Electrodes for Semiconductor Gas Sensors
Abstract
:1. Introduction
2. Electrode Types of Semiconductor Gas Sensors
- (1)
- They should be chemically and mechanically stable on the substrates.
- (2)
- The connection to the lead-out terminals should be easy.
- (3)
- The sensing film should not be damaged during electrode formation.
- (4)
- They must have a geometry that is suitable for sensor construction.
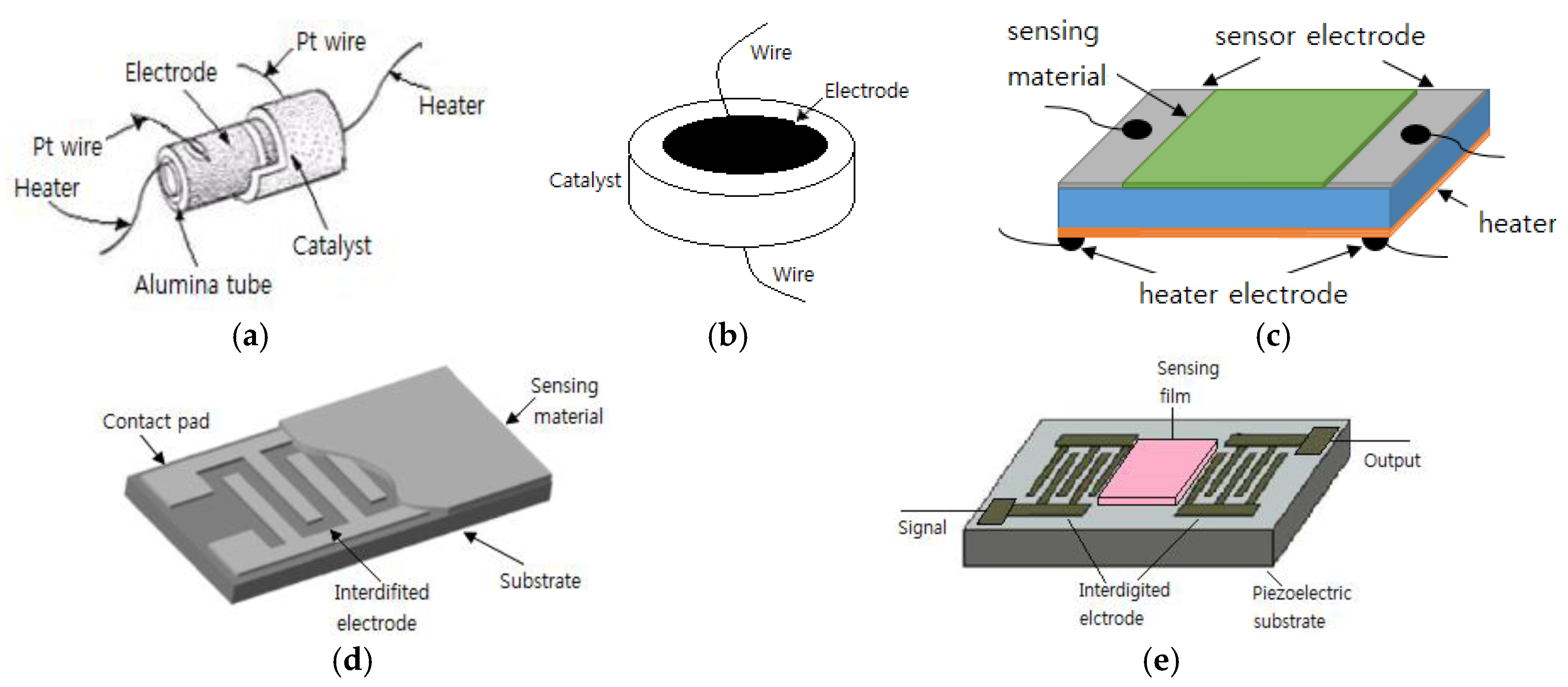
2.1. Two-Electrode Configuration
2.2. One-Electrode Configuration
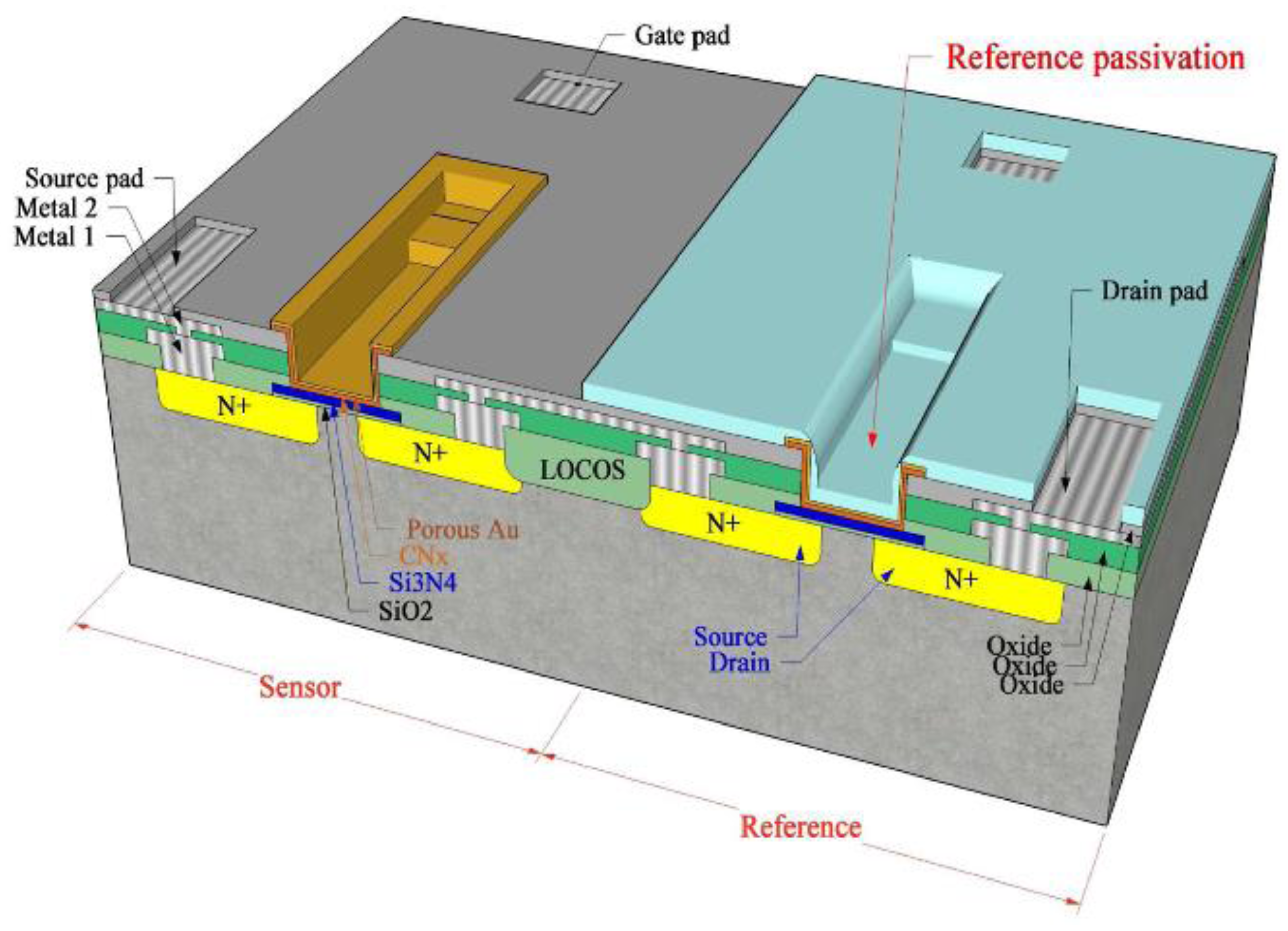
2.3. Gate Electrode
2.4. Electrode Geometry
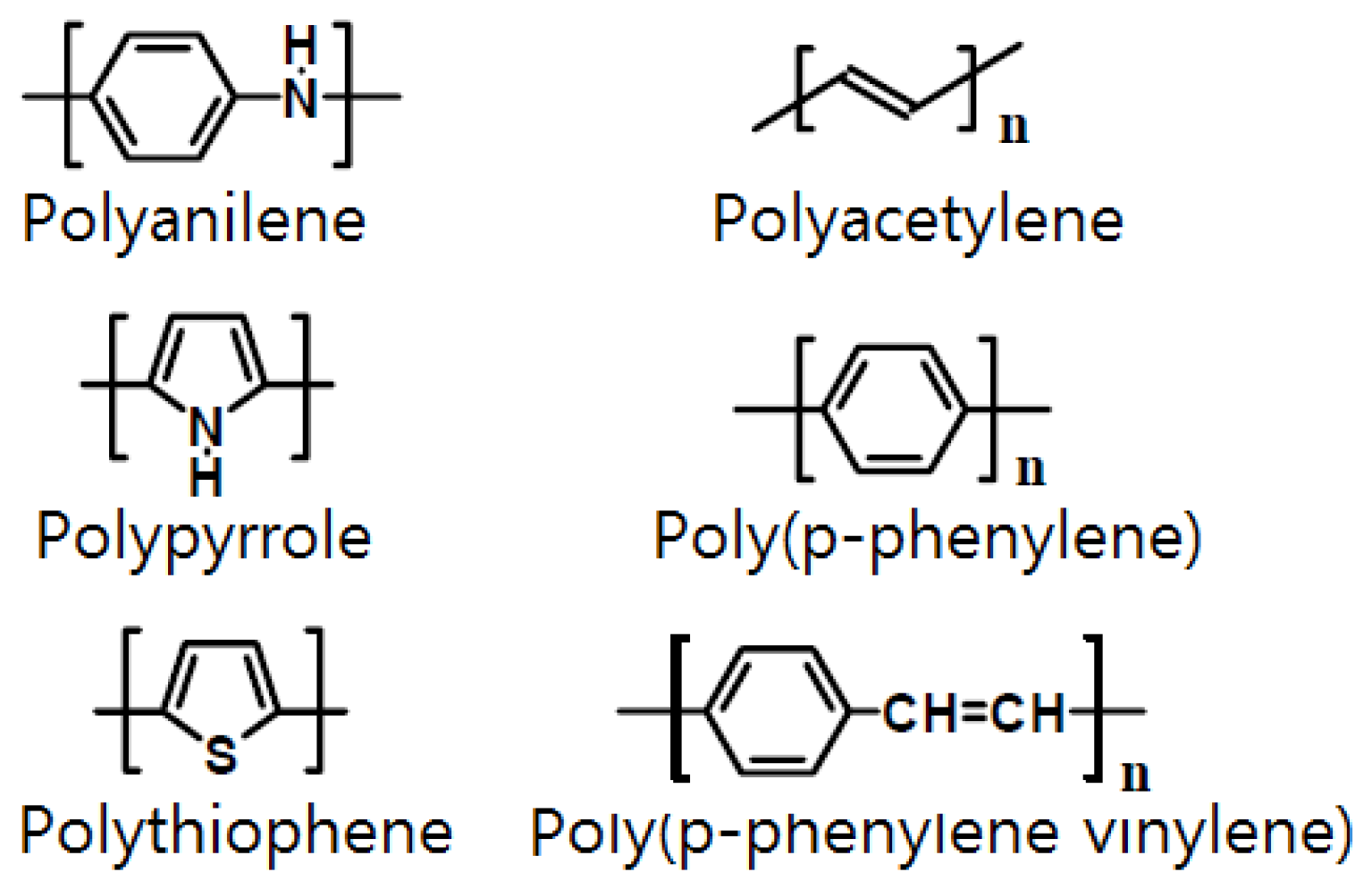
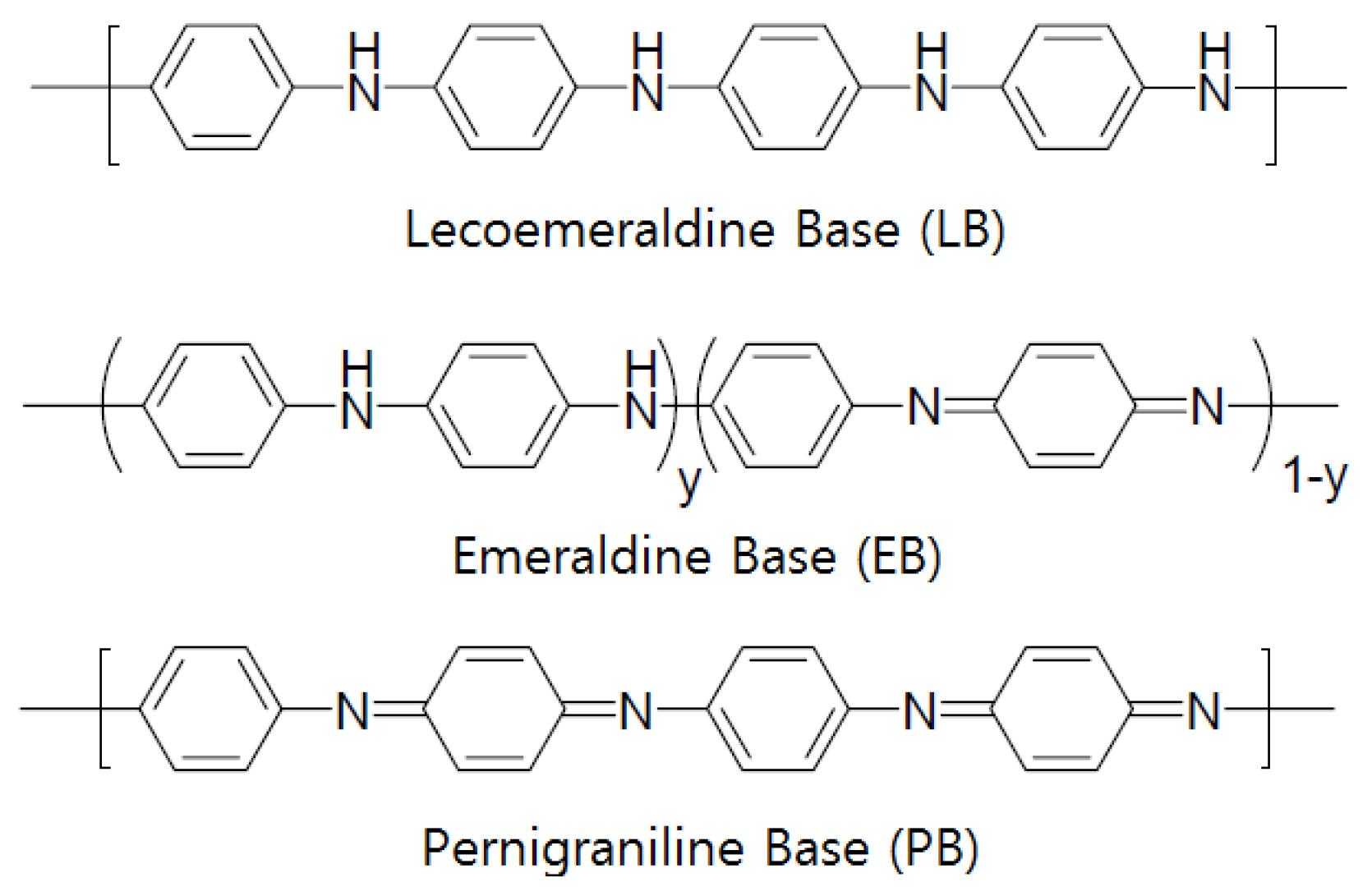
3. Electrode Materials for Semiconductor Gas Sensors
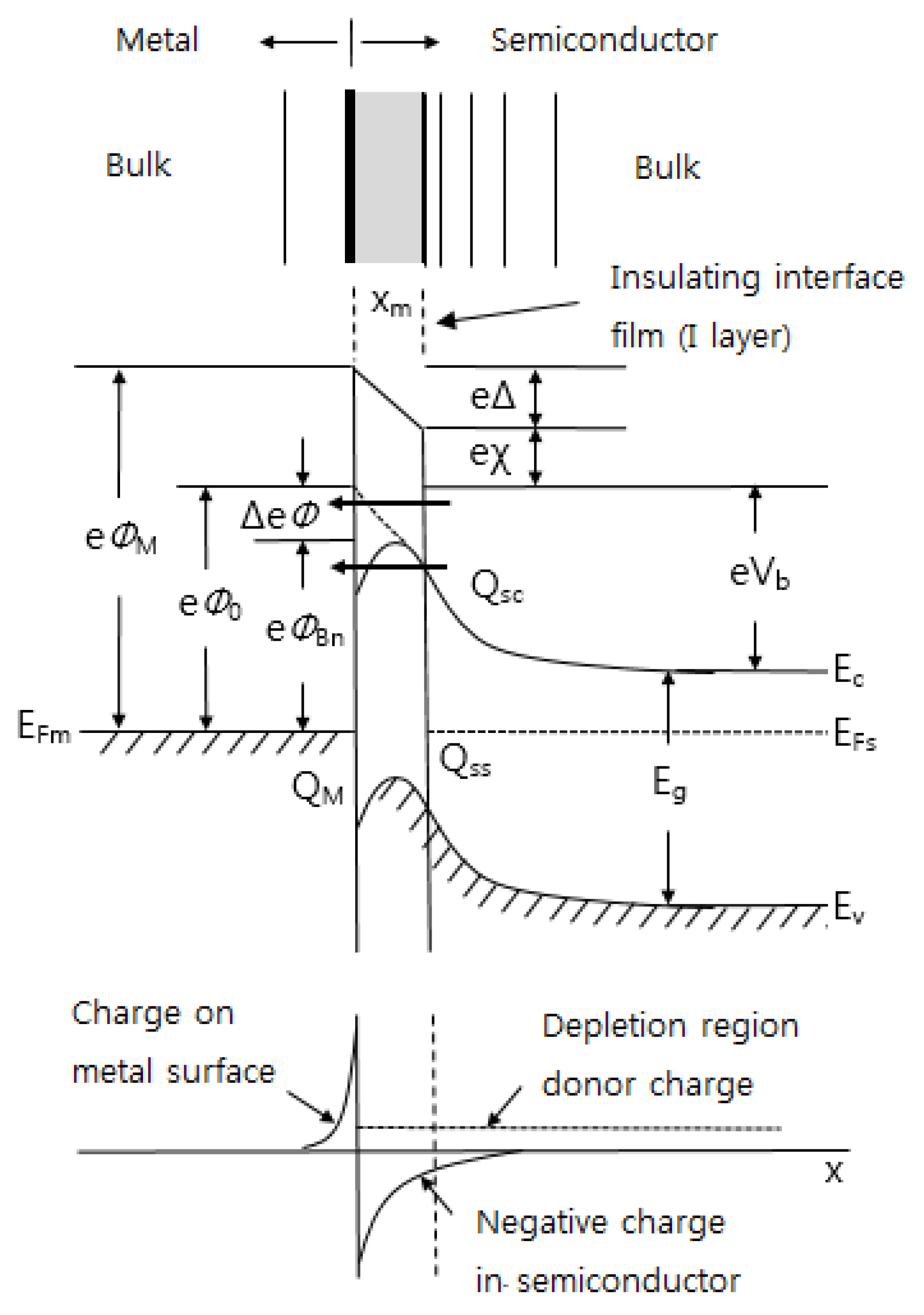
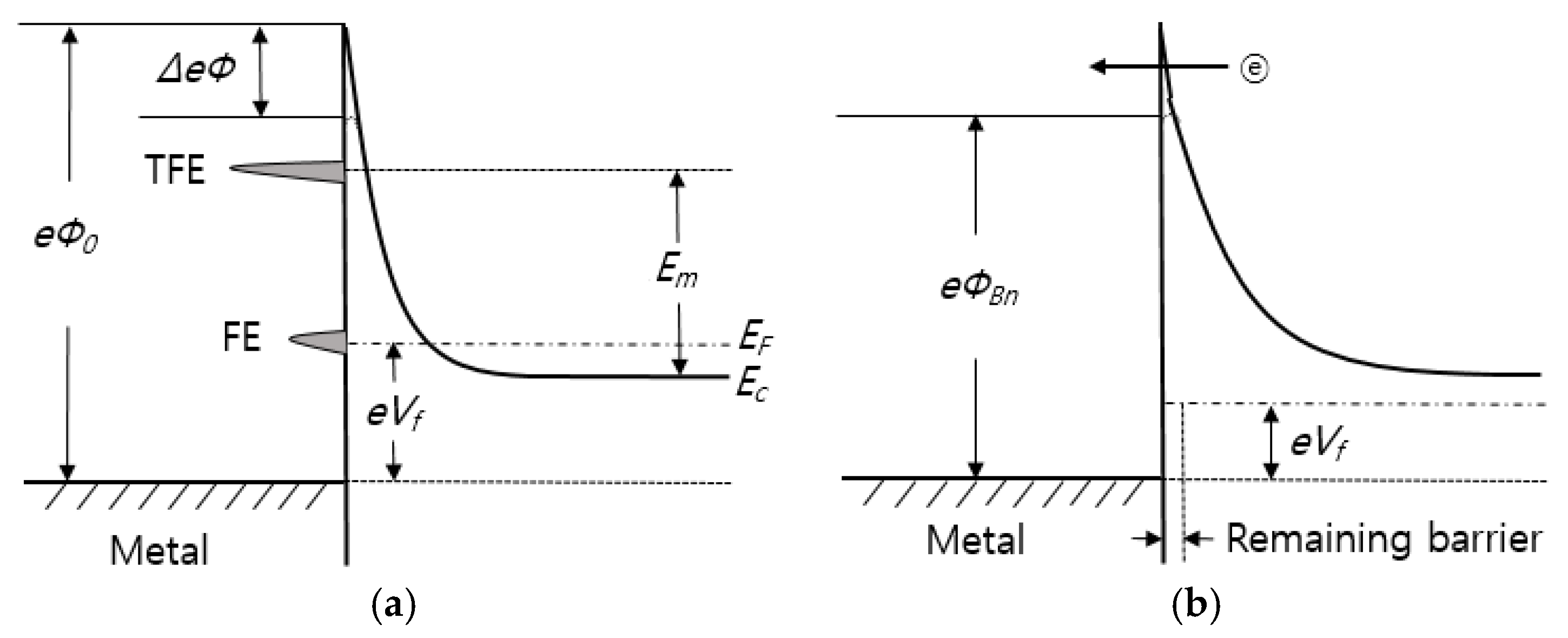
4. Interfacial Zones of Electrode-Semiconductor Contact
- (a)
- Building up the metal layer by layer by evaporation deposition
- (b)
- Pressing two bulk pieces together to form a point contact
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Seiyama, T.; Kato, A.; Fulishi, K.; Nagatani, M. A new detector for gaseous components using semiconductive thin films. Anal. Chem. 1962, 34, 1502–1503. [Google Scholar] [CrossRef]
- López-Gándara, C.; Ramos, F.M.; Cirera, A. YSZ-Based Oxygen Sensors and the Use of Nanomaterials: A Review from Classical Models to Current Trends. J. Sens. 2009, 2009, 1–15. [Google Scholar] [CrossRef]
- Moos, R. A brief overview on automotive exhaust gas sensors based on electroceramics. Int. J. Appl. Ceram. Technol. 2005, 2, 401–413. [Google Scholar] [CrossRef]
- Riegel, J.; Neumann, H.; Wiedenmann, H.M. Exhaust gas sensors for automotive emission control. Solid State Ionics 2002, 152–153, 783–800. [Google Scholar] [CrossRef]
- Ivers-Tiffèe, E.; Härdtl, K.H.; Menesklou, W.; Riegel, J. Principles of solid state oxygen sensors for lean combustion gas control. Electrochim. Acta. 2001, 47, 807–814. [Google Scholar] [CrossRef]
- Anderson, T.; Ren, F.; Pearton, S.; Kang, B.S.; Wang, H.-T.; Chang, C.-Y.; Lin, J. Advances in hydrogen, carbon dioxide, and hydrocarbon gas sensor technology using GaN and ZnO-based devices. Sensors 2009, 9, 4669–4694. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-C.; Bai, H.; Li, S.-N.; Kuo, C.-N. Bromocresol green/mesoporous silica adsorbent for ammonia gas sensing via an optical sensing instrument. Sensors 2011, 11, 4060–4072. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tong, M.M.; Zhang, D.; Gao, Z. Improving the performance of catalytic combustion type methane gas sensors using nanostructure elements doped with rare earth cocatalysts. Sensors 2011, 11, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Wongchoosuk, C.; Wisitsoraat, A.; Phokharatkul, D.; Tuantranont, A.; Kerdcharoen, T. Multi-walled carbon nanotube-doped tungsten oxide thin films for hydrogen gas sensing. Sensors 2010, 10, 7705–7715. [Google Scholar] [CrossRef] [PubMed]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.P. Micro-humidity sensor system using CMOS technology. Sens. Lett. 2011, 9, 1–6. [Google Scholar]
- Song, K.; Wang, Q.; Liu, Q.; Zhang, H.; Cheng, Y. A wireless electronic nose system using a Fe2O3 gas sensing array and least squares support vector regression. Sensors 2011, 11, 485–505. [Google Scholar] [CrossRef] [PubMed]
- Lilienthal, A.J.; Loutfi, A.; Duckett, T. Airborne chemical sensing with mobile robots. Sensors 2006, 6, 1616–1678. [Google Scholar] [CrossRef]
- Gonzalez-Jimenez, J.; Monroy, J.G.; Blanco, J.L. The multi-chamber electronic nose—An improved olfaction sensor for mobile robotics. Sensors 2011, 11, 6145–6164. [Google Scholar] [CrossRef] [PubMed]
- Endres, H.E.; Göttler, W.; Hartinger, R.; Drost, S.; Hellmich, W.; Müller, G.; Braunmühl, C.B.; Krenkow, A.; Perego, C.; Sberveglieri, G. A thin-film SnO2 sensor system for simultaneous detection of CO and NO2 with neural signal evaluation. Sens. Actuators B 1996, 36, 353–357. [Google Scholar] [CrossRef]
- Lee, S.P. Synthesis and characteristics of carbon nitride for micro humidity sensors. Sensors 2008, 8, 1508–1518. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lei, S.; Chen, Y. A single polyaniline nanofiber field effect transistor and its gas sensing mechanisms. Sensors 2011, 11, 6509–6516. [Google Scholar] [CrossRef] [PubMed]
- Schüler, M.; Sauerwald, T.; Schütze, A. Metal oxide semiconductor gas sensor self-test using Fourier-based impedance spectroscopy. J. Sens. Syst. 2014, 3, 213–221. [Google Scholar]
- Kang, K.S.; Lee, S.P. CO Gas Sensors Operating at Room Temperature. J. Mater. Sci. 2003, 38, 4319–4323. [Google Scholar] [CrossRef]
- Hagleitner, C.; Lange, D.; Hierlemann, A.; Brand, O.; Baltes, H. CMOS single-chip gas detection system comprising capacitive, calorimetric and mass-sensitive microsensors. IEEE J. Solid-St. Circ. 2002, 37, 1867–1878. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Li, S.; Liu, M.; He, S. Advances in SAW gas sensors based on the condensate-adsorption effect. Sensors 2011, 11, 11871–11884. [Google Scholar] [CrossRef] [PubMed]
- Marr, I.; Reiß, S.; Hagen, G.; Moos, R. Planar zeolite film-based potentiometric gas sensors manufactured by a combined thick-film and electroplating technique. Sensors 2011, 11, 7736–7748. [Google Scholar] [CrossRef] [PubMed]
- Chinvongamorn, C.; Pinwattana, K.; Praphairaksit, N.; Imato, T.; Chailapakul, O. Amperometric determination of sulfite by gas diffusion-sequential injection with boron-doped diamond electrode. Sensors 2008, 8, 1846–1857. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.R. Selectivity in semiconductor gas sensors. Sens. Actuators B Chem. 1987, 12, 425–440. [Google Scholar] [CrossRef]
- Morrison, S.R. Mechanism of semiconductor gas sensor operation. Sens. Actuators B Chem. 1987, 11, 283–287. [Google Scholar] [CrossRef]
- Barsan, N.; Huebner, M.; Weimar, U. Conduction mechanism in semiconducting metal oxide sensing film: Impact on transition. In Semiconductor Gas Sensors; Jaaniso, R., Tan, O.K., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 64–113. [Google Scholar]
- Ho, K.-C.; Hung, W.-T.; Yang, J.-C. On the Electrooxidation and amperometric detection of no gas at the Pt/nafion® electrode. Sensors 2003, 3, 290–303. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. Review A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Potje-Kamloth, K. Semiconductor junction gas sensors. Chem. Rev. 2008, 108, 367–399. [Google Scholar] [CrossRef] [PubMed]
- Hubert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Korotcenkov, G. Practical aspects in design of one-electrode semiconductor gas sensors: Status report. Sens. Actuators B Chem. 2007, 121, 664–678. [Google Scholar] [CrossRef]
- Pascoe, K.J. Properties of Materials for Electrical Engineers; John Wiley & Sons: London, UK, 1973. [Google Scholar]
- Rhoderick, E.H. Metal-Semiconductor Contacts; Clarendon Press: Oxford, UK, 1978. [Google Scholar]
- Sze, S.M. Physics of Semiconductor Devices; John Wiley & Sons: New York, NY, USA, 1981. [Google Scholar]
- Sze, S. Semiconductor Devices; John Wiley & Sons: New York, NY, USA, 2002. [Google Scholar]
- Zhou, X.; Xu, Y.; Cao, Q.; Niu, S. Metal–semiconductor ohmic contact of SnO2-based ceramic gas sensors. Sens. Actuators B Chem. 1997, 41, 163–167. [Google Scholar] [CrossRef]
- Schierbaum, K.D. Engineering of oxide surfaces and metal/oxide interfaces for chemical sensors: Recent trends. Sens. Actuators B Chem. 1995, 24–25, 239–247. [Google Scholar] [CrossRef]
- Zhang, W.; Vascincelos, E.A.; Uchida, H.; Katsube, T.; Nakatsubo, T.; Nishioka, Y. A study of silicon Schottky diode structures for NOx gas detection. Sens. Actuators B Chem. 2000, 65, 154–156. [Google Scholar] [CrossRef]
- Irokawa, Y. Hydrogen sensors using nitride-based semiconductor diodes: The role of metal/semiconductor interfaces. Sensors 2011, 11, 674–695. [Google Scholar] [CrossRef] [PubMed]
- Schalwig, J.; Müller, G.; Karrer, U.; Eickhoff, M.; Ambacher, O.; Stutzmann, M.; Görgens, L.; Dollinger, G. Hydrogen response mechanism of Pt–GaN Schottky diodes. Appl. Phys. Lett. 2002, 80, 1222–1224. [Google Scholar] [CrossRef]
- Petty, M.C. Conduction mechanisms in Pd/SiO2/n-Si Schottky diode hydrogen detectors. Solid State Electron. 1986, 29, 89–97. [Google Scholar] [CrossRef]
- Matsuo, K.; Negoro, N.; Kotani, J.; Hashizume, T.; Hasegawa, H. Pt Schottky diode gas sensors formed on GaN and AlGaN/GaN heterostructure. Appl. Surf. Sci. 2005, 244, 273–276. [Google Scholar] [CrossRef]
- Soo, M.T.; Cheong, K.Y.; Noor, A.F.M. Advances of SiC-based MOS capacitor hydrogen sensors for harsh environment applications. Sens. Actuators B Chem. 2010, 151, 39–55. [Google Scholar] [CrossRef]
- Toohey, M.J. Electrodes for nano dot-based gas sensors. Sens. Actuators B Chem. 2005, 105, 232–250. [Google Scholar] [CrossRef]
- Song, K.; Joo, B.; Choi, N.; Lee, Y.; Lee, S.; Huh, J.; Lee, D. A micro hot-wire sensors for gas sensing applications. Sens. Actuators B Chem. 2004, 102, 1–6. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Boris, I.; Brinzari, V.; Lychkovsky, Y.; Karkotsky, G.; Golovanov, V.; Cornet, A.; Rossinyol, E.; Rodriguez, J.; Cirera, A. Gas sensing characteristics of one-electrode gas sensors on the base of doped In2O3ceramics. Sens. Actuators B Chem. 2004, 103, 13–22. [Google Scholar] [CrossRef]
- Jones, E. The Pellistor Catalytic Gas Sensor, in Moseley P and Tofield B (Eds.); Solid-state Gas Sensors, Institute of Physics: Bristol, UK, 1987; pp. 17–31. [Google Scholar]
- Fukui, K. Detection and measurements of odour by sintered tin oxide. Sens. Actuators B Chem. 1991, 5, 27–32. [Google Scholar] [CrossRef]
- Malchenko, S.; Lychkovski, Y.; Baykov, M. In2O3-based gas sensors. Sens. Actuators B Chem. 1993, 13–14, 159–161. [Google Scholar] [CrossRef]
- Malchenko, S.; Lychkovski, Y.; Baykov, M. One electrode semiconductor sensors for detection of toxic and explosive gases in air. Sens. Actuators B Chem. 1992, 7, 505–506. [Google Scholar] [CrossRef]
- Golovanov, V.; Liu, C.C.; Kiv, A.; Fuk, D.; Ivanovskaya, M. Microfabricated one-electrode In2O3 and Fe2O3 composite sensors. Solid State Phys. 2009, 13, 68–73. [Google Scholar]
- Faglia, G.; Comini, E.; Sberveglieri, G.; Rella, R.; Siciliano, P.; Vasanelli, L. Square and collinear four probe array and Hall measurements on metal oxide thin film gas sensors. Sens. Actuators B Chem. 1998, 53, 69–75. [Google Scholar] [CrossRef]
- Williams, D. Semiconducting oxides as gas-sensitive resistors. Sens. Actuators B Chem. 1999, 57, 1–16. [Google Scholar] [CrossRef]
- Gerlich, M.; Kornely, S.; Fleischer, M.; Meixner, H.; Kassing, R. Selectivity enhancement of a WO3/TiO2 gas sensor by the use of a four-point electrode structure. Sens. Actuators B Chem. 2003, 93, 503–508. [Google Scholar] [CrossRef]
- Bläser, D.; Rühl, T.; Diehl, C.; Ulrich, M.; Kohl, D. Nanostructured semiconductor gas sensors to overcome sensitivity limitations due to percolation effects. Phys. A 1999, 266, 218–223. [Google Scholar] [CrossRef]
- Lundstrom, I.; Shivaraman, M.S.; Svensson, S. A hydrogen sensitive Pd-gate MOS transistors. J. Appl. Phys. 1975, 46, 3876–3881. [Google Scholar] [CrossRef]
- Lundstrom, I. Hydrogen sensitive MOS structures: Part 1: Principles and applications. Sens. Actuators B Chem. 1981, 1, 403–426. [Google Scholar] [CrossRef]
- Abe, H.; Esashi, M.; Matsuo, T. ISFET’s using inorganic gate thin films. IEEE Trans. Electron Devices 1979, ED-26, 1932–1939. [Google Scholar]
- Sibbald, A.; Whalley, P.; Covington, A. A miniature flow-through cell with a four-junction chemFET integrated circuit. Anal. Chem. Acta 1984, 159, 47–62. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an ion-sensitive solid state device for neuro-physiological measurements. IEEE Trans. Biomed. Eng. 1990, BME-17, 70–71. [Google Scholar]
- Mariucci, L.; Fortunato, G.; Pecora, A.; Bearzotti, A.; Carelli, P.; Leone, R. Hydrogenated amorphous silicon technology for chemical sensing thin film transistors. Sens. Actuators B Chem. 1992, 6, 29–33. [Google Scholar] [CrossRef]
- Litovchenko, V.G.; Gorbanyuk, T.I.; Efremov, A.A.; Evtukh, A.A. Effect of macrostructure and composition of the top metal electrode on properties of MIS gas sensors. Microelectron. Reliabil. 2000, 40, 821–824. [Google Scholar] [CrossRef]
- Qiu, Y.Y.; Azeredo-Leme, C.; Alcacer, L.R.; Franca, J.E. A CMOS humidity sensor with on-chip calibration. Sens. Actuators A Phys. 2001, 92, 80–87. [Google Scholar] [CrossRef]
- Gu, L.; Huang, Q.; Qin, M. A novel capacitive-type humidity sensor using CMOS fabrication technology. Sens. Actuators B Chem. 2004, 99, 491–498. [Google Scholar] [CrossRef]
- Eriksson, M.; Salomonsson, A.; Lundström, I. The influence of the insulator surface properties on the hydrogen response of field-effect gas sensors. J. Appl. Phys. 2005, 98, 0349031–0439036. [Google Scholar] [CrossRef]
- Fukuda, H.; Kasama, K.; Nomura, S. Highly sensitive MISFET sensors with porous Pt–SnO2 gate electrode for CO gas sensing applications. Sens. Actuators B Chem. 2000, 64, 163–168. [Google Scholar] [CrossRef]
- Trinchi, A.; Kandasamy, S.; Wlodarski, W. High temperature field effect hydrogen and hydrocarbon gas sensors based on SiC MOS devices. Sens. Actuators B Chem. 2008, 133, 705–716. [Google Scholar] [CrossRef]
- Johnson, C.L.; Wise, K.D.; Schwank, J.W. A Thin-film Gas Detector for Semiconductor Process Gases. IEDM Tech. Dig. 1988, 662–663. [Google Scholar]
- Suehle, J.S.; Cavicchi, R.E.; Gaitan, M.; Semancik, S. Tin oxide gas sensor fabricated using CMOS micro-hotplates and in situ processing. IEEE Electron Device Lett. 1993, 4, 118–120. [Google Scholar] [CrossRef]
- Chan, P.C.H.; Yan, G.; Sheng, L.Y.; Sharma, R.K.; Tang, Z.; Sin, J.K.O.; Hsing, I.M.; Wang, Y.Y. An integrated gas sensor technology using surface micro-machining. In Proceedings of the MEMS 2001, Interlaken, Switzerland, 21–25 January 2001; pp. 543–546. [Google Scholar]
- Yan, G.; Tang, Z.; Chan, P.C.H.; Sin, J.K.O.; Hsing, I.M.; Wang, Y. An experimental study on high-temperature metallization for micro-hotplate-based integrated gas sensors. Sens. Actuators B Chem. 2002, 86, 1–11. [Google Scholar] [CrossRef]
- Dai, C.L.; Xiao, F.Y.; Juang, Y.Z.; Chiu, C.F. An approach to fabricating microstructures that incorporate circuits using a post-CMOS process. J. Micromech. Microeng. 2005, 15, 98–103. [Google Scholar] [CrossRef]
- Dai, C.L. A capacitive humidity sensor integrated with micro heater and ring oscillator circuit fabricated CMOS-MEMS technique. Sens. Actuators B Chem. 2007, 122, 375–380. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Suspended gate field effect transistors modified with polypyrrole as alcohol sensor. Anal. Chem. 1986, 58, 514–517. [Google Scholar]
- Hauptman, P. Sensors: Principles and Applications; Prentice Hall: Hertfordshire, UK, 1991. [Google Scholar]
- Lee, S.P.; Park, K.J. Humidity sensitive field effect transistors. Sens. Actuators B Chem. 1996, 35–36, 80–84. [Google Scholar] [CrossRef]
- Lee, K.N.; Seo, Y.T.; Yoon, S.; Lee, M.H.; Kim, Y.K.; Seong, W.K. Chemical gating experiment of a nano-field-effect transistor sensor using the detection of negative ions in air. Sens. Actuators B Chem. 2016, 236, 654–658. [Google Scholar] [CrossRef]
- Jain, U.; Harker, A.; Stoneham, A.; Williams, D. Effect of electrode geometry on sensor response. Sens. Actuators B Chem. 1990, 2, 111–114. [Google Scholar] [CrossRef]
- Williams, D.; Pratt, K. Theory of self-diagnostic sensor array devices using gas-sensitive resistors. J. Chem. Soc. Faraday Trans. 1995, 91, 1961–1966. [Google Scholar] [CrossRef]
- Vilanova, X.; Llobet, E.; Brezmes, J.; Calderer, J.; Correig, X. Numerical simulation of the electrode geometry and position effects on semiconductor gas sensor response. Sens. Actuators B Chem. 1998, 48, 425–431. [Google Scholar] [CrossRef]
- Gardner, J. Intelligent gas sensing using an integrated sensor pair. Sens. Actuators B Chem. 1995, 26–27, 261–266. [Google Scholar] [CrossRef]
- VitaeTamaki, J.; Miyaji, A.; Makinodan, J.; Ogura, S.; Konishi, S. Effect of micro-gap electrode on detection of dilute NO2 using WO3 thin film microsensors. Sens. Actuators B Chem. 2005, 108, 202–206. [Google Scholar]
- Shaalan, N.M.; Yamazaki, T.; Kikuta, T. Influence of morphology and structure geometry on NO2 gas-sensing characteristics of SnO2 nanostructures synthesized via a thermal evaporation method. Sens. Actuators B Chem. 2011, 153, 11–16. [Google Scholar] [CrossRef]
- Hoefer, U.; Steiner, K.; Wagner, E. Contact and sheet resistances of SnO2 thin films from transmission-line model measurements. Sens. Actuators B Chem. 1995, 26, 59–63. [Google Scholar] [CrossRef]
- Hoefer, U.; Böttner, H.; Felske, A.; Kühner, G.; Steiner, K.; Sulz, G. Thin-film SnO2 sensor arrays controlled by variation of contact potential––A suitable tool for chemometric gas mixture analysis in the TLV range. Sens. Actuat. B 1997, 44, 429–433. [Google Scholar] [CrossRef]
- Capone, S.; Siciliano, P.; Quaranta, F.; Rella, R.; Epifani, M.; Vasanelli, L. Moisture influence of geometry effect of Au and Pt electrodes on CO sensing response of SnO2 microsensors based on sol-gel thin film. Sens. Actuators B Chem. 2001, 77, 503–511. [Google Scholar] [CrossRef]
- Gourari, H.; Lumbreras, M.; Van Landschoot, R.; Schoonman, J. Electrode nature effects on stannic oxide type layers prepared by electrostatic spray deposition. Sens. Actuators B Chem. 1999, 58, 365–369. [Google Scholar] [CrossRef]
- Saukko, S.; Latto, V. Influence of electrode material on properties of SnO2-based gas sensor. Thin Solid Film 2003, 436, 137–140. [Google Scholar] [CrossRef]
- Durrani, S.M. The influence of electrode metals and its configuration on the response of tin oxide thin film CO sensor. Talanta 2006, 68, 732–1735. [Google Scholar] [CrossRef] [PubMed]
- Pijolat, C. Etude des pRoprietes Physico-Chimiques et des Proprietes Electriques du Dioxyde d’etain en Fonction de l’atmosphere Gazeuse Environnante, Application a la Detection Selective des Gaz. Ph.D. Thesis, IN Polytechnique de Grenoble, Grenoble, France, 1986. [Google Scholar]
- Bertrand, J.; Koziej, D.; Barsan, N.; Viricelle, J.P.; Pijolat, C.; Weimar, U. Influence of the nature of the electrode on the sensing performance of SnO2 sensors; Impedance spectroscopy studies. In Proceedings of the Eurosensors XX, Goeteborg, Sweden, 17–20 September 2006; pp. 100–101. [Google Scholar]
- Ylinampa, A.; Lantto, V.; Leppävuori, S. Some differences between Au and Pt electrodes in SnO2 thick-film gas sensors. Sens. Actuators B Chem. 1993, 13–14, 602–604. [Google Scholar] [CrossRef]
- Lin, H.M.; Tzeng, S.J.; Hsiau, P.J.; Tsai, W.I. Electrode effects on gas sensing properties of nanocrystalline zinc oxide. Nanostruct. Mater. 1998, 10, 465–477. [Google Scholar]
- RBender, F.; Kim, C.; Mlsna, T.; Vetelino, J.F. Characterization of a WO3 thin film chlorine sensor. Sens. Actuators B Chem. 2001, 77, 281–286. [Google Scholar]
- Rank, S.; Hafner, S.; Barsan, N.; Weimar, U. The impact of the nature of the electrode material on SnO2 thick Film sensor performance: Influence on oxygen adsorption. Proc. Eng. 2012, 47, 514–517. [Google Scholar] [CrossRef]
- Sofian, M.; Oussama, M.E.; Imad, A.A.; Marsha, C.K. Semiconducting metal oxide based sensors for selective gas pollutant detection. Sensors 2009, 9, 8158–8196. [Google Scholar]
- Ahmad, I.A. Metal/metal-oxide nanoclusters for gas sensor applications. J. Nanomater. 2016, 16, 1–717. [Google Scholar] [CrossRef]
- Barsan, N.; Schweizer-Berberich, M.; Göpel, W. Fundamental and practical aspects in the desgin of nanoscaled SnO2 gas sensors: A status report. J. Anal Chem. 1999, 365, 287–304. [Google Scholar] [CrossRef]
- Steele, M.; MacIver, B. Palladium/cadmium-sulfide Schottky diodes for hydrogen detection. Appl. Phys. Lett. 1976, 28, 687–1687. [Google Scholar] [CrossRef]
- Ito, K. Hydrogen sensitive Schottky barrier diodes. Surf. Sci. 1979, 86, 345–352. [Google Scholar] [CrossRef]
- Cheng, C.; Tsai, Y.; Lin, K.; Chen, H.; Hsu, W.; Chuang, H.; Chen, C.; Liu, W. Hydrogen sensing characteristics of Pd- and Pt-Al0.3Ga0.7As metal semiconductor(MS) Schottky diodes. Semicond. Sci. Tech. 2004, 19, 778–782. [Google Scholar] [CrossRef]
- Kim, J.; Gila, B.; Chung, G.; Abernathy, C.; Pearton, S.; Ren, F. Hydrogen sensitive GaN Schottky diodes. Solid State Electron. 2003, 47, 1069–1073. [Google Scholar] [CrossRef]
- Basu, S.; Roy, S.; Jacob, C. Ruthenium as Schottky metal for SiC-based high temperature hydrogen sensors. Mater. Technol. Hydrogen Economy 2004, 801, 193–198. [Google Scholar] [CrossRef]
- Salehi, A.; Nazerian, V. Characterization of magnetic Ni/n-Si Schottky contact for hydrogen gas sensing applications. Sens. Actuators B Chem. 2007, 122, 572–577. [Google Scholar] [CrossRef]
- Pandis, C.; Brilis, N.; Bourithis, E.; Tsamakis, D.; Ali, H.; Krishnamoorthy, S.; Iliadis, A.; Kompitsas, M. Low-temperature hydrogen sensors based on Au nanoclusters and Schottky contacts on ZnO films deposited by pulsed laser deposition on Si and SiO2 substrates. IEEE Sens. J. 2007, 7, 448–454. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Purahmad, M. Wide dynamic range hydrogen sensing using silver-rutile Schottky diode. In Proceedings of the Third International Conference on Sensing Technology, Taipei, Taiwan, 30 November–3 December 2008; pp. 72–75. [Google Scholar]
- Song, J.; Lu, W.; Flynn, J.; Brandes, G. AlGaN/GaN Schottky diode hydrogen sensor performance at high temperatures with different catalytic metals. Solid State Electron. 2005, 49, 1330–1334. [Google Scholar] [CrossRef]
- Meixner, H.; Lampe, U. Metal oxide sensors. Sens. Actuators B Chem. 1996, 33, 198–202. [Google Scholar] [CrossRef]
- Korotcenkov, G. Handbook of Gas Sensor Materials; Springer: New York, NY, USA, 2013. [Google Scholar]
- Hoefer, U.; Kühner, G.; Schweizer, W.; Sulz, G.; Steiner, K. CO and CO2 thin-film SnO2 gas sensors on Si substrates. Sens. Actuators B Chem. 1994, 22, 115–119. [Google Scholar] [CrossRef]
- Michel, H.J.; Leiste, H.; Halbritter, J. Structural and electrical characterization of PVD-deposited SnO2 films for gas –sensor application. Sens. Actuators B Chem. 1995, 24–25, 568–572. [Google Scholar] [CrossRef]
- Sozza, A.; Dua, C.; Kerlain, A.; Brylinski, C.; Zanoni, E. Long-term stability of Ti-Pt-Au metallization system for Schottky contact and first-level metallization on SiC MESFET. Microelectron. Reliab. 2004, 44, 1109–1113. [Google Scholar] [CrossRef]
- Capone, S.; Epifani, M.; Francioso, L.; Kaciulis, S.; Mezzi, A.; Siciliano, P.; Taurino, A. Influence of electrodes ageing on the properties of the gas sensors based on SnO2. Sens. Actuators B Chem. 2006, 115, 396–402. [Google Scholar] [CrossRef]
- Lee, J.; Kim, E. Effect of structural and morphological changes on the conductivity of stretched PANI-DBSA/HIPS film. Bull. Korean Chem. Soc. 2011, 32, 2661–2665. [Google Scholar] [CrossRef]
- Skotheim, T.A. Handbook of Conducting Polymers; Marcel Dekker: New York, NY, USA, 1986. [Google Scholar]
- Ray, S.; Easteal, A.J.; Cooney, R.P.; Edmonds, N.R. Structure and properties of melt-processed PVDF/PMMA/polyaniline blends. Mat. Chem. Phys. 2009, 113, 829–838. [Google Scholar] [CrossRef]
- Singh, S.K.; Gupta, R.K.; Singh, R.A. Optical, mechanical, and electrical studies of polymer composites based on charge transfer complex of phenothiazine-iodine with poly(vinyl chloride). Synth. Met. 2009, 159, 2478–2485. [Google Scholar] [CrossRef]
- Zhang, D.H. On the conductivity measurement of polyaniline pellets. Polym. Test. 2007, 26, 9–13. [Google Scholar] [CrossRef]
- Ansari, R.; Raofie, F. Removal of mercuric ion from aqueous solutions using sawdust coated by polyaniline. E-J. Chem. 2006, 3, 35–43. [Google Scholar] [CrossRef]
- Holmes, P.; Loasby, R. Handbook of Thick Film Technology; Electrochemical Pub.: Glasgow, UK, 1976. [Google Scholar]
- Heine, V. Theory of surface states. Phys. Rev. 1965, A138, 1689–1696. [Google Scholar] [CrossRef]
- Louie, S.G.; Chelikowsky, J.R.; Cohen, M.L. Ionicity and the theory of Schottky barriers. Phys. Rev. B 1977, 15, 2154–2162. [Google Scholar] [CrossRef]
- Mead, C. Metal-semiconductor surface barriers. Solid State Electron. 1966, 9, 1023–1032. [Google Scholar] [CrossRef]
- Andrew, J.; Phillips, J. Chemical bonding and structure of metal-semiconductor interfaces. Phys. Rev. Lett. 1969, 22, 1433–1436. [Google Scholar]
- Cowley, A.; Sze, S. Surface states and barrier height of metal-semiconductor systems. J. Appl. Phys. 1965, 36, 3212–3220. [Google Scholar] [CrossRef]
- Wada, O.; Majerfeld, A.; Robson, P. InP Schottky contacts with increased barrier height. Solid State Electron. 1982, 25, 381–387. [Google Scholar] [CrossRef]
- Lee, S.P. Electrode materials and electrode-oxide interfaces in semiconductor gas sensors. In Semiconductor Gas Sensors; Jaaniso, R., Tan, O.K., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 64–113. [Google Scholar]
- Bachrach, R.Z. Interface Chemistry and Structure of Schottky Barrier Formation, in Sharma B, Metal-Semiconductor Schottky Barrier Junction and Their Application; Plenum: New York, NY, USA, 1984. [Google Scholar]
- Neamen, D. Semiconductor Physics and Devices; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Tyagi, M. Physics of Schottky Barrier Junction, in Sharma B, Metal-Semiconductor Schottky Barrier Junction and Their Application; Plenum: New York, NY, USA, 1984. [Google Scholar]
- Wagner, C. Theory of current rectifier. Phys. Z. 1931, 32, 641–645. [Google Scholar]
- Schottky, W.; Spenke, E. Quantitative treatment of the space charge and boundary-layer theory of the crystal rectifier. Wiss. Veroff. Siemens-Werken 1939, 18, 225–291. [Google Scholar]
- Crowell, C.; Sze, S. Current transport in metal-semiconductor barrier. Solid State Electron. 1966, 9, 1035–1048. [Google Scholar] [CrossRef]
- Halas, S.; Durakiewicz, T. Work functions of elements expressed in terms of the Fermi energy and the density of free electrons. J. Phys. Conden. Matt. 1999, 10, 10815–10825. [Google Scholar] [CrossRef]
- Methfessel, M.; Hennig, D.; Scheffler, M. Trends of the surface relaxations, surface energies, and work functions of the 4d transition metals. Phys. Rev. 1992, B13, 4816–4829. [Google Scholar] [CrossRef]
- Akbi, M. A method for measuring the photoelectric work function of contact materials versus temperature. IEEE Trans. Compon. Packag. Manuf. Technol. 2014, 4, 1293–1302. [Google Scholar] [CrossRef]
- Padovani, F.; Stratton, R. Field and thermionic-field emission in Schottky barriers. Solid State Electron. 1966, 9, 695–707. [Google Scholar] [CrossRef]
- Crowell, C.; Rideout, V. Normalized thermionic-field emission in metal-semiconductor barriers. Solid State Electron. 1969, 12, 89–105. [Google Scholar]
- Schottky, W. Zur Halbleitertheorie der Sperrschicht- und Spitzengleichrichter. Phys. Z. 1939, 113, 367–414. [Google Scholar] [CrossRef]
- Bardeen, J. Surface States and Rectification at a Metal Semi-Conductor Contact. Phys. Rev. 1947, 71, 717–727. [Google Scholar] [CrossRef]

| Electrode Materials | Sensing Materials | Target Gases | References |
|---|---|---|---|
| Pd, Pt | ZnO, SnO2, In2O3, KTaO3 | H2 | Ito [100] |
| Au, Pd, Pt | SnO2 | H2 | Gourari [87] |
| Ru | SiC | H2 | Basu et al. [103] |
| Pt, IrPt, PdAg | Al GaN-GaN | H2 | Song et al. [107] |
| Ni | Si | H2 | Salehi et al. [104] |
| Au | ZnO | H2 | Pandis et al. [105] |
| Au, Pt | SnO2 | H2, CO | Rank et al. [95] |
| Au, Pt | SnO2 | H2, CO | Saukko et al. [88] |
| Au, Pt | SnO2 | CO | Capone et al. [86] |
| Au, Pt | SnO2 | CO | Bertland et al. [91] |
| Ag, Al, Au, Pt | SnO2 | CO | Durrani [89] |
| Au | Fe2O3-In2O3 | CO | Golovanov et al. [51] |
| Ag, Au | ZnO | CO, NO2 | Lin et al. [93] |
| Au | WO3 | NO2 | Tamaki et al. [82] |
| Au | SnO2 | NO2 | Shaalan et al. [83] |
| Au, Pt | SnO2 | Benzene | Pijolat [90] |
| Pt, Au, Pt-Au | SnO2 | H2O | Ylinampa et al. [92] |
| Al | WO3 | Cl2 | Bender et al. [94] |
| Materials | Electrical Properties | Advantages | Disadvantages |
|---|---|---|---|
| Silver |
|
|
|
| Gold |
|
|
|
| Platinum |
|
|
|
| Palladium-Silver |
|
|
|
| Platinum-Silver |
|
| |
| Platinum-Gold |
|
|
|
| Palladium-Gold |
|
|
|
|
| Metal | Work Function (eV) | Metal | Work Function (eV) |
|---|---|---|---|
| Pt | 5.65 | Zn | 4.33 |
| Ni | 5.25 | Al | 4.28 |
| Pd | 5.12 | Ag | 4.26 |
| Au | 5.1 | Pb | 4.25 |
| Cu | 4.65 | Ta | 4.25 |
| W | 4.55 | Cd | 4.22 |
| Cr | 4.5 | Ga | 4.2 |
| Hg | 4.49 | In | 4.12 |
| Sn | 4.42 | Zr | 4.05 |
| Ti | 4.33 | Cs | 2.14 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.P. Electrodes for Semiconductor Gas Sensors. Sensors 2017, 17, 683. https://doi.org/10.3390/s17040683
Lee SP. Electrodes for Semiconductor Gas Sensors. Sensors. 2017; 17(4):683. https://doi.org/10.3390/s17040683
Chicago/Turabian StyleLee, Sung Pil. 2017. "Electrodes for Semiconductor Gas Sensors" Sensors 17, no. 4: 683. https://doi.org/10.3390/s17040683





