1. Introduction
In recent decades, the overall consumption of chicken and turkey meat has increased considerably and it is expected that in the coming years, chicken will become the most produced type of meat in the world. It has been estimated that in 2020, global chicken meat production will be around 122.5 million tons [
1]. The two main reasons that are driving the success of poultry meat are the low price and the healthy nutritional profile compared to pork and beef.
As a result of increasing demand, there has been an intensification in poultry production, where modern hybrid broilers show a pectoral development greater than 20% of the body weight [
2]. This production intensification has increased the incidence of abnormalities in the pectoralis muscle [
3,
4,
5]. The most common malformations are deep pectoral myopathy [
6,
7] and white striping [
8,
9].
White striping disease is a serious, emerging issue characterized by the appearance of white stripes (WS) parallel to the muscle fiber on the surface of the
pectoralis major muscle [
10]. Some authors have classified the incidence into three categories, according to the intensity and thickness of the WS: NORMAL when the breast is not affected, MODERATE when the thickness of the WS is less than 1 mm and SEVERE when the WS covers most of the surface and the thickness is greater than 1 mm [
11,
12].
The presence of WS on the surface of chicken breasts affects the visual appearance of the product and decreases the degree of consumer acceptance [
11]. However, the negative visual impact is not the only problem; white striping also affects the chemical composition: protein content decreases as the degree of affection increases, while there is an opposite trend in fat content, thus the presence of this hypertrophy decreases the nutritional value of the meat [
13].
For this reason, white striping breasts (WSB) should not be commercialized as normal breast (NB), and should be used in the formulation of meat products such as sausages and nuggets, however, when the chicken is sold whole, the presence of the skin means that it is not possible to see the effects of this disorder. Considering this issue, it is necessary to find a reliable, efficient industrial system to detect the presence of white striping; for this reason, non-invasive sensors based on spectrophotometry at range of radiofrequency and microwaves could be a good tool to meet this challenge.
Dielectric properties expressed as permittivity can be explained as vector, polar or complex numbers. As a complex number, permittivity (ε) is composed of two terms, the dielectric constant ε′ and the loss factor ε″, which are the real and imaginary terms of permittivity, respectively. The dielectric constant is related to the tissue’s ability to absorb and store electric energy, and the loss factor is related to the dissipation of the electric energy into other energies such as thermal or mechanical energies.
In the radiofrequency and microwave range, the interaction of the photon flux with biological tissue produces three main dispersions: α, β, and γ [
14]. In particular, α-dispersion (from a few Hz to a few kHz), also called the counterion effect, is induced by the orientation of mobile charges in a dielectric medium [
15]. β-Dispersion (from kHz to tens of MHz) is related to the orientation of fixed charges in macromolecules such as proteins [
16]. At higher frequencies of the radiofrequency range, β-dispersion results due to the surface tension charges, this phenomenon is called the Maxwell-Wagner effect. Finally, in the microwave range, γ-dispersion occurs at GHz frequencies and is due to the orientation and induction of dipolar molecules such as water [
17,
18,
19]. Another important effect in the microwave range is ionic conductivity. It only affects the loss factor, as it produces a repulsion of charged molecules, transforming electric energy into other types [
20].
The usefulness of spectrophotometry at low frequencies in the food industry has been demonstrated as a monitoring technique in a wide range of the permittivity spectra: radiofrequency, microwave and infrared (from Hz to THz). Talens et al. [
20] developed a dielectric isotherm technique capable of predicting the water activity in dried orange peel using the ε′ at 20 GHz (microwave range); Shang, Guo, and Nelson [
21] identified the apple varieties in the radiofrequency range. Castro-Giráldez et al. [
22] demonstrated the usefulness of two dielectric ageing indexes at different frequencies (140 Hz, 500 Hz and 300 kHz) to determine pork meat ageing. Also, Trabelsi and Roelvink [
23] and Damez et al. [
24] demonstrated that dielectric spectroscopy is able to predict chicken and beef ageing using microwave and radiofrequency ranges, respectively. Finally, Cuibus et al. [
25] and Traffano-Schiffo et al. [
26] demonstrated that infrared is a good non-destructive technique to monitor freezing and drying processes.
The aim of this research was to develop a sensor based in spectrophotometry to detect white striping physiopathy in chicken breast meat in whole chicken carcass with skin.
3. Results and Discussion
All the samples presented a pH value between 5.7 and 6.1, which were classified as NORMAL according to the Zhang and Barbut [
28] classification. In
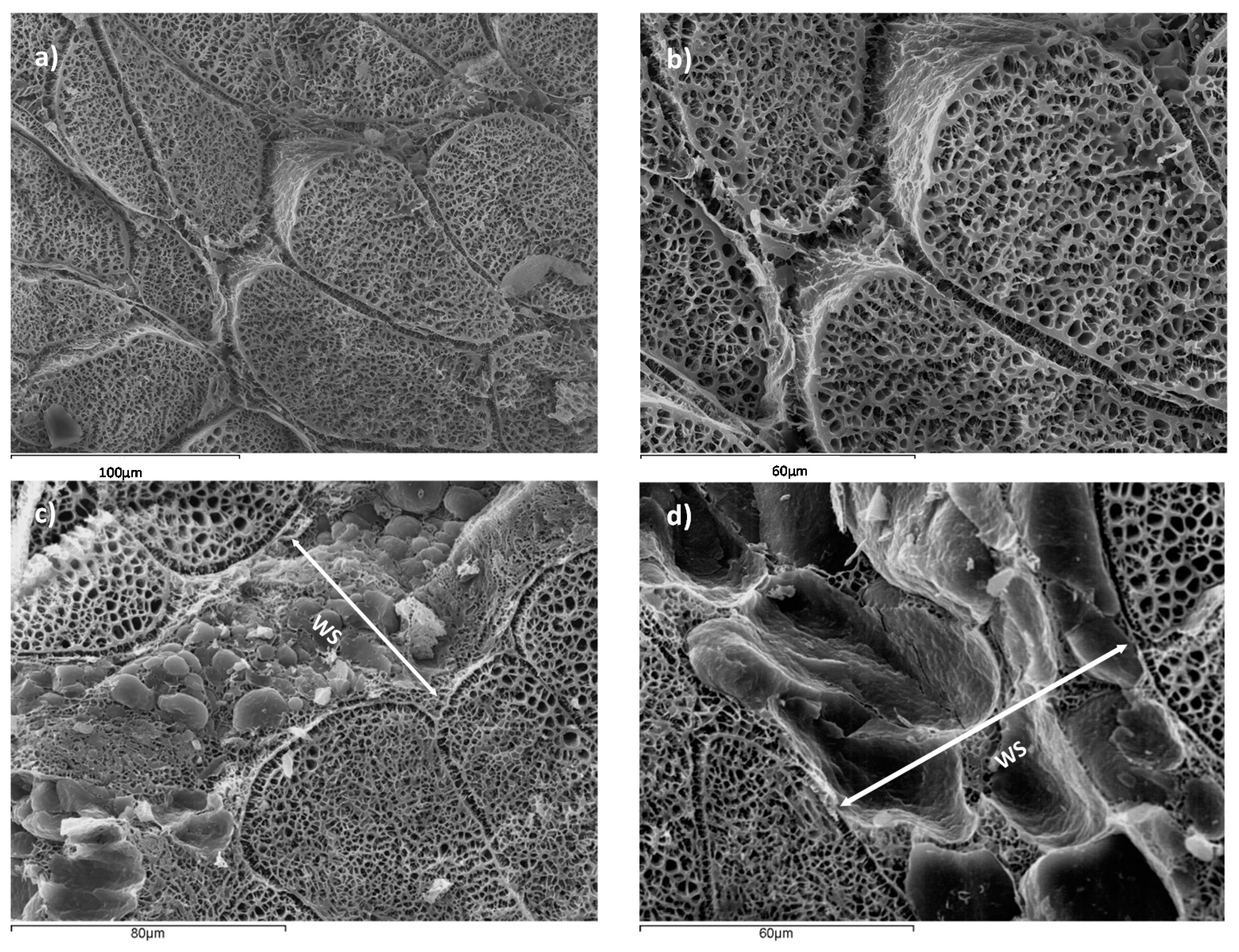
Figure 2, a microstructural analysis of NB and WSB samples is shown.
Figure 2a,b correspond to NB, where muscle tissue with correct packaging of the myofibrils is observed. However, in
Figure 2c,d, it is possible to observe the deposition of adipocytes, conforming a new adipose tissue surrounded by muscle tissue, defined as white stripe (WS). This deposition of adipocytes is produced in areas which have suffered muscle breakdown. Other authors suggested that there is also an accumulation of collagen in the WS [
13,
29].
Figure 3 shows a detail of WS in chicken breast, where a muscular rupture, from the surface breast to the internal muscle, can be appreciated, induced by forces perpendicular to the fissure, and filled with fat tissue, in order to maintain the breast integrity. Thus, the accelerated muscular growth induced by pectoral hypertrophy has caused a partial muscular rupture and the deposition of adipose tissue in this area.
Adipose tissue maintains the cohesion between muscle fibers and the elasticity of muscle tissue and therefore its activity of contraction-relaxation, conferring to the muscle the ability to transmit mechanical tension.
In addition, it is possible to appreciate that tissue breakdown is generated from the outside to the inside, so the quantity of adipose tissue is higher at the surface of the breast than the interior. Major muscle breakdown is in the external surface because the maximum tension is caused by the pectoral activity of poultry during flapping. This phenomenon produces a pectoralis major expansion, being maximum in the surface (reaching in this area the breakdown tension level).
Compared to NB, the WSB contains higher quantities of fatty acids, which cause the diminution of the protein content [
30]. In particular, collagen increases due to hypertrophy as it is necessary to maintain the muscle structure, however, sarcoplasmic and myofibrillar proteins decrease [
13].
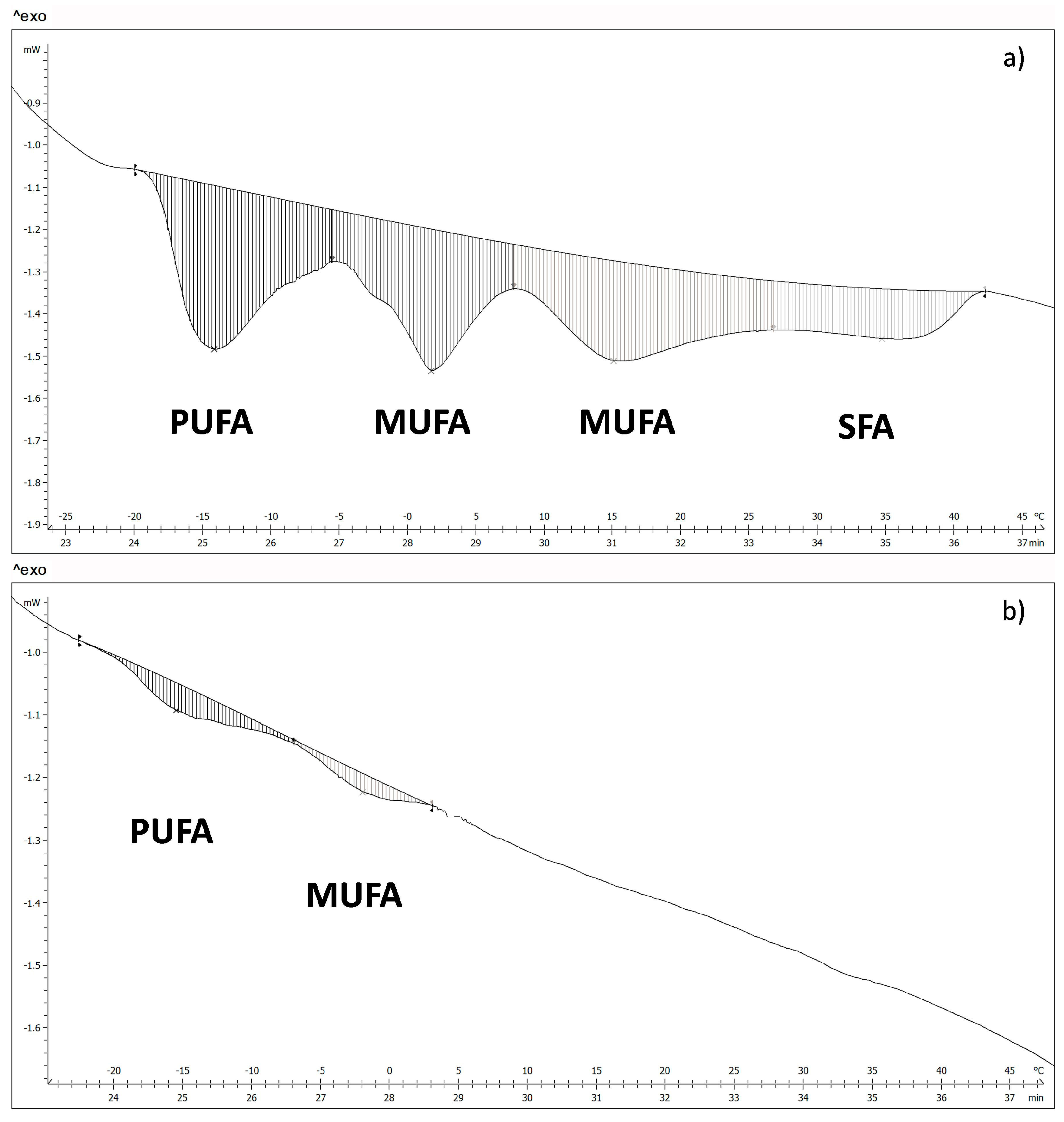
The fatty acids of WSB and NB, in the surface, were measured by differential scanning calorimetry. The thermograms obtained show four transitions for the WSB (
Figure 4a). According to the melting temperatures, it was possible to relate each transition to the different groups of fatty acids [
31,
32], where polyunsaturated fatty acids (PUFA) show a melting point in the range between −17 °C and −5 °C; monounsaturated fatty acids (MUFA) between −5 °C and 27 °C and saturated fatty acids (SFA) between 27 °C and 40 °C.
Figure 4b shows a thermogram corresponding to a NB, where it is possible to appreciate that there are only two transition peaks associated with PUFA and MUFA, which suggests that there is a big difference in fatty acids content between NB and WSB. Energy values and the temperature of the different transitions for both groups of samples are shown in
Table 1 and
Table 2, respectively.
According to Kuttappan et al. [
31] and Knothe and Dunn [
32], the majority of fatty acids per groups are PUFA: linoleic acid (18:2n-6), MUFA: palmitoleic (16:1c) and oleic acids (18:1c), and finally SFA: palmitic acid (16:0). The transition temperatures of these fatty acids correspond to the temperatures 1st, 2nd, 3rd and 4th, respectively, from
Table 1 and
Table 2.
Mass fraction of fatty acids of NB and WSB (
Table 3) were obtained from the transition energies and the latent heat of fusion of the major fatty acids in each transition [
32] according to Equation (4):
Being the mass fraction of fatty acids (kg/kg), E the transition energy of specific fatty acids group (J/g) and the ΔHf the latent heat of fusion of specific fatty acid (J/g).
As can be appreciated in the table, WSB show a much higher total fatty acid content than NB.
The fatty acid profile also differs between NB and WSB. WSB showed approximately 5% SFA, 23% PUFA and a 72% MUFA (expressed in relation to total fatty acids); while samples NB showed 76% PUFA, 24% MUFA and no presence of SFA (expressed in relation to total fatty acids). The change in the fatty acid profile of WSB is very important, mainly due to the presence of saturated fatty acids, principally palmitic acid. Numerous studies have shown that the consumption of saturated fat increases blood cholesterol levels, especially the LDL fraction [
35,
36,
37]. Furthermore, the presence of WS produces, in tissue surface, an increase in total fatty acid content from 0.54% to 10.1%, so the chicken breast can no longer be considered a totally lean meat.
Permittivity was measured with different sensors. A small sensor with two needles with blunt-ended was used at radiofrequency and a coaxial probe was used in the microwave range in order to characterize the dielectric properties of the different parts of breast: muscle and adipose tissues, in NB and WSB, and WS in WSB.
One of the main problems to fit the full spectrum of radiofrequency and microwave is the appearance of three dispersions on a very large frequency range with sigmoidal shape. The Debye model is a physic model that explains the electric behaviors in this specific frequency range. This model uses different parameters to define each relaxation [
38], but it is tedious to handle and difficult to fit with statistic tools. Others authors have used different math models trying to approach the Debye model [
39,
40]. A powerful sigmoidal model used in biological systems is the Gompertz model [
41].
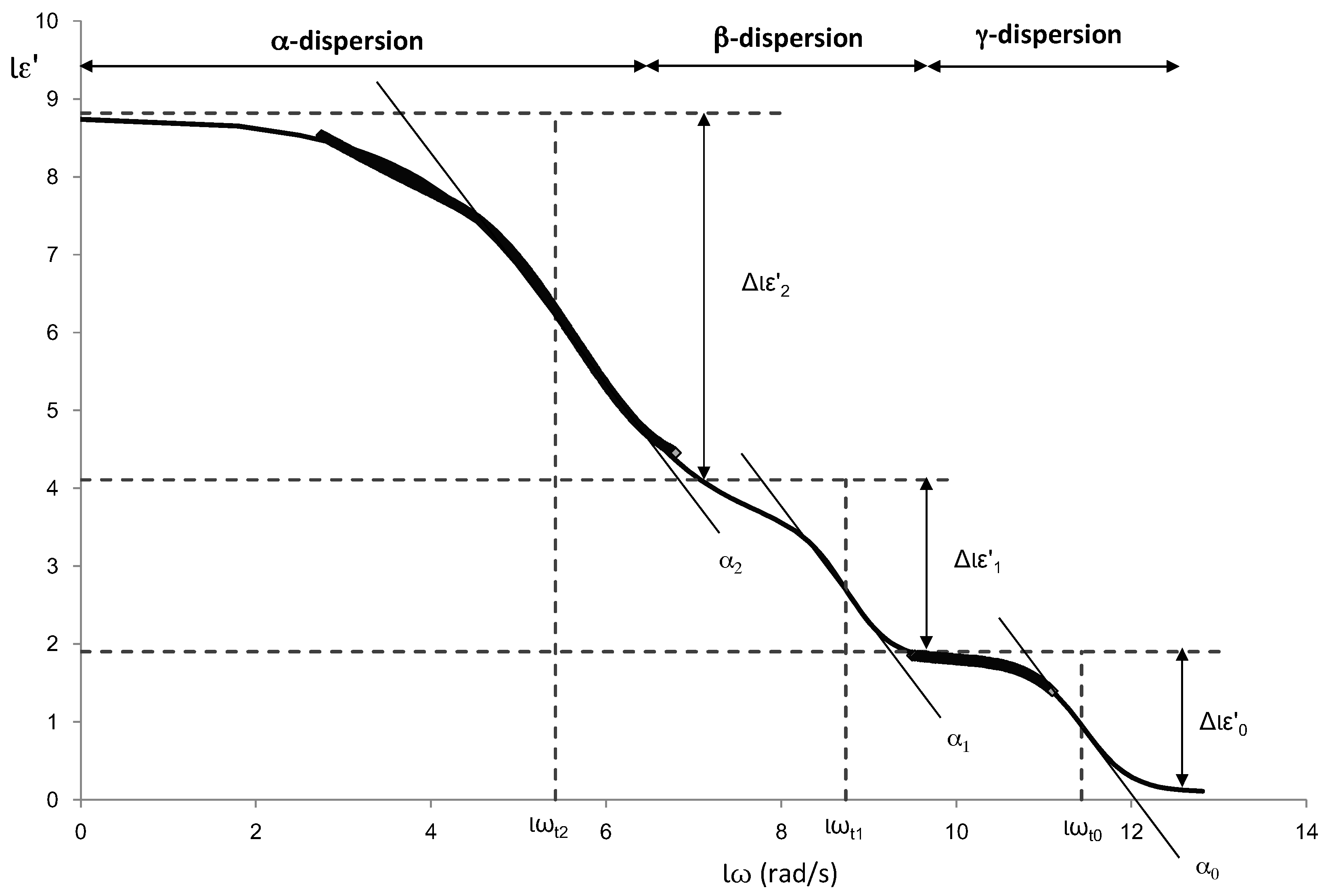
The dispersions shown in the dielectric constant spectra, α, β and γ, are similar to the sigmoidal models aforementioned. Therefore, dielectric constant (ε′) was modelled adjusting the experimental data using an own adaptation of the modified Gompertz model (Equation (5)) in order to obtain information on each of the dispersions:
where
represents the decimal logarithm of the dielectric constant,
the logarithm of the dielectric constant at high frequencies,
represents the decimal logarithm of the angular velocity (obtained from the frequency),
(
=
the magnitude of the dispersion,
the logarithm of the angular velocity at relaxation time for each dispersion
n, and
are the dispersion slopes.
Figure 5 shows an example of the different dispersions that have been modelled. In the figure the parameters of the Gompertz model are also represented.
From the Gompertz parameters, it is possible to determine the relaxation frequencies and dielectric constants of each relaxation (Equations (6)–(9)):
Being i for Equation (9) each dispersion (α, β and γ).
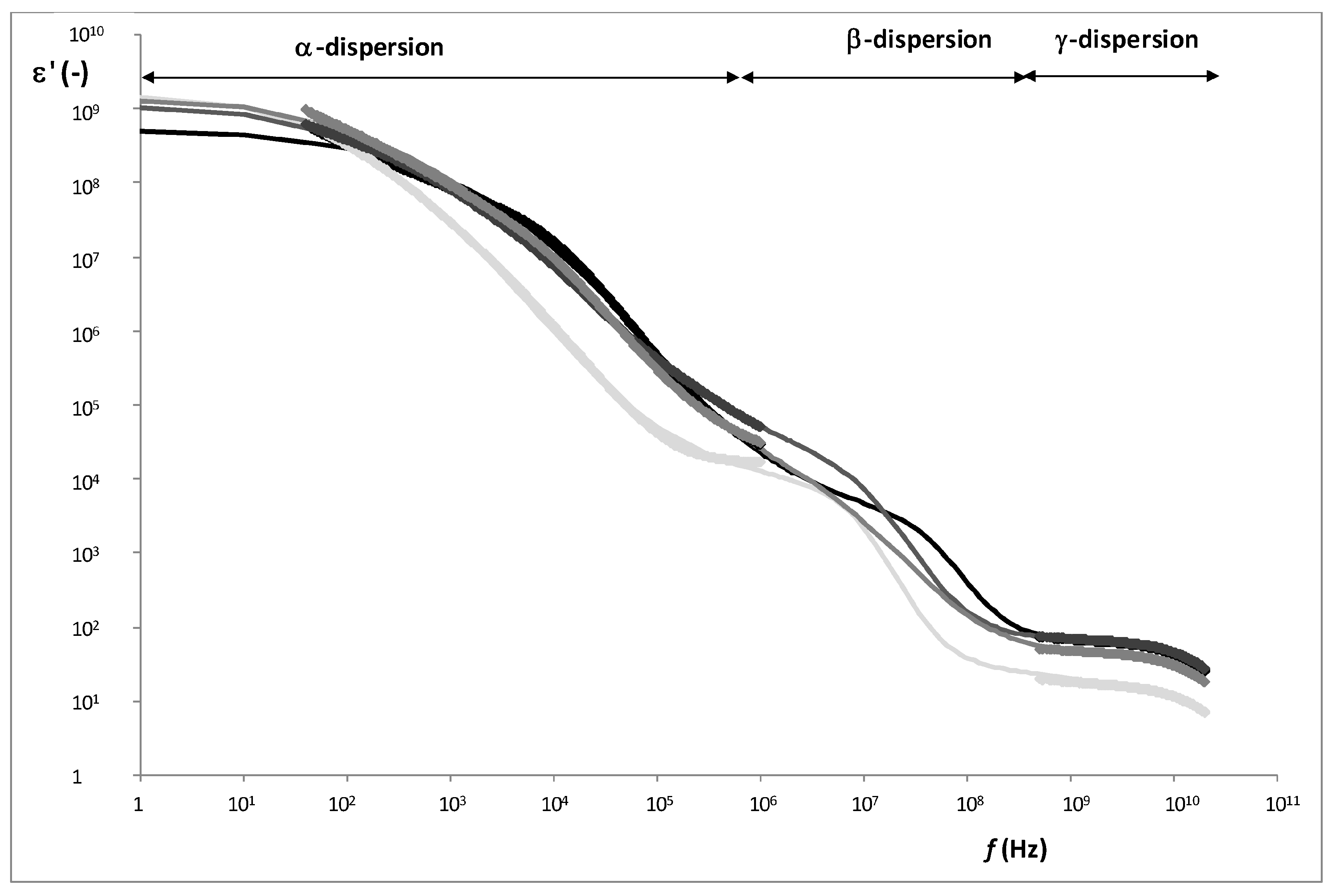
Figure 5 shows an example of radiofrequency and microwave spectra for each tissue. From the Gompertz adjustment and using Equations (6)–(9), it was possible to obtain the relaxation dielectric constants and the relaxation frequencies for each dispersion (
Table 4 and
Table 5).
As
Figure 6 shows, three relaxations per tissue can be appreciated. In α-dispersion, the relaxation dielectric constant of muscles tissues (WSB and NB) shows a significant higher value (
p < 0.05) than the fatty tissues (WS and adipose). The main mobile charges in muscle tissue are Ca
2+, K
+, Na
+ and Mg
2+, which have different functions, for example as the second messenger of ATP signaling; these electrolytes are solved in liquid phase, and their mobility is high. However, in fatty tissues, the electrolytes suffer the high attraction of surface tension of fatty globules, thus electrolytes maintain their orientation ability although this ability is reduced. Moreover, the white stripe sample shows a significantly higher value (
p < 0.05) than the adipose tissue because white stripe has an accumulation of electrolytes in the interface between the partially breakdown muscle tissue and the fat globules deposited in this fissure (
Table 4), where the MUFA predominance increases the adsorption of electrolytes. In order to understand the nature and interaction of the electrolytes responsible of α dispersion, the relaxation frequencies were analyzed (
Table 5). The fatty tissues show the electrolytes in the same state (spin orientation); nevertheless, in case of muscle tissues, the muscle in WSB shows a similar state of electrolytes as fatty tissues. This could be due to the effect of the partial breakdown of muscle in WSB, where the nature of the electrolytes associated with the interface between fat globules and muscle tissue is different from the electrolytes in the liquid phase. This is shown clearly in the very significant difference (
p < 0.001) of the relaxation frequency (state of electrolytes) between the muscle tissue in NB (muscle fibers without fat interactions) and the muscle in WSB.
With regard to β-relaxation, the dielectric constant of muscles tissues (WSB and NB) shows a significant higher value (p < 0.05) than the fatty tissues (WS and adipose), being also significant (p < 0.05) the difference between WS and adipose tissue.
The β-relaxation phenomena are explained by the orientation of fixed charges of the dielectric media. In muscle tissue, the structural proteins have active sites, fixed charges with orientation capacity; moreover, the main of these charges with orientation capacity are the charges involved in the actin-myosin complex [
42]. This phenomenon is produced in the low MHz range as
Table 5 shows, where muscles tissues have 8 ± 4 and 13 ± 5 MHz, for WSB and NB, respectively, without significant differences. Nevertheless, in the high MHz range another phenomenon occurs which is associated to the surface tension, this phenomenon is called the Maxwell-Wagner effect [
15,
43], and produces less absorption of energy than the effect of the active sites of proteins. In case of adipose tissue, as the relaxation frequency shows (110 ± 7 MHz), the high surface tension of the fat globules is the main contributor to β-relaxation, being the relaxation dielectric constant the lowest. In the case of WS, the breakdown of the muscle tissue and the formation of the white stripes by fat globule deposition produces both effects: the breakdown of the structural proteins of muscle tissue produces a partial degradation of myosin-actin complex and new active sites with orientation capacity, and the effect of the orientation of surface charges of fat globules. Comparing the dielectric constant of muscle tissue and adipose tissue (
Table 4), it is possible to observe that the orientation capacity of active protein sites produces more absorption of energy than the capacity of orientation of surface charges of the fat globules. Taking into account that the WS is composed by broken muscle tissue and fat globules, then the β-relaxation of WS should be affected by the stronger effect, which is the orientation of active sites of proteins. This theory is strengthened by observing the β-relaxation frequency in range of the effect of protein charges (10 ± 1 MHz) and an intermediate dielectric constant value between muscles tissues and adipose tissue. The partial denaturalization of myosin-actin complex, the protein breakdown and the reduction of the electric field space occupied by proteins (
Figure 3) produces a reduction of the absorption energy capacity in the WS with regard to the muscles tissues, as
Table 4 shows.
Finally, regarding the γ-dispersion, the dielectric constant of the muscle tissues is higher than that of the adipose tissues (
Table 4). Orientation of the dipolar molecules occurs at these frequencies, the main dipolar molecule in animal tissue is water; the adipose tissue shows less interaction with water molecules than muscle tissue due to its hydrophobic character. Water molecules in adipose tissue interact with adsorbed electrolytes of the fat, while in muscle tissue the water can be in liquid phase and also adsorbed to the tissue. Therefore the relaxation frequency is larger in muscle tissue with regard to the fat tissue since water has a higher mobility in liquid phase than adsorbed in the solid matrix (
Table 5).
The permittivity of NB and WSB samples were also measured using a sensor with two flat circular plates. This kind of sensor with a raised surface was used in order to increase the penetration and to measure the permittivity at radiofrequency through the chicken skin.
Table 6 shows the average values of the dielectric constant of α and β dispersions after adjusting the data with the adapted modified Gompertz model (Equation (5)). The ANOVA performed on the WS samples compared to the normal samples reveals that the differences in the dielectric constant are significant (
p < 0.05). As was explained in the analysis of the different tissues, in α and β dispersion, the muscle tissue produces the major effect in the dielectric constant. The measurements made in the whole breast (including the skin) follows the same behaviors, where the NB (
Table 6) behaves as the muscle of the NB (
Table 4) and the WSB (
Table 6) behaves as a mixture between muscle of the WSB and WS (
Table 4). Therefore, using sensors in the radio frequency range, it is possible to segregate chickens with white striping physiopathy before dismembering, carrying out the measurements on whole chickens.