Investigation on Inter-Limb Coordination and Motion Stability, Intensity and Complexity of Trunk and Limbs during Hands-Knees Crawling in Human Adults
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Crawling Scheme
2.2. Crawling Data Acquisition Based on Accelerometers and Force Sensors
2.3. Crawling Data Analysis
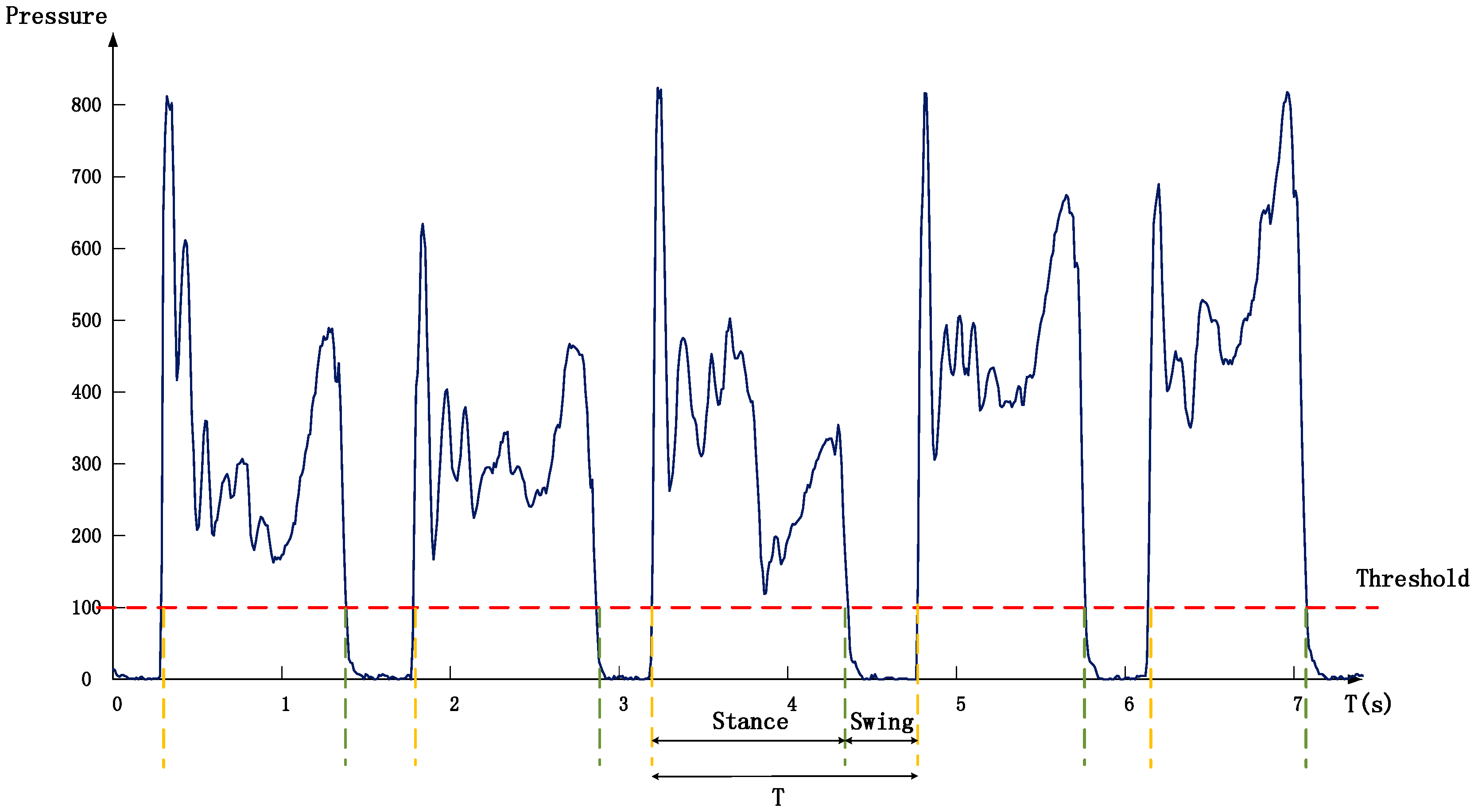
2.3.1. Data Pre-Processing and Stride Segmentation
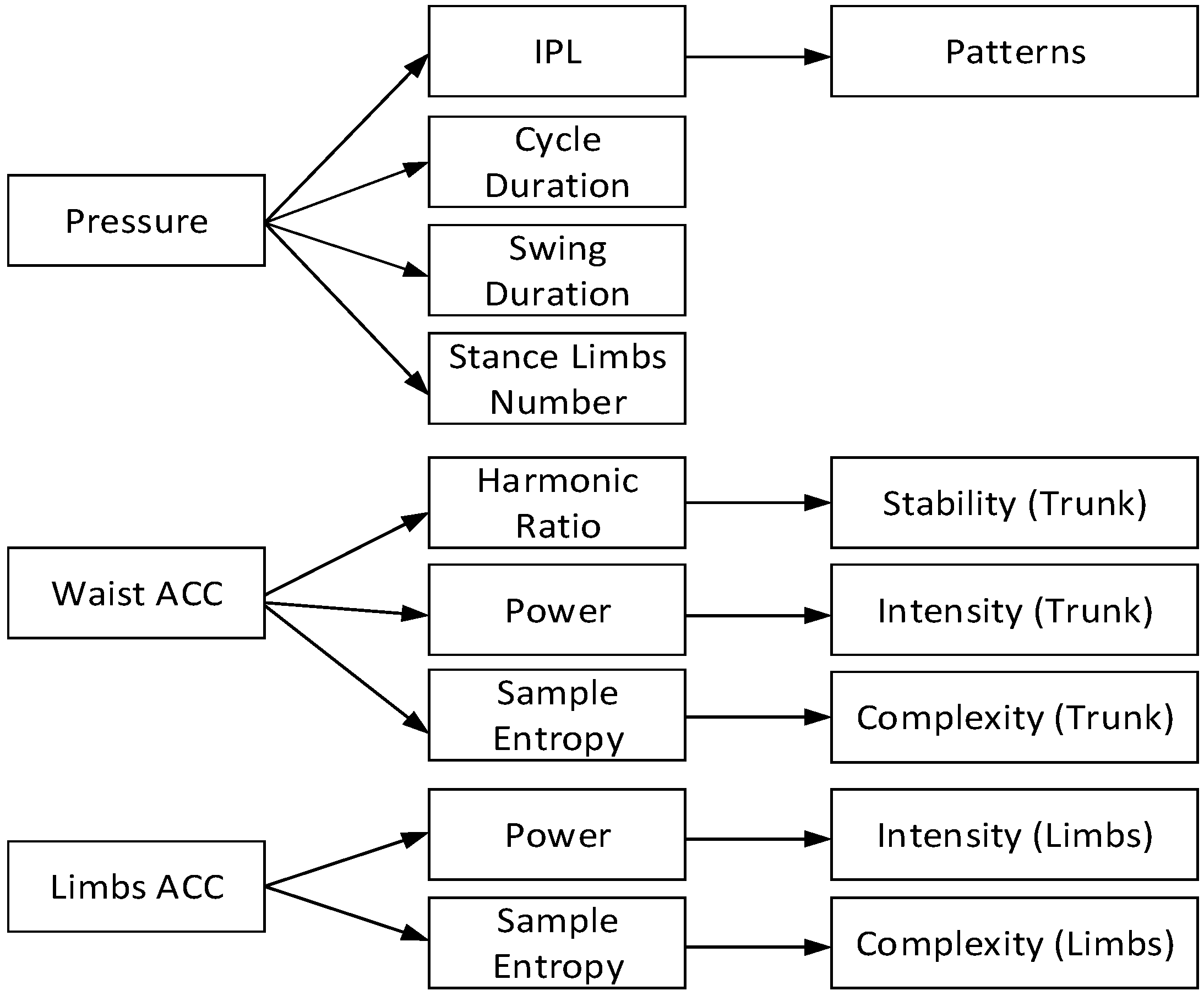
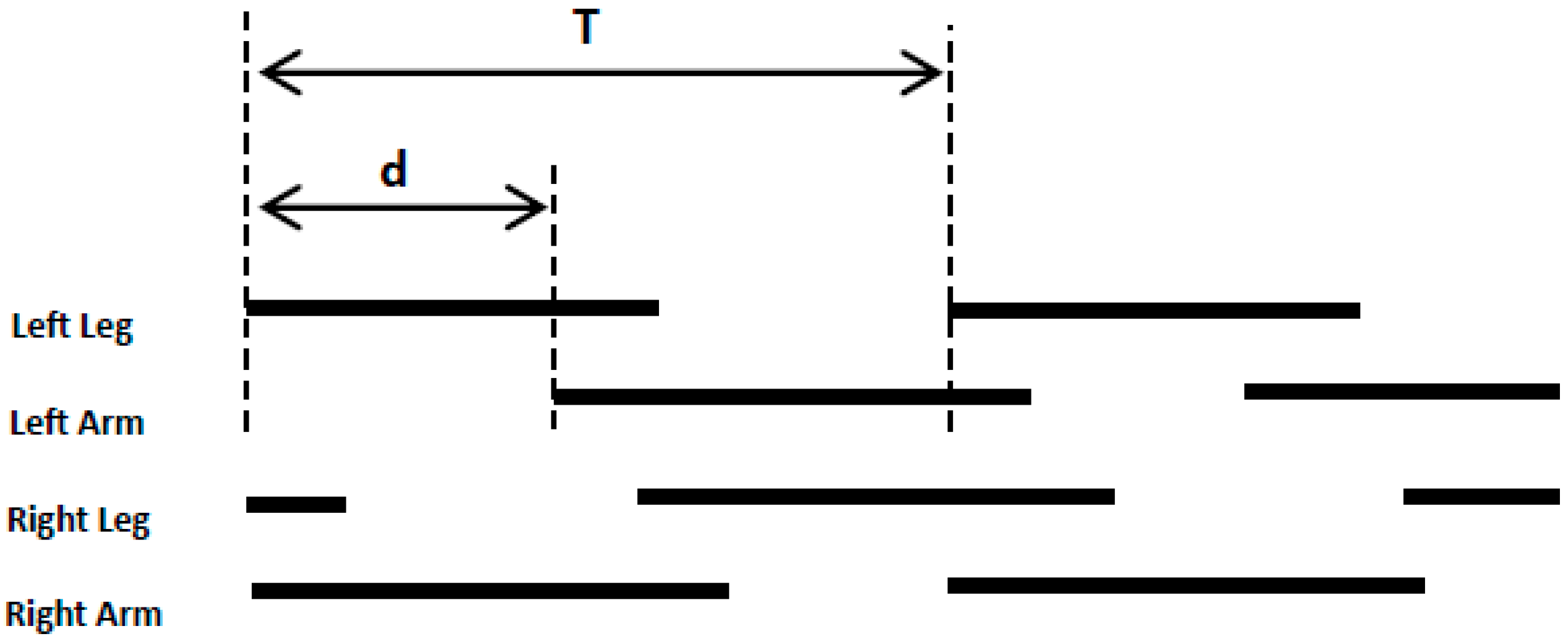
2.3.2. Crawling Movement Features Extraction
3. Results
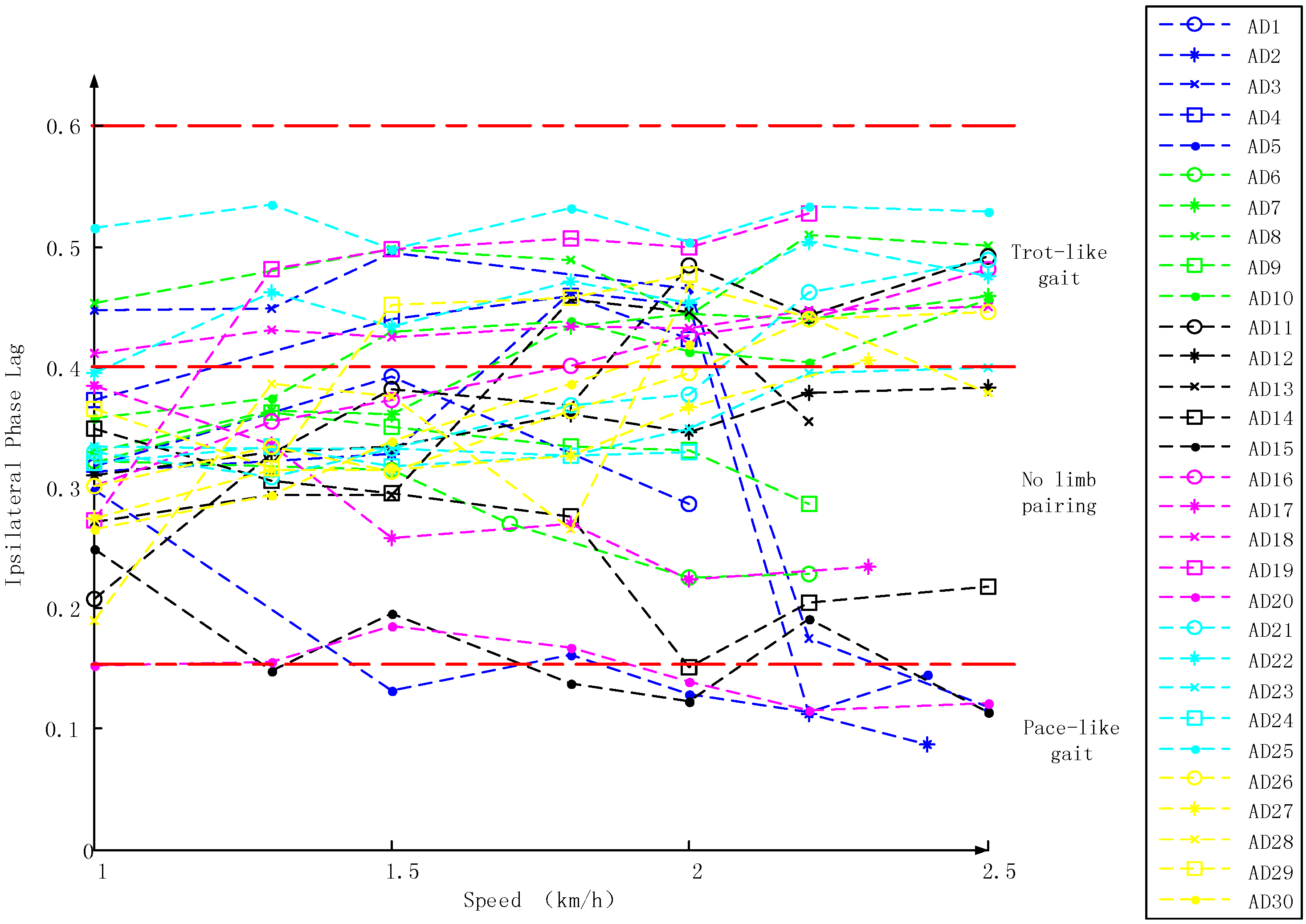
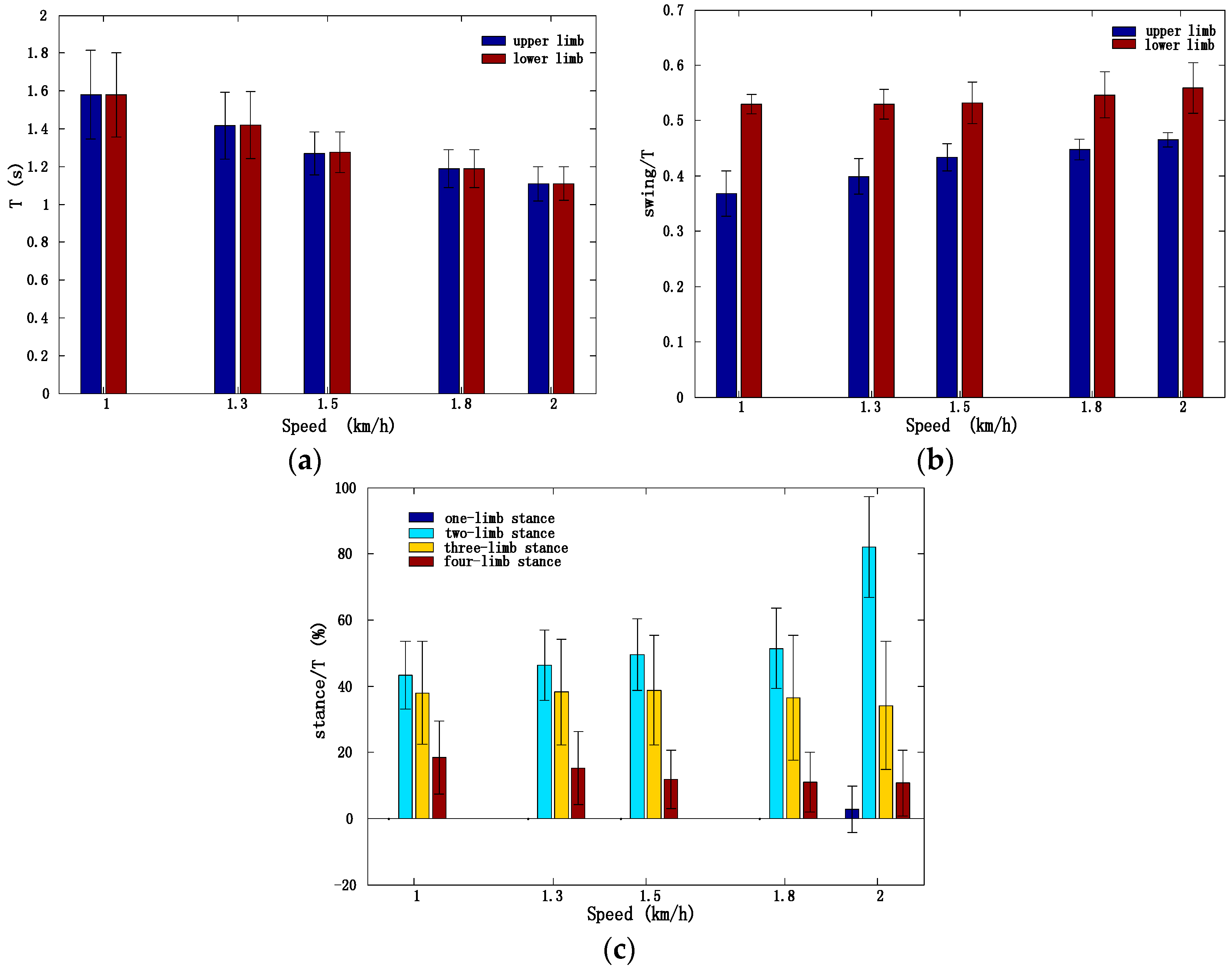
3.1. Inter-Limb Coordination Variations under Different Crawling Speeds
3.2. Motion Intensity, Stability, and Complexity Variations of Trunk under Different Speeds
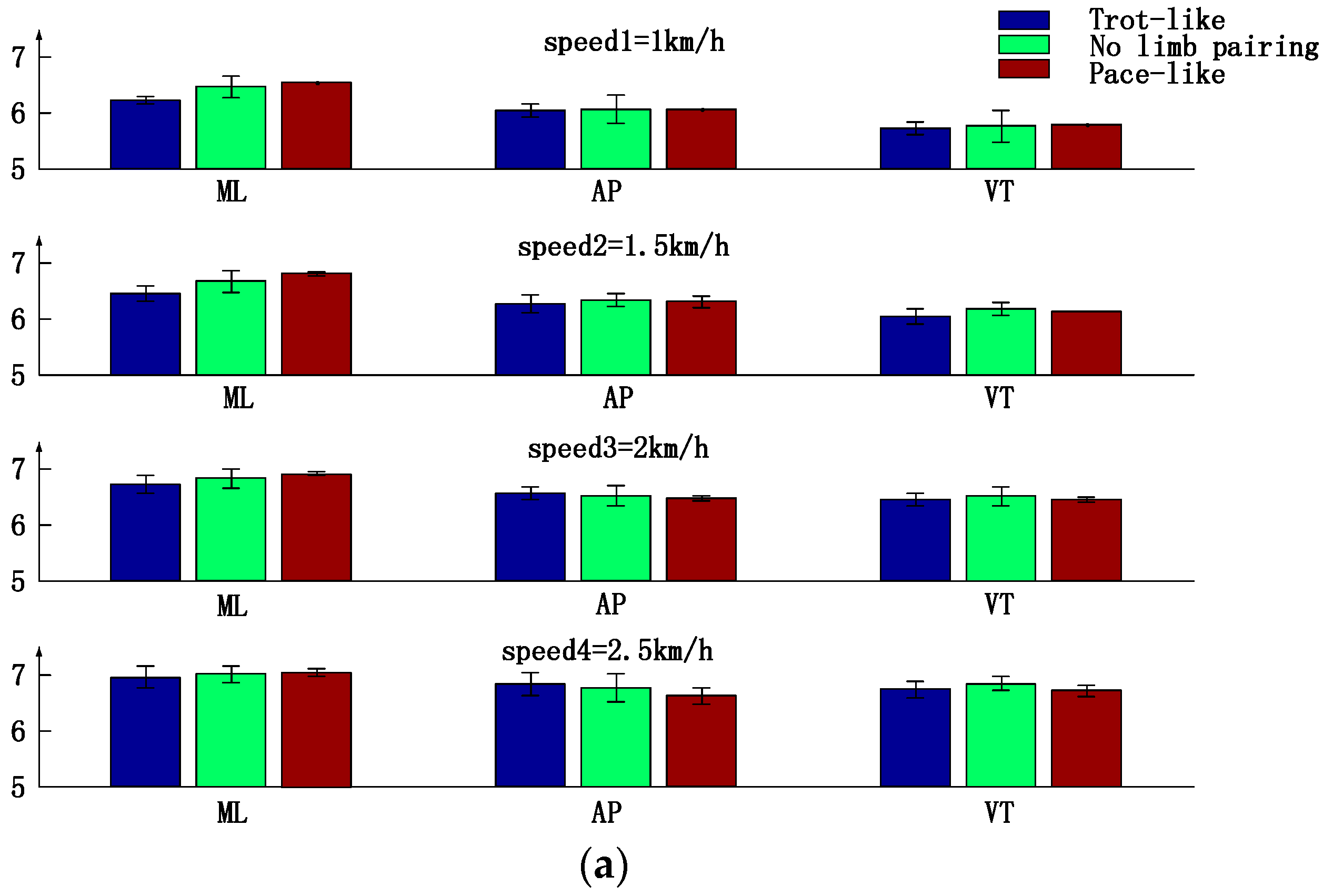
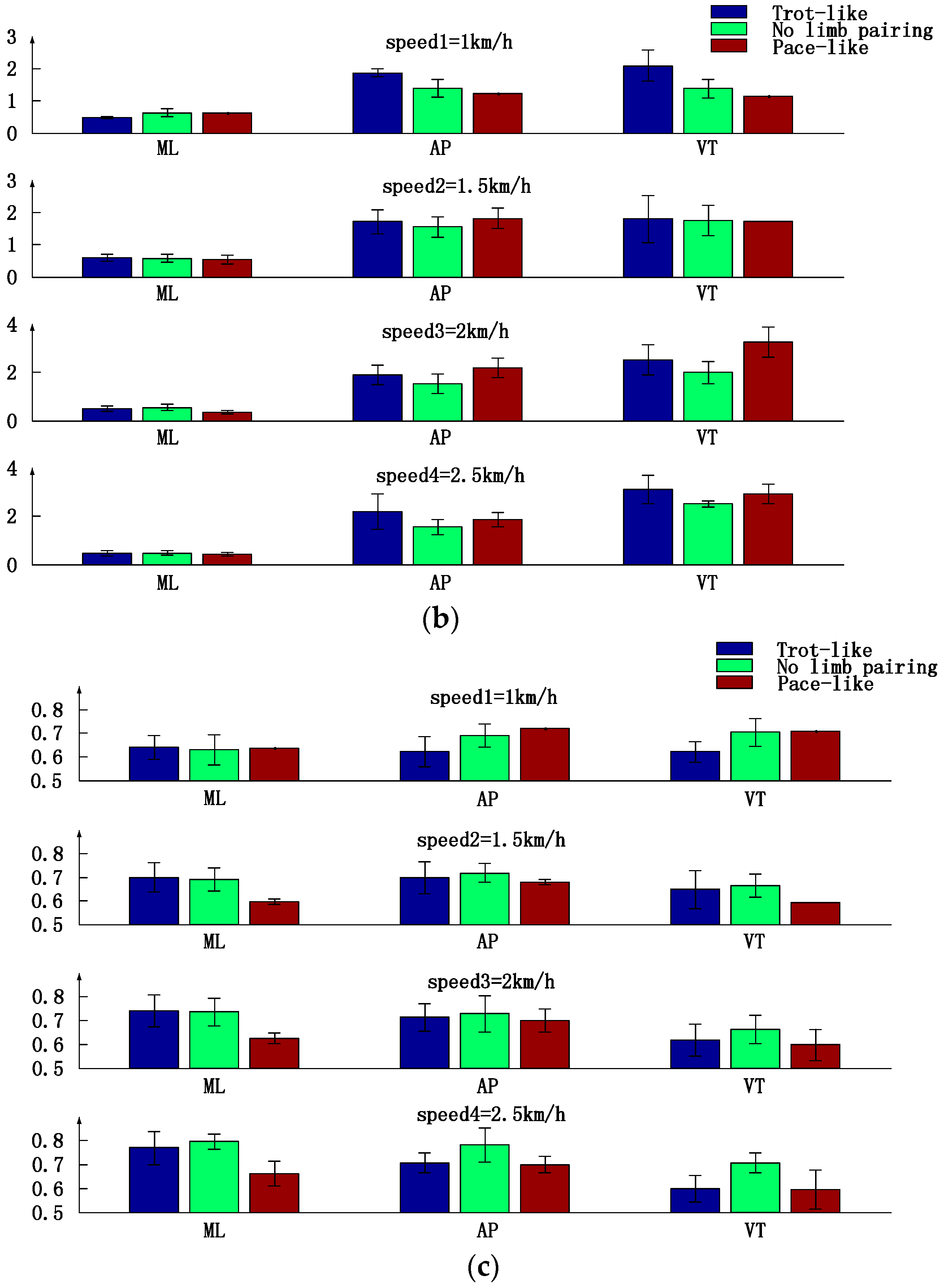
3.3. Trunk Motion Intensity, Stability and Complexity Parameters Comparison between Three Types of Inter-Limb Coordination Patterns under Different Crawling Speeds
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Calanca, A.; Piazza, S.; Fiorini, P. A motor learning oriented, compliant and mobile Gait Orthosis. Appl. Bionics Biomech. 2012, 9, 15–27. [Google Scholar] [CrossRef]
- Patrick, S.K.; Noah, J.A.; Yang, J.F. Developmental constraints of quadrupedal coordination across crawling styles in human infants. J. Neurophysiol. 2012, 107, 3050–3061. [Google Scholar] [CrossRef] [PubMed]
- MacLellan, M.J.; Ivanenko, Y.P.; Cappellini, G.; Labini, F.S.; Lacquaniti, F. Features of hand-foot crawling behavior on human adults. J. Neurophysiol. 2012, 107, 114–128. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, M.A.; Nash, M.D.; Fagg, A.H.; Ding, L.; Kolobe, T.H.A.; Miller, D.P. Novel assistive device for teaching crawling skills to infants. In Springer Tracts in Advanced Robotics; Springer: Berlin/Heidelberg, Germany, 2016; pp. 593–605. [Google Scholar]
- Ozer, K. Guidence for rehabilitation cerebral palsy. In Proceedings of the Ninth International Scientific Conference, Durres, Albania, 17–19 June 2016. [Google Scholar]
- Patrick, S.K.; Noah, J.A.; Yang, J.F. Interlimb coordination in human crawling reveals similarities in development and neural control with quadrupeds. J. Neurophysiol. 2009, 101, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.J.; Cole, W.G.; Young, J.W.; Raichlen, D.A.; Robinson, S.R.; Adolph, K.E. Human Quadrupeds, Primate Quadrupedalism, and Uner Tan Syndrome. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Owaki, D.; Kano, T.; Nagasawa, K.; Tero, A.; Ishiguro, A. Simple robot suggests physical interlimb communication is essential for quadruped walking. J. R. Soc. Interface 2012, 10. [Google Scholar] [CrossRef] [PubMed]
- Burnside, L.H. Coordination in the locomotion of infants. Genet. Psychol. Monogr. 1927, 2, 279–372. [Google Scholar]
- Schmitt, D. Insights into the evolution of human bipedalism from experimental studies of humans and other primates. J. Exp. Biol. 2003, 206, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M. Symmetrical Gaits of Horses. Science 1965, 150, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Courtine, G.; Roy, R.R.; Hodgson, J.; McKay, H.; Raven, J.; Zhong, H.; Yang, H.; Tuszynski, M.H.; Edgerton, V.R. Kinematic and EMG determinants in quadrupedal locomotion of a non-human primate. J. Neurophysiol. 2005, 93, 3127–3145. [Google Scholar] [CrossRef] [PubMed]
- Cartmill, M.; Lemelin, P.; Schmitt, D. Understanding the adaptive value of diagonal-sequence gaits in primates. Am. J. Phys. Anthropol. 2007, 133, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.J.; Raichlen, D.A. Lateral sequence walking in infant papio cynocephalus: Implications for the evolution of diagonal sequence walking inprimates. Am. J. Phys. Anthropol. 2005, 126, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.J.; Young, J.W. Is primate-like quadrupedalism necessary for fine-branch locomotion? A test using sugar gliders (Petaurus breviceps). J. Hum. Evol. 2010, 58, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, L.J.; Young, J.W.; Souther, A. Quadrupedal Locomotion of Saimiri boliviensis: A Comparison of Field and Laboratory-based Kinematic Data. In Primate Locomotion; D’Aout, K., Vereecke, E.E., Eds.; Springer: New York, NY, USA, 2010; pp. 335–356. [Google Scholar]
- Young, J.W. Gait Selection and the Ontogeny of Quadrupedal Walking in Squirrel Monkeys (Saimiri boliviensis). Am. J. Phys. Anthropol. 2012, 147, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Pontzer, H.; Raichlen, D.A. Bipedal and quadrupedal locomotion in chimpanzees. J. Hum. Evol. 2014, 66, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Wannier, T.; Bastiaanse, C.; Colombo, G.; Dietz, V. Arm to leg coordination in humans during walking, creeping, and swimming activities. Exp. Brain Res. 2001, 141, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Getchell, N.; Forrester, L.; Whitall, J. Individual differences and similarities in the stabilities, timing consistency, and natural frequency of rhythmic coordinated actions. Res. Q. Exerc. Sport. 2001, 72, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.; Pollard, J.; Porter, W.L. Locomotion in restricted space: Kinematic and electromyographic analysis of stoopwalking and crawling. Gait Posture 2011, 33, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, W.A.; Newel, K.M. The coordination and control of human creeping with increase in speed. Behav. Brain Res. 1994, 63, 151–158. [Google Scholar] [CrossRef]
- Heglund, N.C.; Taylor, C.R.; McMahon, T.A. Scaling stride frequency and gait to animal size: Mice to Horses. Science 1974, 186, 1112–1113. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, D.F.; Taylor, C.R. Gait and the energetics of locomotion in horse. Nature 1981, 292, 239–240. [Google Scholar] [CrossRef]
- Karantonis, D.M.; Narayanan, M.R.; Mathie, M.; Lovell, N.H.; Celler, B.G. Implementation of a real-time human movement classifier using a triaxial accelerometer for ambulatory monitoring. IEEE Trans. Inf. Technol. Biomed. 2006, 10, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Yack, H.J.; Berger, R.C. Dynamic stability in the elderly: Identifying a possible measure. J. Gerontol. 1993, 48, 225–230. [Google Scholar] [CrossRef]
- Sejdic, E.; Lowry, K.A.; Bellanca, J.; Redfern, M.S.; Brach, J.S. A Comprehensive Assessment of Gait Accelerometry Signals in Time, Frequency and Time-Frequency Domains. IEEE Trans. Neural. Syst. Rehabil. Eng. 2014, 22, 603–612. [Google Scholar] [CrossRef] [PubMed]
- Bellanca, J.L.; Lowry, K.A.; VanSwearingen, J.M.; Brach, J.S.; Redfern, M.S. Harmonic ratios: A quantification of step to step symmetry. J. Biomech. 2013, 46, 828–831. [Google Scholar] [CrossRef] [PubMed]
- Auvinet, B.; Berrut, G.; Touzard, C.; Moutel, L.; Collet, N.; Chaleil, D.; Barrey, E. Reference data for normal subjects obtained with an accelerometric device. Gait Posture 2002, 16, 124–134. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time series analysis using approximate entropy and sample entropy. Am. J. Physiol. 2000, 278, 2039–2049. [Google Scholar]
- Aktaruzzaman, M.; Sassi, R. Parametric estimation of sample entropy in heart rate variability analysis. Biomed. Signal Process. Control 2014, 14, 141–147. [Google Scholar] [CrossRef]
- Latash, M.L. The bliss (not the problem) of motor abundance (not redundancy). Exp. Brain Res. 2012, 217, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.H.; Chiel, H.J.; Trumbower, R.D.; Allen, J.L.; McKay, J.L.; Hackney, M.E.; Kesar, T.M. Neuromechanical principles underlying movement modularity and their implications for rehabilitation. Neuron 2015, 86, 38–54. [Google Scholar] [CrossRef] [PubMed]
- Scholz, J.P. Dynamic pattern theory—Some implications for therapeutics. Phys. Ther. 1990, 70, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Chiu, S.L.; Chou, L.S. Effect of walking speed on inter-joint coordination differs between young and elderly adults. J. Biomech. 2010, 45, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Muratori, L.M.; Lamberg, E.M.; Quinn, L.; Duff, S.V. Applying principles of motor learning and control to upper extremity rehabilitation. J. Hand Ther. 2013, 26, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Holt, K.G.; Jeng, S.F.; Ratcliffe, R.; Hamill, J. Energetic Cost and Stability during Human Walking at the Preferred Stride Frequency. J. Motor Behav. 1995, 27, 164–178. [Google Scholar] [CrossRef] [PubMed]
- Sides, D.; Wilson, C. Intra-limb coordinative adaptations in cycling. Sports Biomech. 2012, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Duncan, C.A.; Ingram, T.G.J.; Mansfield, A.; Byrne, J.M.; McIlroy, W.E. Population differences in postural response strategy associated with exposure to a novel continuous perturbation stimuli: would dancers have better balance on a boat? PLoS ONE 2016, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]

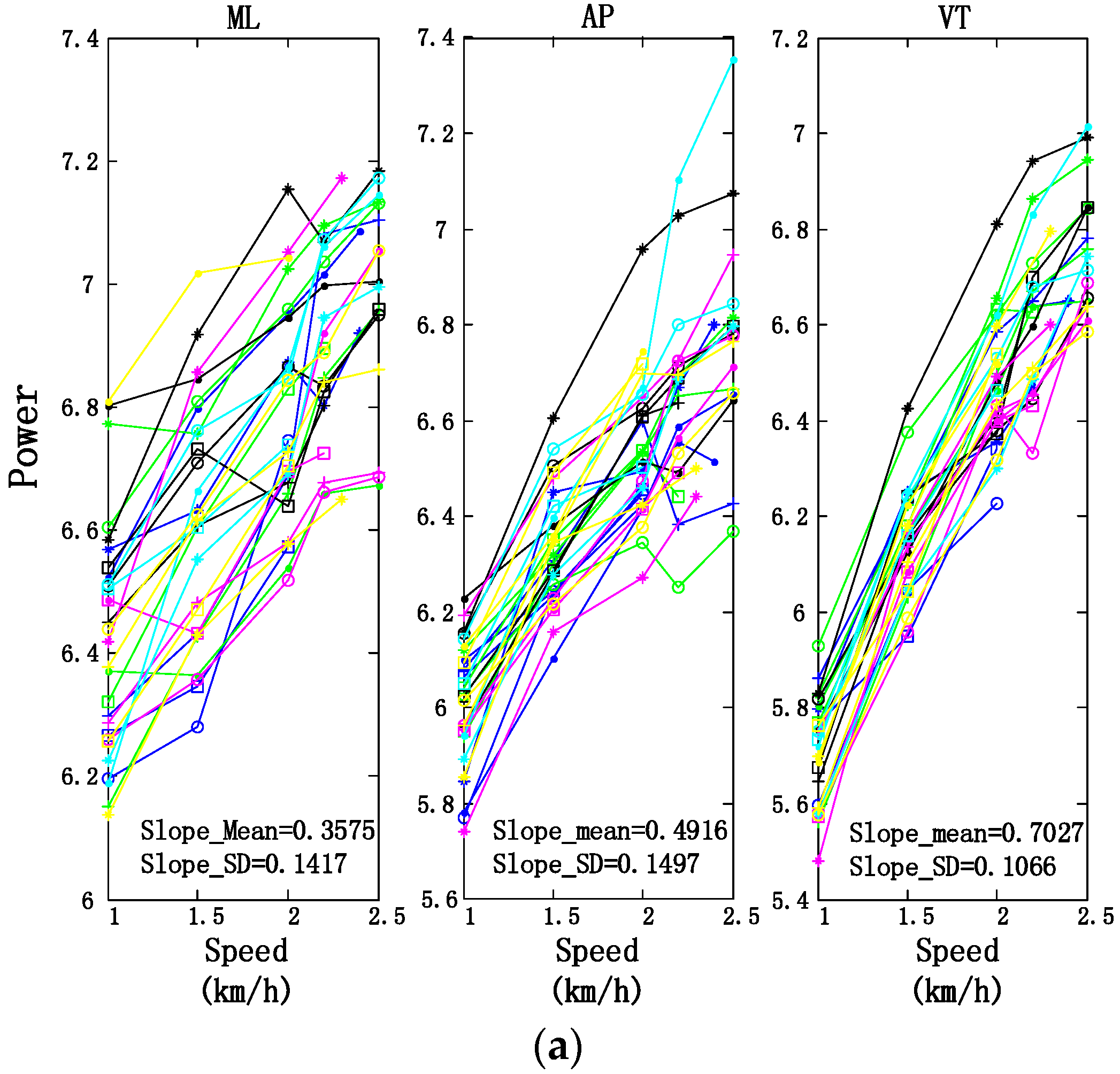
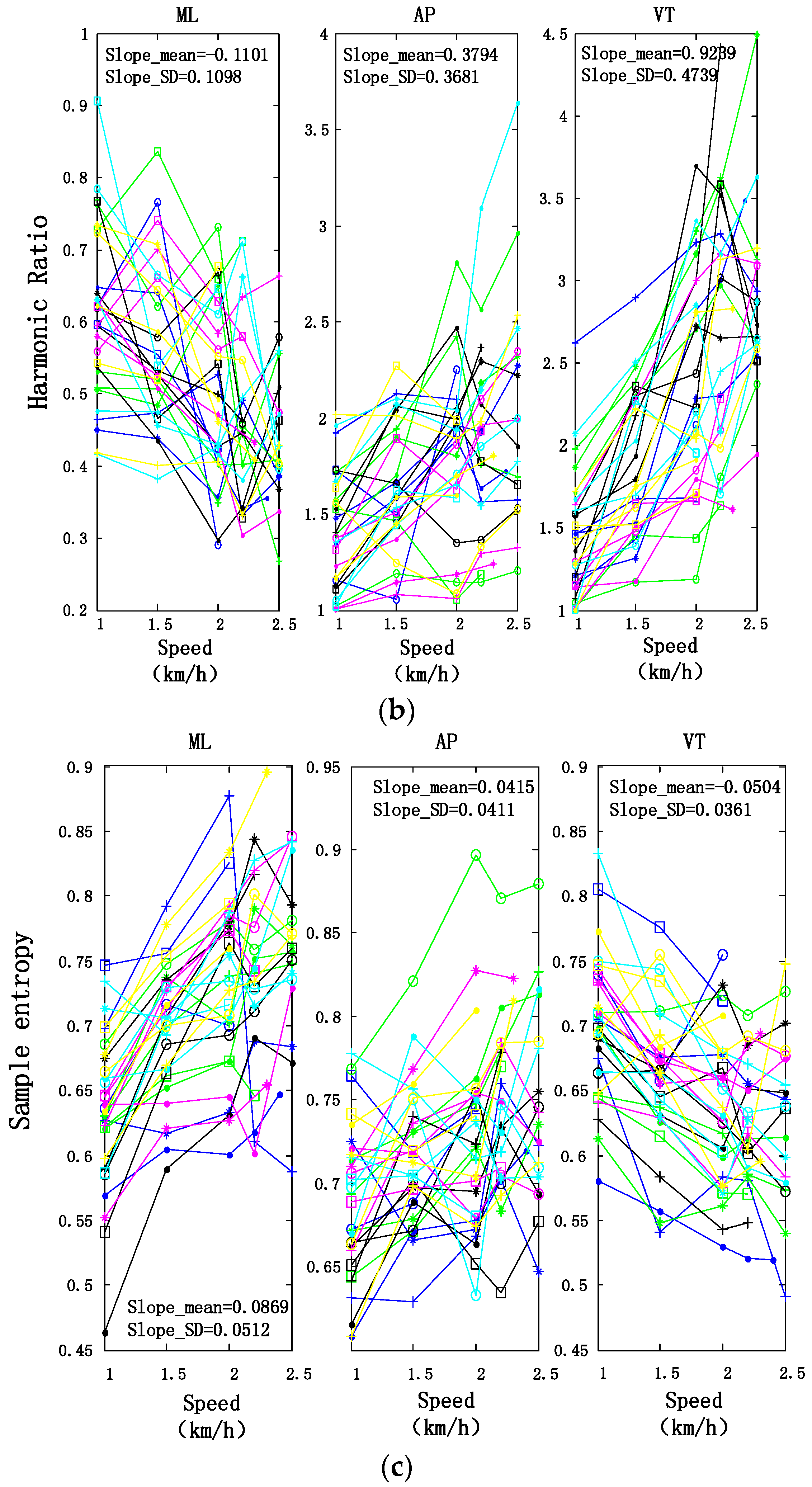
| Slopes of Fitting Lines (Mean ± SD) | ||||
|---|---|---|---|---|
| ML | AP | VT | ||
| Power | Trunk | 0.3575 ± 0.1417 | 0.4916 ± 0.1497 | 0.7027 ± 0.1067 |
| Upper limbs | 0.3092 ± 0.1293 | 0.3992 ± 0.1170 | 0.4283 ± 0.1246 | |
| Lower limbs | 0.2884 ± 0.1936 | 0.2019 ± 0.1752 | 0.1191 ± 0.0438 | |
| SE | Trunk | 0.0869 ± 0.0512 | 0.0415 ± 0.0411 | −0.0504 ± 0.0361 |
| Upper limbs | 0.0467 ± 0.0584 | 0.0202 ± 0.0645 | 0.1586 ± 0.0794 | |
| Lower limbs | 0.0735 ± 0.0540 | 0.0783 ± 0.0858 | 0.0914 ± 0.0629 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Chen, X.; Cao, S.; Yu, Y.; Zhang, X. Investigation on Inter-Limb Coordination and Motion Stability, Intensity and Complexity of Trunk and Limbs during Hands-Knees Crawling in Human Adults. Sensors 2017, 17, 692. https://doi.org/10.3390/s17040692
Ma S, Chen X, Cao S, Yu Y, Zhang X. Investigation on Inter-Limb Coordination and Motion Stability, Intensity and Complexity of Trunk and Limbs during Hands-Knees Crawling in Human Adults. Sensors. 2017; 17(4):692. https://doi.org/10.3390/s17040692
Chicago/Turabian StyleMa, Shenglan, Xiang Chen, Shuai Cao, Yi Yu, and Xu Zhang. 2017. "Investigation on Inter-Limb Coordination and Motion Stability, Intensity and Complexity of Trunk and Limbs during Hands-Knees Crawling in Human Adults" Sensors 17, no. 4: 692. https://doi.org/10.3390/s17040692






