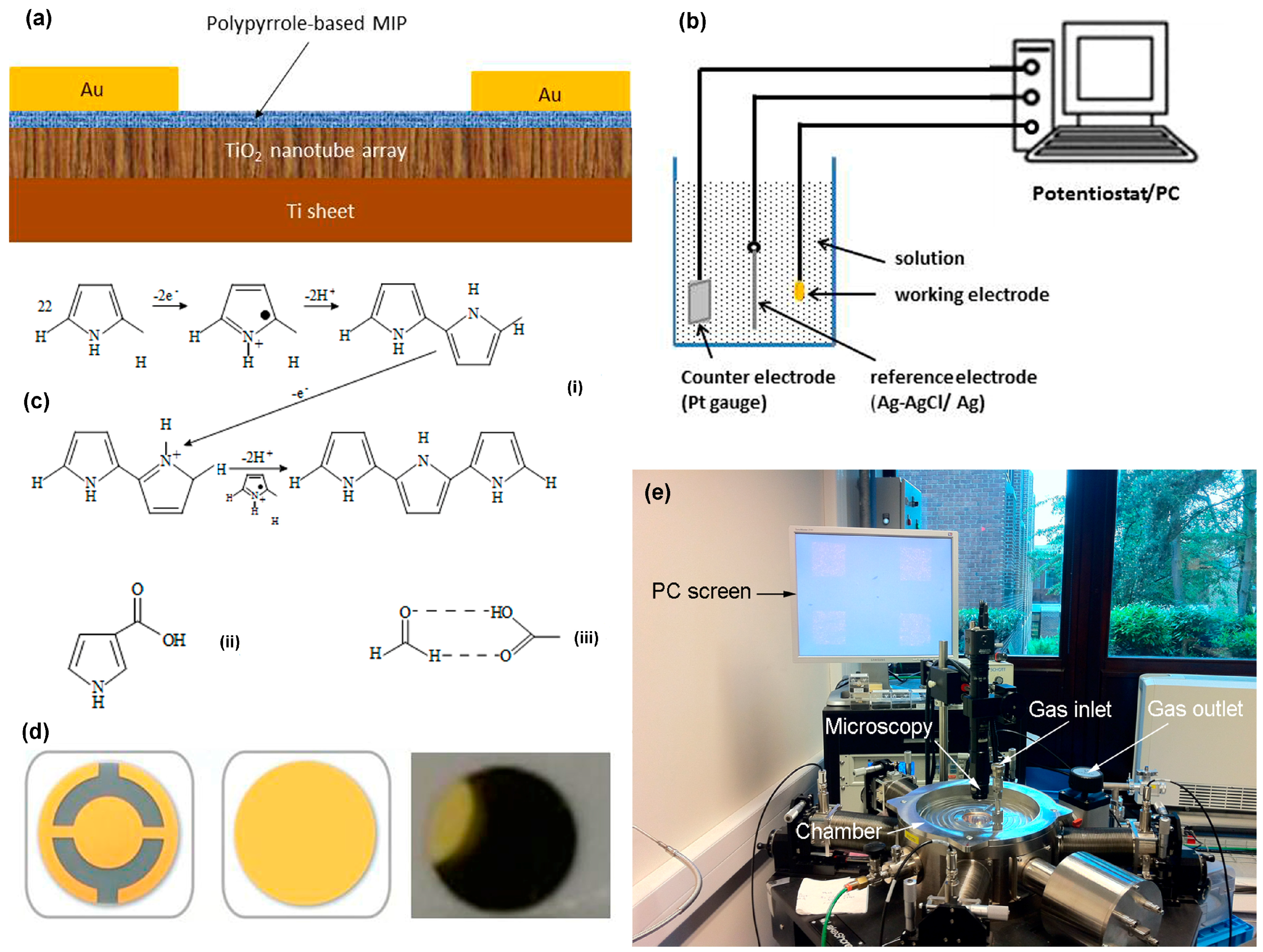
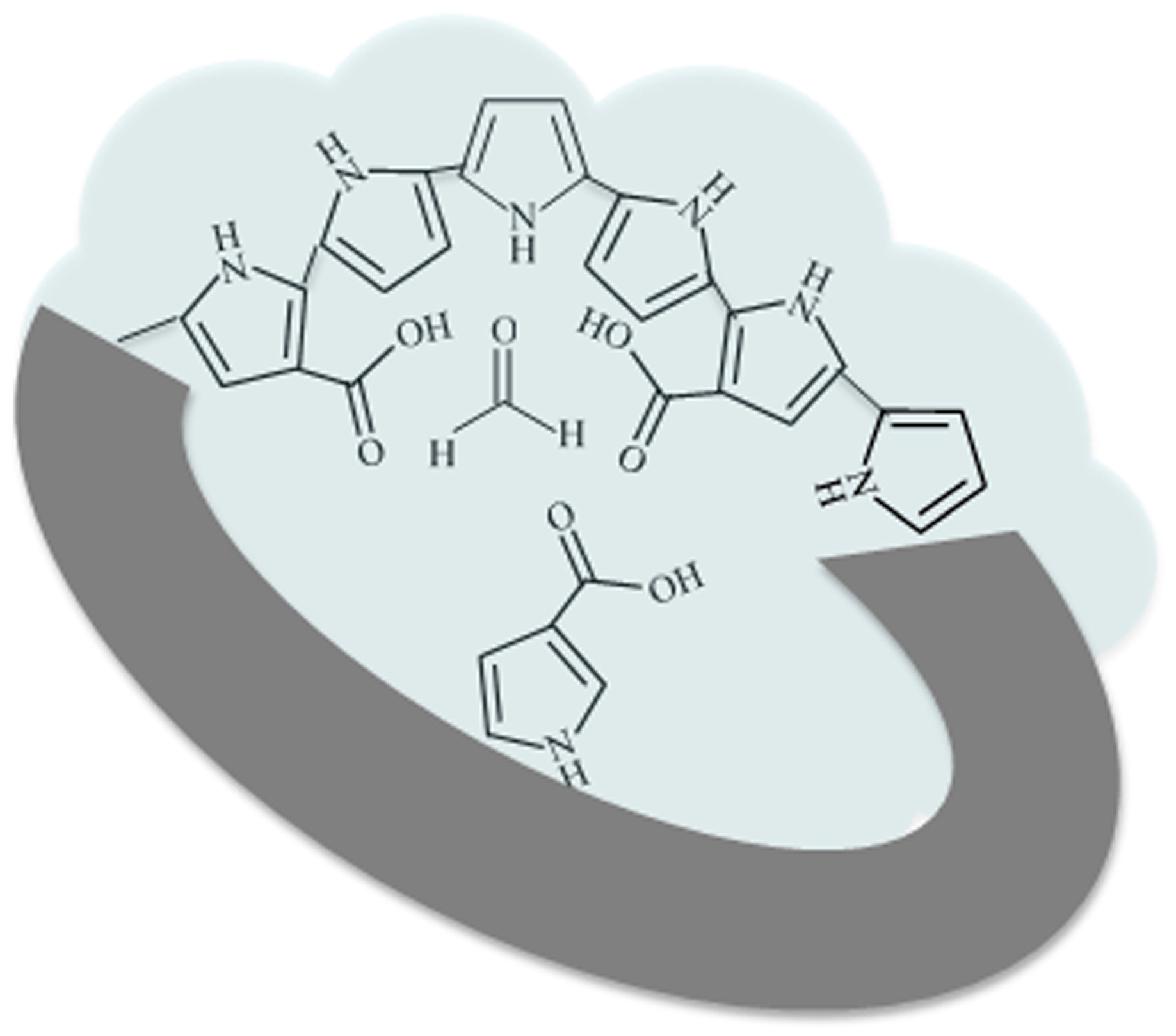
2.1. Synthesis of Polypyrrole-Based Molecularly-Imprinted Polymer
2.2. Physical Characterization System and Electric Sensing Setup
3. Results
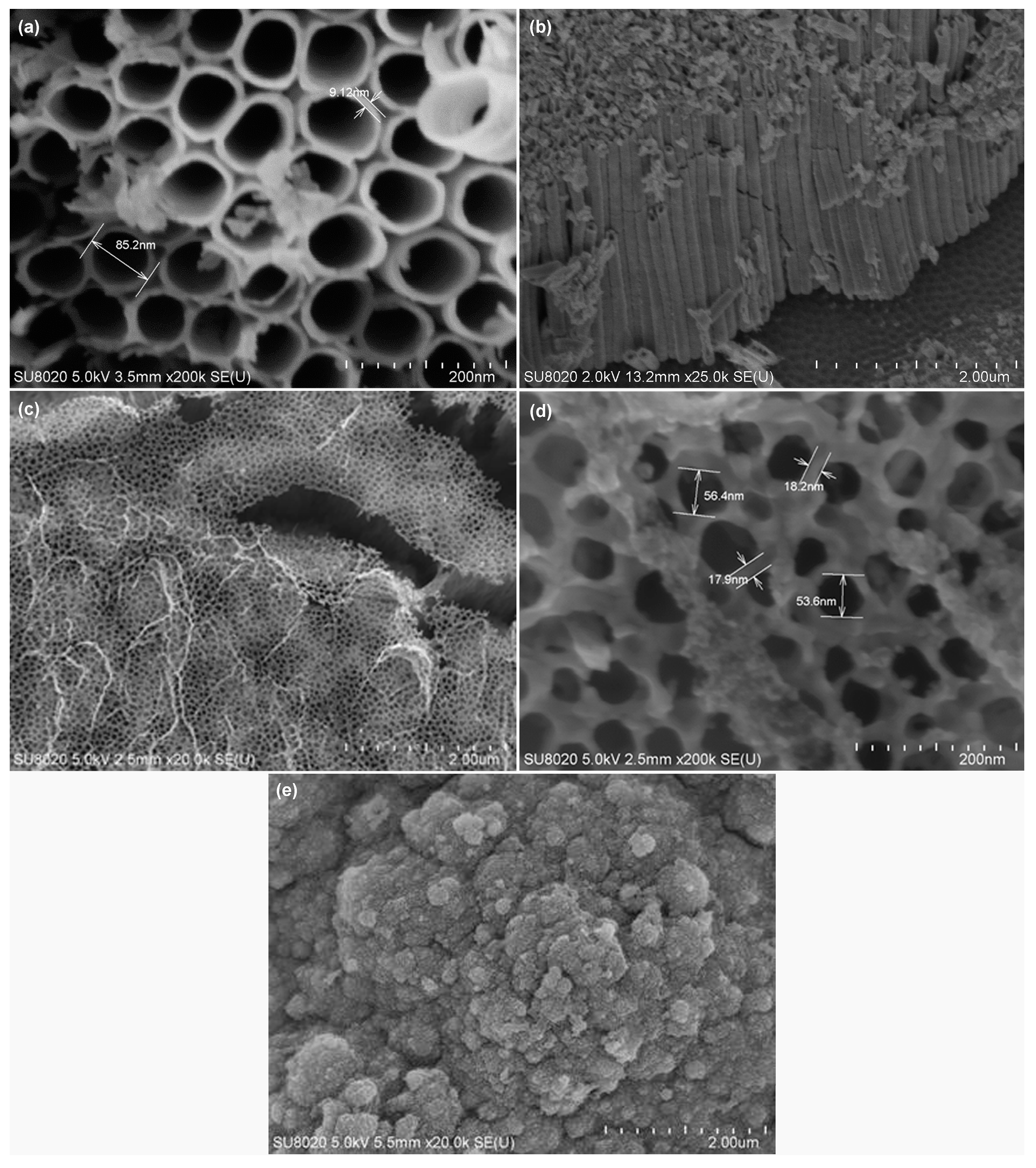
3.1. Physical Characterization of Materials
3.2. Chemical Compositions of Materials
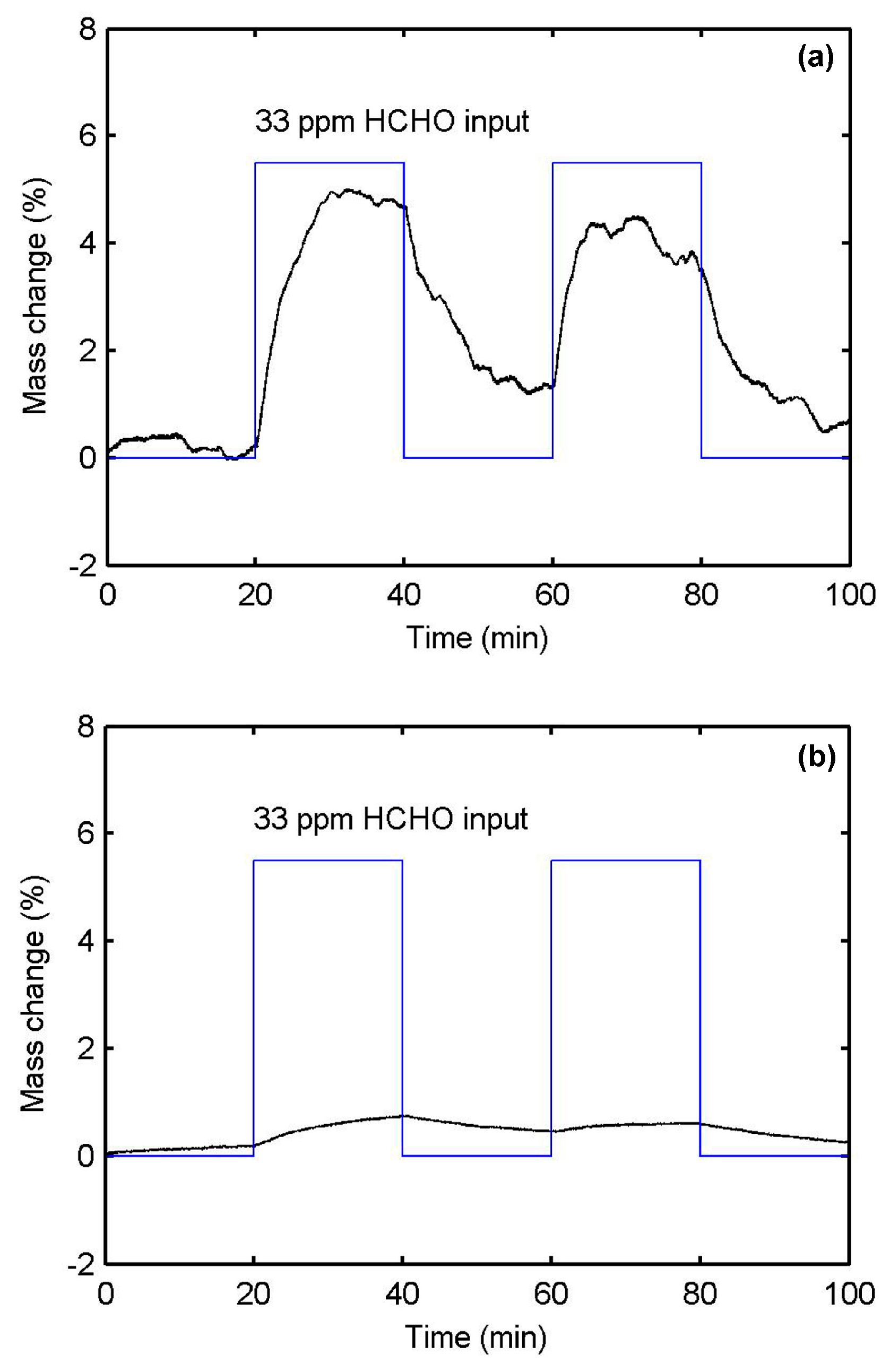
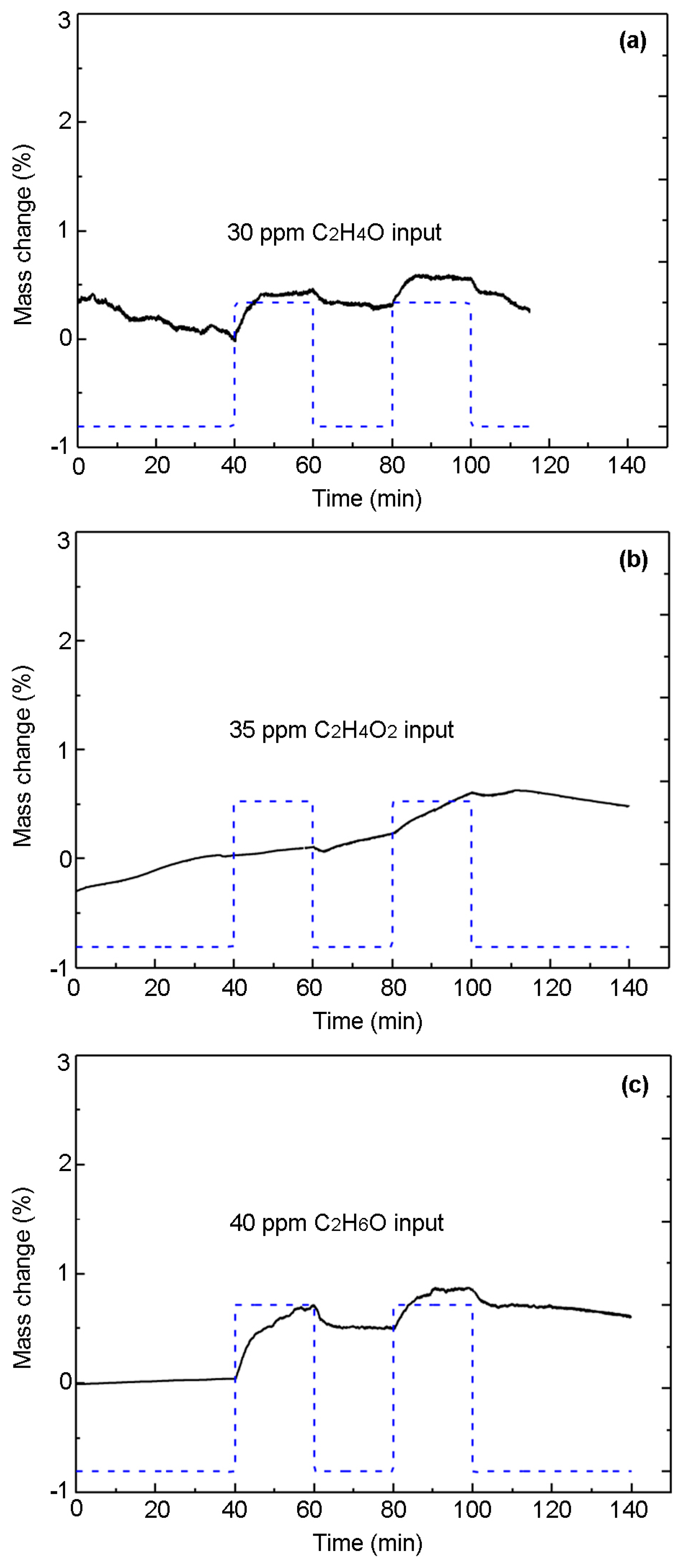
3.3. Specific Adsorption of PPy-Based MIP Film
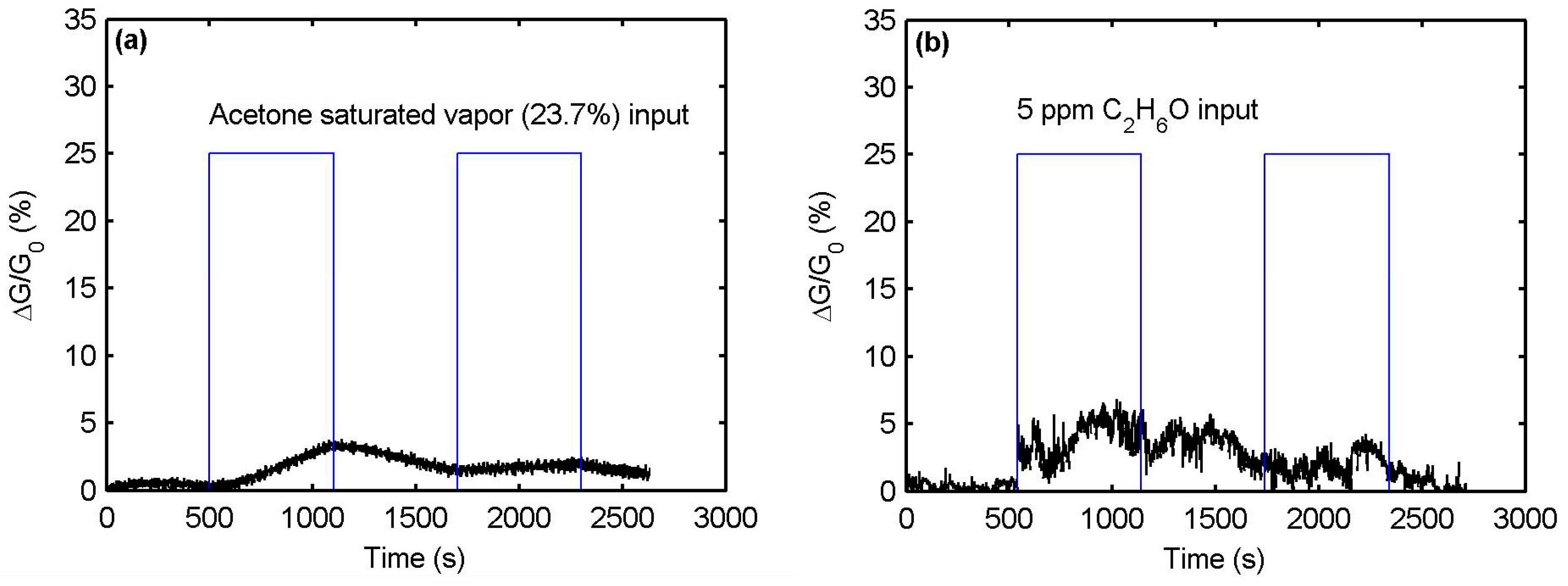
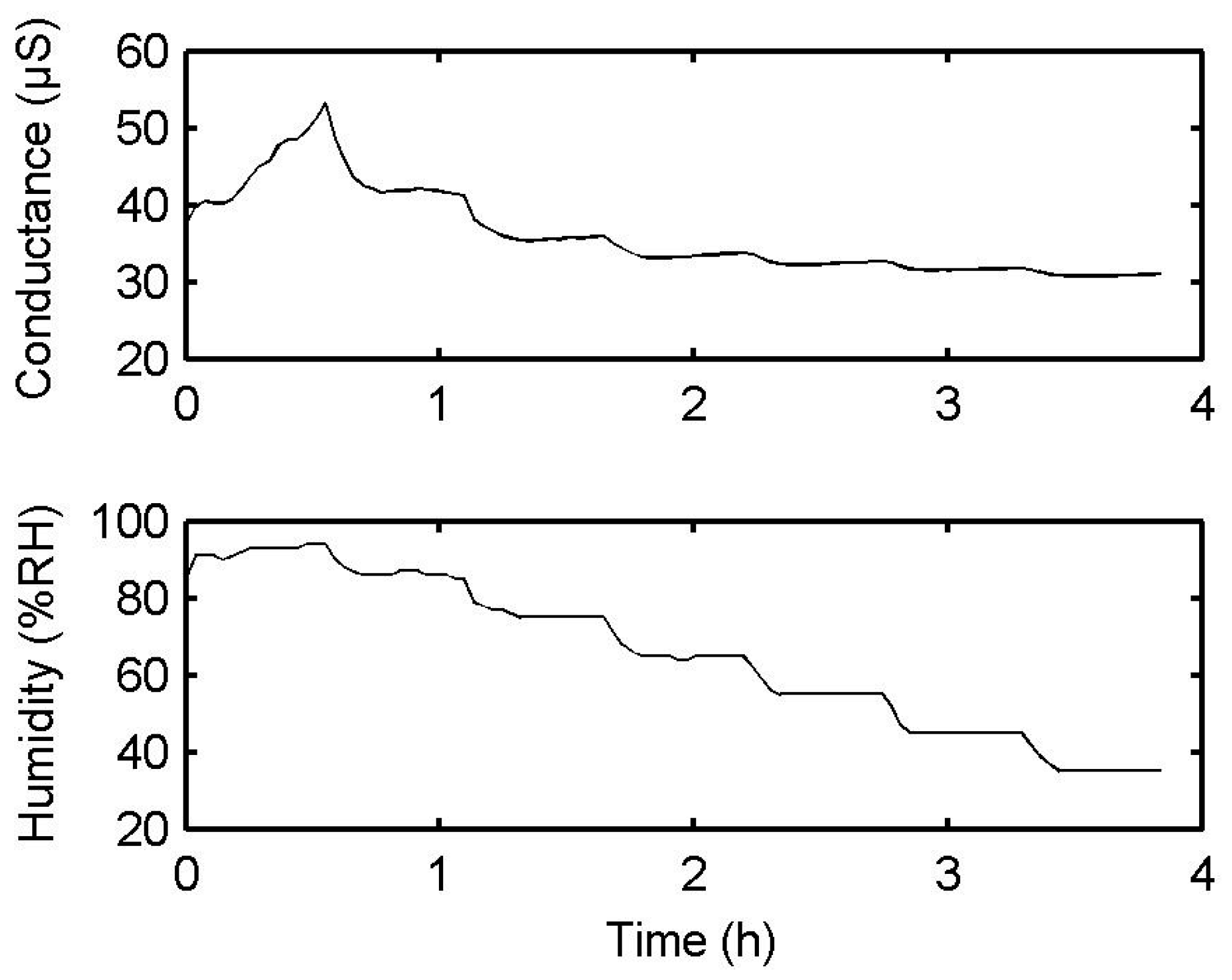
3.4. Electrical Sensing Measurement Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, S.-C.; Guo, H.; Li, W.-M.; Chan, L.-Y. Inter-comparison of air pollutant concentrations in different indoor environments in Hong Kong. Atmos. Environ. 2002, 36, 1929–1940. [Google Scholar] [CrossRef]
- Zhai, L.; Zhao, J.; Xu, B.; Deng, Y.; Xu, Z. Influence of Indoor Formaldehyde Pollution on Respiratory System Health in the Urban Area of Shenyang, China. Afr. Health Sci. 2013, 13, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, F.X.R.; Krzyzanowski, M. Air Quality Guidelines, 2nd ed.; WHO Regional Office for Europe: Copenhagen, Denmark, 2001. [Google Scholar]
- Occupational Safety and Health Guideline for Formaldehyde Potential Human Carcinogen; U.S. Department of Health and Human Services: Washington, DC, USA, 1988. Available online: https://www.cdc.gov/niosh/docs/81-123/pdfs/0293.pdf (accessed on 22 March 2017).
- Yasri, N.G.; Seddik, H.; Mosallb, M.A. Spectrophotometric Determination of Formaldehyde Based on the Telomerization Reaction of Tryptamine. Arab. J. Chem. 2015, 8, 487–494. [Google Scholar] [CrossRef]
- Mann, B.; Grayeski, M.L. New Chemiluminescent Derivatizing Agent for the Analysis of Aldehydes and Ketones by High-Performance Liquid Chromatography with Peroxyoxalate Chemiluminescence. J. Chromatogr. A 1987, 386, 149–158. [Google Scholar] [CrossRef]
- Descamps, M.N.; Bordy, T.; Hue, J.; Mariano, S.; Nonglaton, G.; Schultz, E.; Tran-Thi, T.H.; Vignoud-Despond, S. Real-Time Detection of Formaldehyde by a Fluorescence-Based Sensor. Procedia Eng. 2010, 5, 1009–1012. [Google Scholar] [CrossRef]
- Seo, H.; Jung, S.; Jeon, S. Detection of Formaldehyde Vapor Using Mercaptophenol-Coated Piezoresistive Cantilevers. Sens. Actuators B Chem. 2007, 126, 522–526. [Google Scholar] [CrossRef]
- Hong Chan, W.; Chung, W.C.; Cai, P.X. Differential-Pulse Polarographic Micro-Determination of Formaldehyde via in Situ Derivatization with Girard’s Reagent T. Analyst 1995, 120, 2233–2236. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Zhou, X.; Hu, J. The Fabrication and Characterization of a Formaldehyde Odor Sensor Using Molecularly Imprinted Polymers. J. Colloid Interface Sci. 2005, 284, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Flueckiger, J.; Ko, F.K.; Cheung, K.C. Microfabricated Formaldehyde Gas Sensors. Sensors 2009, 9, 9196–9215. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Tang, Z.A.; Yu, J.; Zhang, F.T.; Wei, G.F.; Huang, Z.X.; Hu, Y. Study on a Micro-Gas Sensor with SnO2–NiO Sensitive Film for Indoor Formaldehyde Detection. Sens. Actuators B Chem. 2008, 132, 74–80. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Cai, Z.; Chen, M.; Cheng, F. A Novel Electrochemical Sensor for Formaldehyde Based on Palladium Nanowire Arrays Electrode in Alkaline Media. Electrochim. Acta 2012, 68, 172–177. [Google Scholar] [CrossRef]
- Xie, H.; Sheng, C.; Chen, X.; Wang, X.; Li, Z.; Zhou, J. Multi-Wall Carbon Nanotube Gas Sensors Modified with Amino-Group to Detect Low Concentration of Formaldehyde. Sens. Actuators B Chem. 2012, 168, 34–38. [Google Scholar] [CrossRef]
- Chung, P.-R.; Tzeng, C.-T.; Ke, M.-T.; Lee, C.-Y. Formaldehyde Gas Sensors: A Review. Sensors 2013, 13, 4468–4484. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Vasapollo, G. Phthalocyanine-Based Molecularly Imprinted Polymers as Nucleoside Receptors. Met. Based Drugs 2008, 2008, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Whitcombe, M.J.; Kirsch, N.; Nicholls, I.A. Molecular Imprinting Science and Technology: A Survey of the Literature for the Years 2004–2011. J. Mol. Recognit. 2014, 27, 297–401. [Google Scholar] [PubMed]
- Shiomi, T.; Matsui, M.; Mizukami, F.; Sakaguchi, K. A Method for the Molecular Imprinting of Hemoglobin on Silica Surfaces Using Silanes. Biomaterials 2005, 26, 5564–5571. [Google Scholar] [CrossRef] [PubMed]
- Bossi, A.; Bonini, F.; Turner, A.P.F.; Piletsky, S.A. Molecularly Imprinted Polymers for the Recognition of Proteins: The State of the Art. Biosens. Bioelectron. 2007, 22, 1131–1137. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, C.; Qader, A.A.; Halvorsen, T.G.; Sellergren, B.; Reubsaet, L. Antibody-Free Biomarker Determination: Exploring Molecularly Imprinted Polymers for Pro-Gastrin Releasing Peptide. Anal. Chem. 2014, 86, 12291–12298. [Google Scholar] [CrossRef] [PubMed]
- Pichon, V.; Chapuis-Hugon, F. Role of Molecularly Imprinted Polymers for Selective Determination of Environmental pollutants—A Review. Anal. Chim. Acta 2008, 622, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, L.; Ng, T.B.; Wong, J.H.; Yan, Y.; Shi, Z.; Liu, F. Separation and Purification of the Antioxidant Compound Hispidin from Mushrooms by Molecularly Imprinted Polymer. Appl. Microbiol. Biotechnol. 2015, 99, 7569–7577. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Davis, M.E. Molecular imprinting of bulk, microporous silica. Nature 2000, 403, 283–289. [Google Scholar]
- Li, W.; Li, S. Molecular Imprinting: A Versatile Tool for Separation, Sensors and Catalysis. In Oligomers–Polymer Composites–Molecular Imprinting; Springer: Berlin, Germany, 2006; Volume 206, pp. 191–210. [Google Scholar]
- Piletsky, S.A.; Turner, N.W.; Laitenberger, P. Molecularly Imprinted Polymers in Clinical diagnostics—Future Potential and Existing Problems. Med. Eng. Phys. 2006, 28, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Dickert, F.L.; Hayden, O. Molecular Imprinting in Chemical Sensing. TrAC Trends Anal. Chem. 1999, 18, 192–199. [Google Scholar] [CrossRef]
- Blanco-Lopez, M.C.; Gutierrez-Fernandez, S.; Lobo-Castanon, M.J.; Miranda-Ordieres, A.J.; Tunon-Blanco, P. Electrochemical Sensing with Electrodes Modified with Molecularly Imprinted Polymer Films. Anal. Bioanal. Chem. 2004, 378, 1922–1928. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Ma, Y.; Pan, J.; Meng, Z.; Pan, G.; Sellergren, B. Molecularly Imprinted Polymers with Stimuli-Responsive Affinity: Progress and Perspectives. Polymers 2015, 7, 1689–1715. [Google Scholar] [CrossRef]
- Vasapollo, G.; Del Sole, R.; Mergola, L.; Lazzoi, M.R.; Scardino, A.; Scorrano, S.; Mele, G. Molecularly Imprinted Polymers: Present and Future Prospective. Int. J. Mol. Sci. 2011, 12, 5908–5945. [Google Scholar] [CrossRef] [PubMed]
- Kyzas, G.Z.; Bikiaris, D.N. Molecular Imprinting for High-Added Value Metals: An Overview of Recent Environmental Applications. Adv. Mater. Sci. Eng. 2014, 2014, 1–8. [Google Scholar] [CrossRef]
- Antwi-Boampong, S.; BelBruno, J.J. Detection of Formaldehyde Vapor Using Conductive Polymer Films. Sens. Actuators B Chem. 2013, 182, 300–306. [Google Scholar] [CrossRef]
- Hirayama, K.; Sakai, Y.; Kameoka, K.; Noda, K.; Naganawa, R. Preparation of a Sensor Device with Specific Recognition Sites for Acetaldehyde by Molecular Imprinting Technique. Sens. Actuators B Chem. 2002, 86, 20–25. [Google Scholar] [CrossRef]
- Balamurugan, K.; Gokulakrishnan, K.; Prakasam, T. Preparation and Evaluation of Molecularly Imprinted Polymer Liquid Chromatography Column for the Separation of Cathine Enantiomers. Saudi Pharm. J. 2012, 20, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Kotova, K.; Lieberzeit, P. Molecularly Imprinted Polymer Nanoparticles for Formaldehyde Sensing with QCM. Sensors 2016, 16, 1011. [Google Scholar] [CrossRef] [PubMed]
- Cepak, V.M.; Hulteen, J.C.; Che, G.; Jirage, K.B.; Lakshmi, B.B.; Fisher, E.R.; Martin, C.R.; Yoneyama, H. Chemical Strategies for Template Syntheses of Composite Micro- and Nanostructures. Chem. Mater. 1997, 9, 1065–1067. [Google Scholar] [CrossRef]
- Hulteen, J.C.; Martin, C.R. A General Template-Based Method for the Preparation of Nanomaterials. J. Mater. Chem. 1997, 7, 1075–1087. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, W.; Strout, T.; Schempf, A.; Zhang, H.; Li, B.; Cui, J.; Lei, Y. Ammonia Gas Sensor Using Polypyrrole-Coated TiO2/ZnO Nanofibers. Electroanalysis 2009, 21, 1432–1438. [Google Scholar] [CrossRef]
- Waltman, R.J.; Bargon, J. Electrically Conducting Polymers: A Review of the Electropolymerization Reaction, of the Effects of Chemical Structure on Polymer Film Properties, and of Applications towards Technology. Can. J. Chem. 1986, 64, 76–95. [Google Scholar] [CrossRef]
- Yokota, S.; Kitaoka, T.; Wariishi, H. Surface Morphology of Cellulose Films Prepared by Spin Coating on Silicon Oxide Substrates Pretreated with Cationic Polyelectrolyte. Appl. Surface Sci. 2007, 253, 4208–4214. [Google Scholar] [CrossRef]
- Chrisey, D.B.; Piqué, A.; McGill, R.A.; Horwitz, J.S.; Ringeisen, B.R.; Bubb, D.M.; Wu, P.K. Laser Deposition of Polymer and Biomaterial Films. Chem. Rev. 2003, 103, 553–576. [Google Scholar] [CrossRef] [PubMed]
- Decroly, A.; Krumpmann, A.; Debliquy, M.; Lahem, D. Nanostructured TiO2 Layers for Photovoltaic and Gas Sensing Applications. In Green Nanotechnology—Overview and Further Prospects; Larramendy, M.L., Soloneski, S., Eds.; InTech; Available online: https://www.intechopen.com/books/green-nanotechnology-overview-and-further-prospects/nanostructured-tio2-layers-for-photovoltaic-and-gas-sensing-applications (accessed on 22 March 2017). [CrossRef]
- Sauerbrey, G. Verwendung von Schwingquarzen Zur Wägung Dünner Schichten und Zur Mikrowägung. Zeitschrift Für Physik 1959, 155, 206–222. (In German) [Google Scholar] [CrossRef]
- Hao, Q.; Kulikov, V.; Mirsky, V.M. Investigation of Contact and Bulk Resistance of Conducting Polymers by Simultaneous Two- and Four-Point Technique. Sens. Actuators B Chem. 2003, 94, 352–357. [Google Scholar] [CrossRef]
- Riskin, M.; Tel-Vered, R.; Bourenko, T.; Granot, E.; Willner, I. Imprinting of Molecular Recognition Sites through Electropolymerization of Functionalized Au Nanoparticles: Development of an Electrochemical TNT Sensor Based on π-Donor−Acceptor Interactions. J. Am. Chem. Soc. 2008, 130, 9726–9733. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, B.; Sun, M.; Yu, J.; Sun, G. Nanofibrous Polyethyleneimine Membranes as Sensitive Coatings for Quartz Crystal Microbalance-Based Formaldehyde Sensors. Sens. Actuators B Chem. 2010, 144, 11–17. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.; Lin, J.; Ding, B.; Yu, J.; Pan, N. Nanoporous Polystyrene Fibers Functionalized by Polyethyleneimine for Enhanced Formaldehyde Sensing. Sens. Actuators B Chem. 2011, 152, 316–323. [Google Scholar] [CrossRef]
- Wang, X.; Cui, F.; Lin, J.; Ding, B.; Yu, J.; Al-Deyab, S.S. Functionalized Nanoporous TiO2 Fibers on Quartz Crystal Microbalance Platform for Formaldehyde Sensor. Sens. Actuators B Chem. 2012, 171–172, 658–665. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Duan, C.; Dan, W.; Li, X. Development of a Novel Formaldehyde OTFT Sensor Based on P3HT/Fe2O3 Nanocomposite Thin Film. Integr. Ferroelectr. 2013, 144, 15–21. [Google Scholar] [CrossRef]
- Chen, D.; Yuan, Y.J. Thin-Film Sensors for Detection of Formaldehyde: A Review. IEEE Sens. J. 2015, 15, 6749–6760. [Google Scholar] [CrossRef]
- Itoh, T.; Matsubara, I.; Shin, W.; Izu, N. Preparation and Characterization of a Layered Molybdenum Trioxide with Poly(O-Anisidine) Hybrid Thin Film and Its Aldehydic Gases Sensing Properties. Bull. Chem. Soc. Jpn. 2007, 80, 1011–1016. [Google Scholar] [CrossRef]
- Hosono, K.; Matsubara, I.; Murayama, N.; Shin, W.; Izu, N. The Sensitivity of 4-Ethylbenzenesulfonic Acid-Doped Plasma Polymerized Polypyrrole Films to Volatile Organic Compounds. Thin Solid Films 2005, 484, 396–399. [Google Scholar] [CrossRef]
- Khan, A.A.; Rao, R.A.K.; Alam, N.; Shaheen, S. Formaldehyde Sensing Properties and Electrical Conductivity of Newly Synthesized Polypyrrole-zirconium(IV) selenoiodate Cation Exchange Nanocomposite. Sens. Actuators B Chem. 2015, 211, 419–427. [Google Scholar] [CrossRef]
- Chuang, W.-Y.; Yang, S.-Y.; Wu, W.-J.; Lin, C.-T. A Room-Temperature Operation Formaldehyde Sensing Material Printed Using Blends of Reduced Graphene Oxide and Poly(methyl Methacrylate). Sensors 2015, 15, 28842–28853. [Google Scholar] [CrossRef] [PubMed]
- Srinives, S.; Sarkar, T.; Mulchandani, A. Primary Amine-Functionalized Polyaniline Nanothin Film Sensor for Detecting Formaldehyde. Sens. Actuators B Chem. 2014, 194, 255–259. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, G.; Liu, M.; Zhu, Z. A Novel Photoelectrochemical Sensor Based on Molecularly Imprinted Polymer Modified TiO2 Nanotubes and Its Highly Selective Detection of 2,4-Dichlorophenoxyacetic Acid. Electrochem. Commun. 2011, 13, 1404–1407. [Google Scholar] [CrossRef]
- Zhang, Z.; Yuan, Y.; Liang, L.; Cheng, Y.; Xu, H.; Shi, G.; Jin, L. Preparation and Photoelectrochemical Properties of a Hybrid Electrode Composed of Polypyrrole Encapsulated in Highly Ordered Titanium Dioxide Nanotube Array. Thin Solid Films 2008, 516, 8663–8667. [Google Scholar] [CrossRef]
- Ruangchuay, L.; Sirivat, A.; Schwank, J. Electrical Conductivity Response of Polypyrrole to Acetone Vapor: Effect of Dopant Anions and Interaction Mechanisms. Synth. Met. 2004, 140, 15–21. [Google Scholar] [CrossRef]

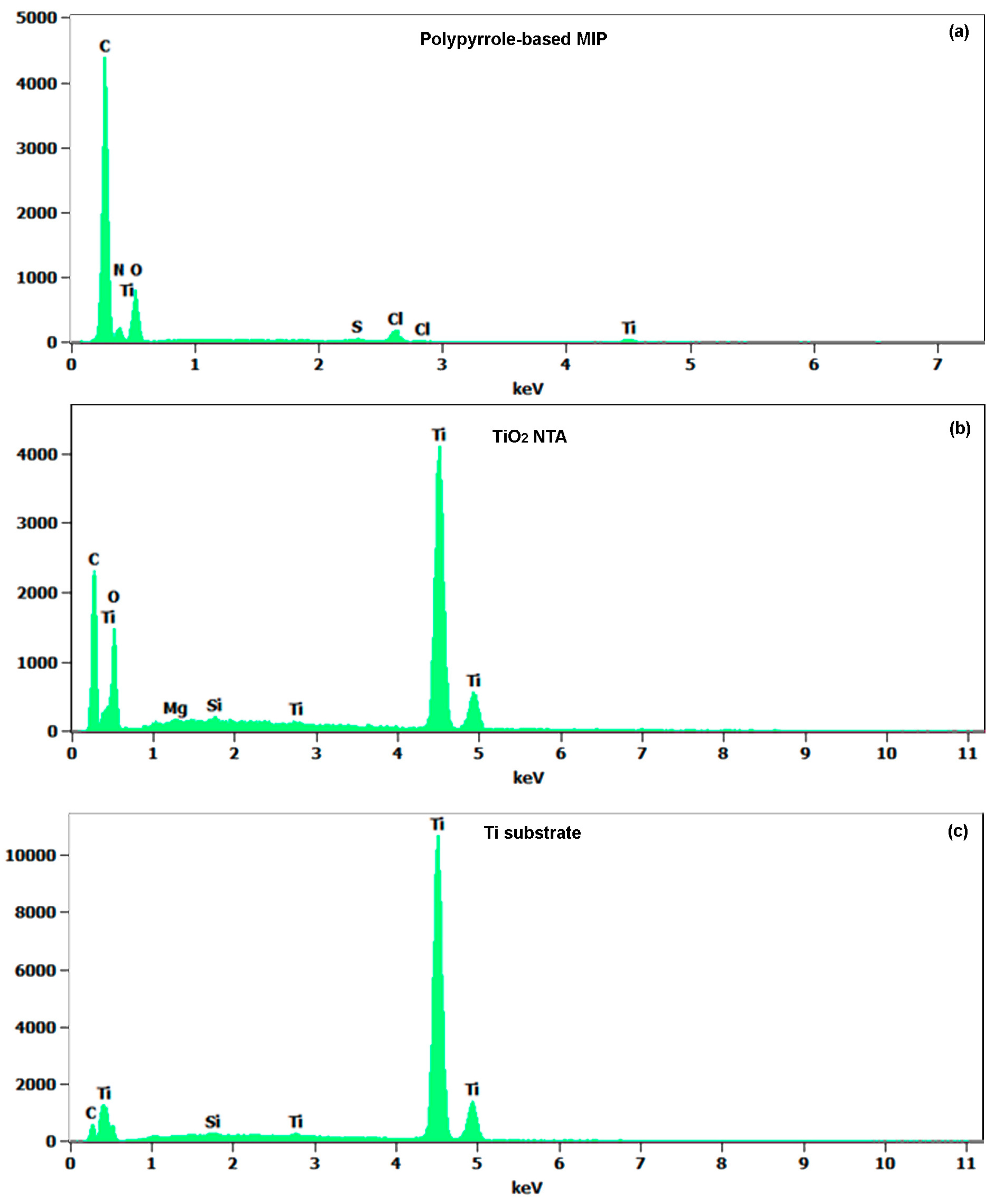

| C-K | O-K | Mg-K | N-K | Si-K | S-K | Ti-K | |
|---|---|---|---|---|---|---|---|
| at % | at % | at % | at % | at % | at % | at % | |
| PPy-MIP | 50.6 ± 0.7 | 29.6 ± 1.3 | 17 ± 2.8 | 0.3 ± 0.1 | 1.1 ± 0.2 | ||
| TiO2 NTA | 24.8 ± 0.5 | 47.8 ± 1.1 | 0.2 ± 0.1 | 0.2 ± 0.0 | 26.9 ± 0.3 | ||
| Ti substrate | 8.4 ± 0.5 | 1.5 ± 0.1 | 91.1 ± 0.8 |
| Sensor Type | Sensing Material | Response Value | Response Time | Recovery Time | Detection Limit | Reference |
|---|---|---|---|---|---|---|
| QCM | PEI/PVA | 0.5 Hz/ppm | 10 ppm | [45] | ||
| QCM | PEI/PS | 1.5 Hz/3 ppm | 3 ppm | [46] | ||
| QCM | PEI/PVA | 0.8 Hz/1 ppm | 120 s | 1 ppm | [47] | |
| QCM | MIP-NP | 24 Hz/100 ppm | 30 s | 0.5 ppm | [34] | |
| OFET | P3HT/Fe2O3 | 16%/100 ppm | [48] | |||
| OFET | P3HT/ZnO | 20%/100 ppm | [49] | |||
| IDE | (PANi)xMoO3 | 8%/50 ppm | [50] | |||
| IDE | PANi/PEI | 3.9%/1 ppm | 20 s | 1 ppm | [31] | |
| IDE | PPy/EBSA | 40%/500 ppm | 300 s | >300 s | [51] | |
| Chemiresistor | PPy/ZSI | 4%/990 ppm | 20 s | [52] | ||
| Chemiresistor | PMMA/RGO | 30.5%/1000 ppm | 150 s | 180 s | 100 ppm | [53] |
| Chemiresistor | PANi/LYS | 15%/75 ppm | 0.4 ppm | [54] | ||
| Chemiresistor | MIP/TiO2 NTA | 13%/1 ppm | 300 s | 300 s | 1 ppm | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Raskin, J.-P.; Lahem, D.; Krumpmann, A.; Decroly, A.; Debliquy, M. A Formaldehyde Sensor Based on Molecularly-Imprinted Polymer on a TiO2 Nanotube Array. Sensors 2017, 17, 675. https://doi.org/10.3390/s17040675
Tang X, Raskin J-P, Lahem D, Krumpmann A, Decroly A, Debliquy M. A Formaldehyde Sensor Based on Molecularly-Imprinted Polymer on a TiO2 Nanotube Array. Sensors. 2017; 17(4):675. https://doi.org/10.3390/s17040675
Chicago/Turabian StyleTang, Xiaohui, Jean-Pierre Raskin, Driss Lahem, Arnaud Krumpmann, André Decroly, and Marc Debliquy. 2017. "A Formaldehyde Sensor Based on Molecularly-Imprinted Polymer on a TiO2 Nanotube Array" Sensors 17, no. 4: 675. https://doi.org/10.3390/s17040675




