Current and Potential Developments of Cortisol Aptasensing towards Point-of-Care Diagnostics (POTC)
Abstract
:1. Diagnosis of Stress
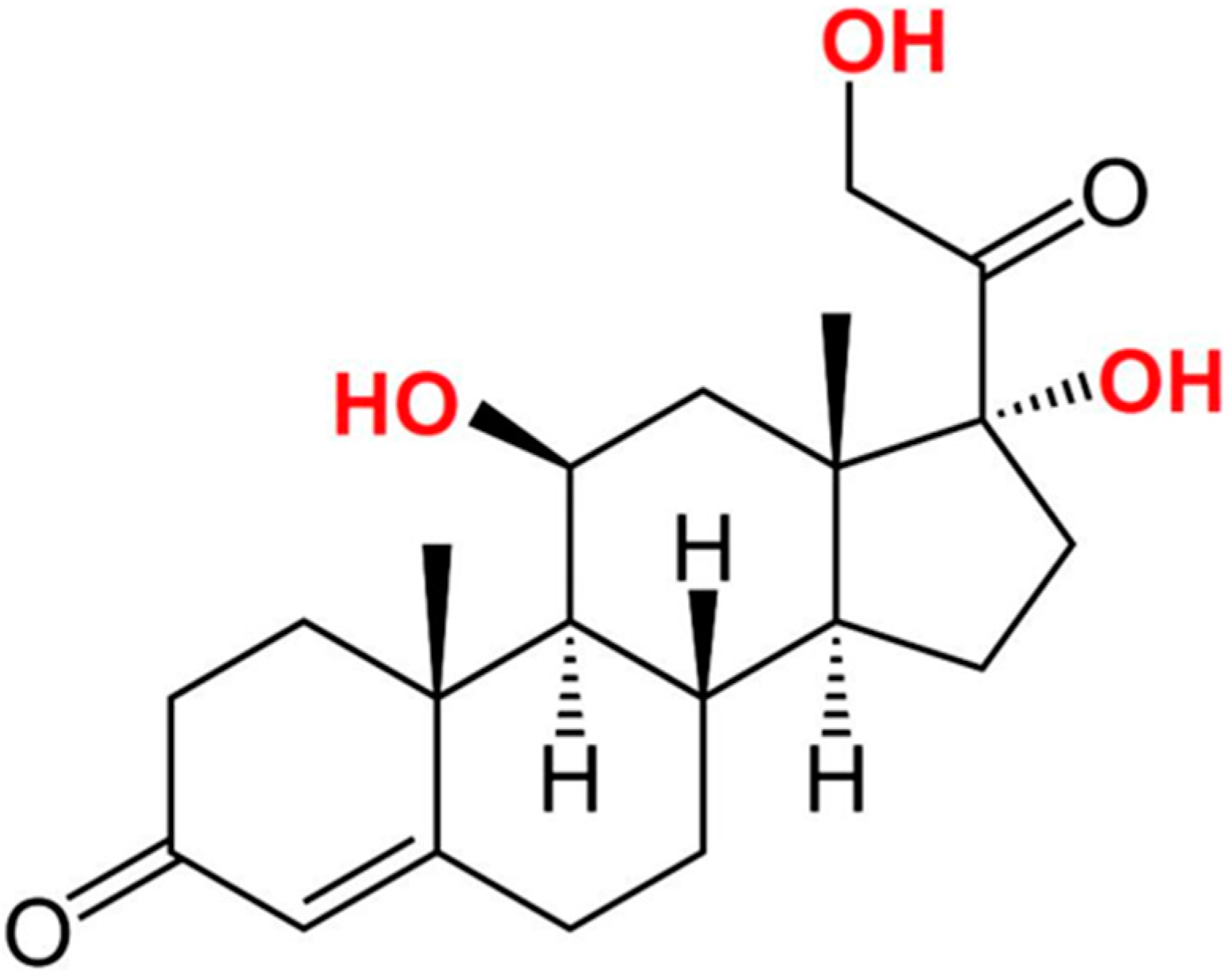
2. Cortisol as a Potential Stress Biomarker
3. Aptamers as a Potential Diagnostic Element That Can Replace Anti-Cortisol Antibodies
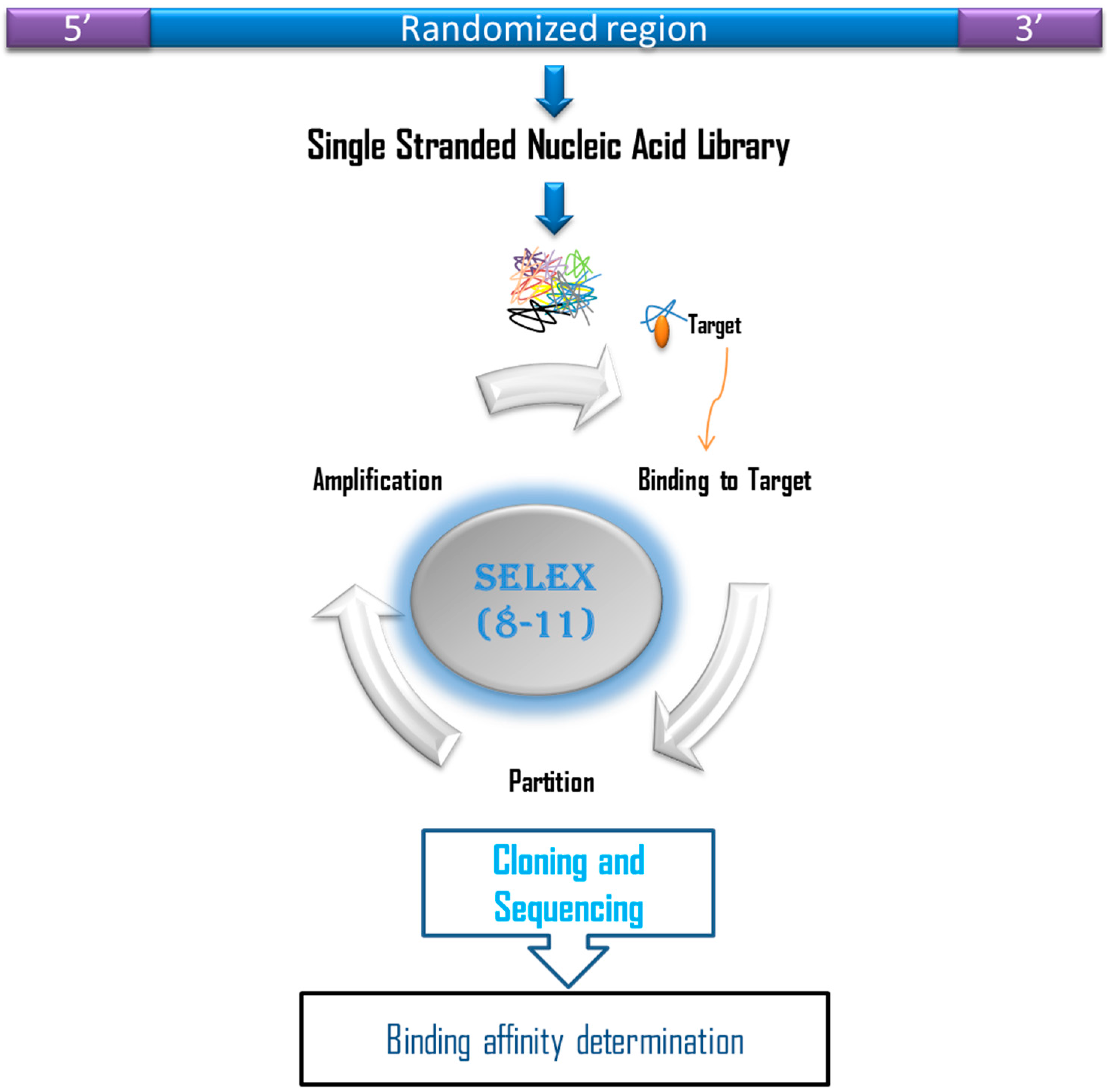
4. In Vitro Isolation of Aptamers Against Cortisol
5. Cortisol Aptasensor towards Point-of-Care Diagnostics
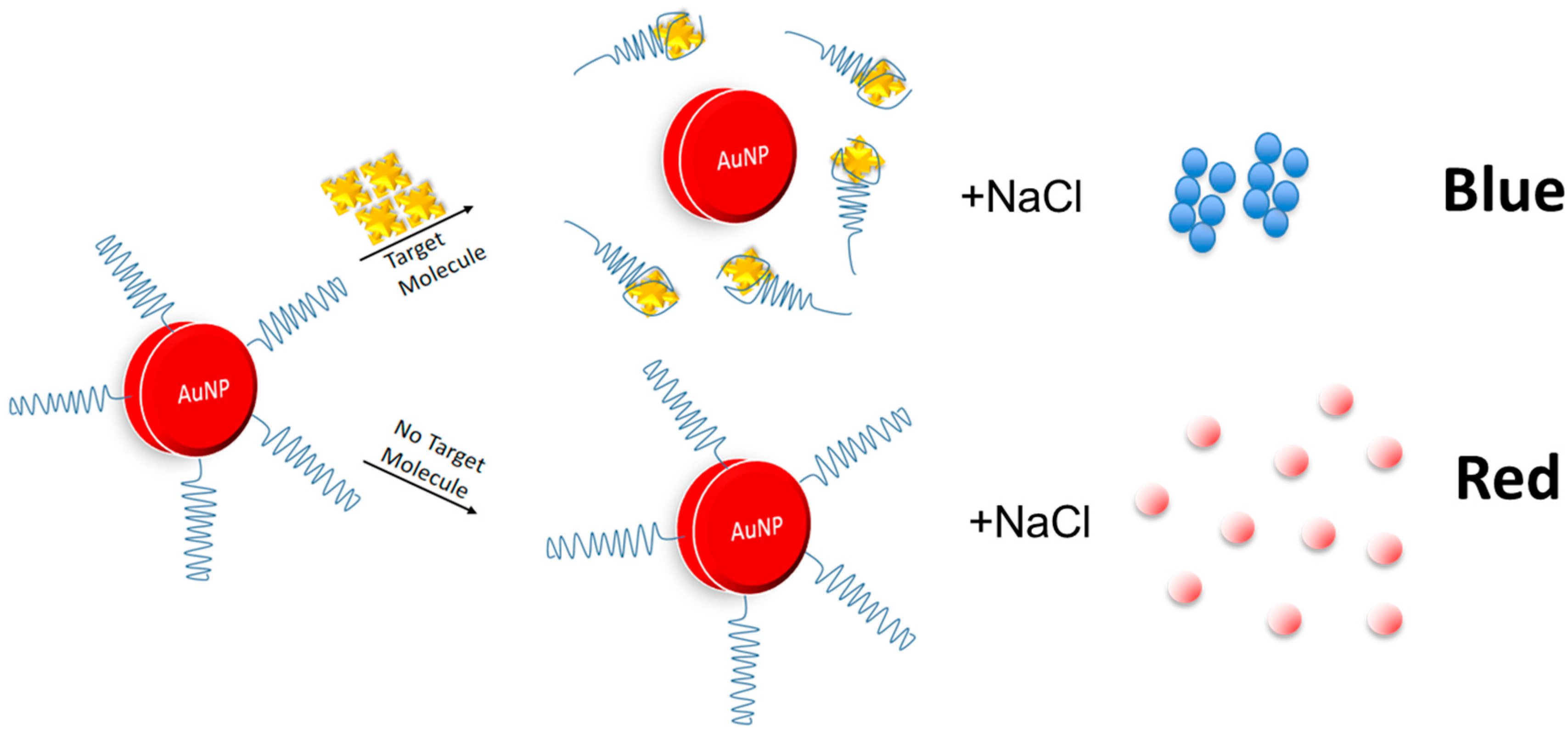
5.1. Gold Nanoparticles
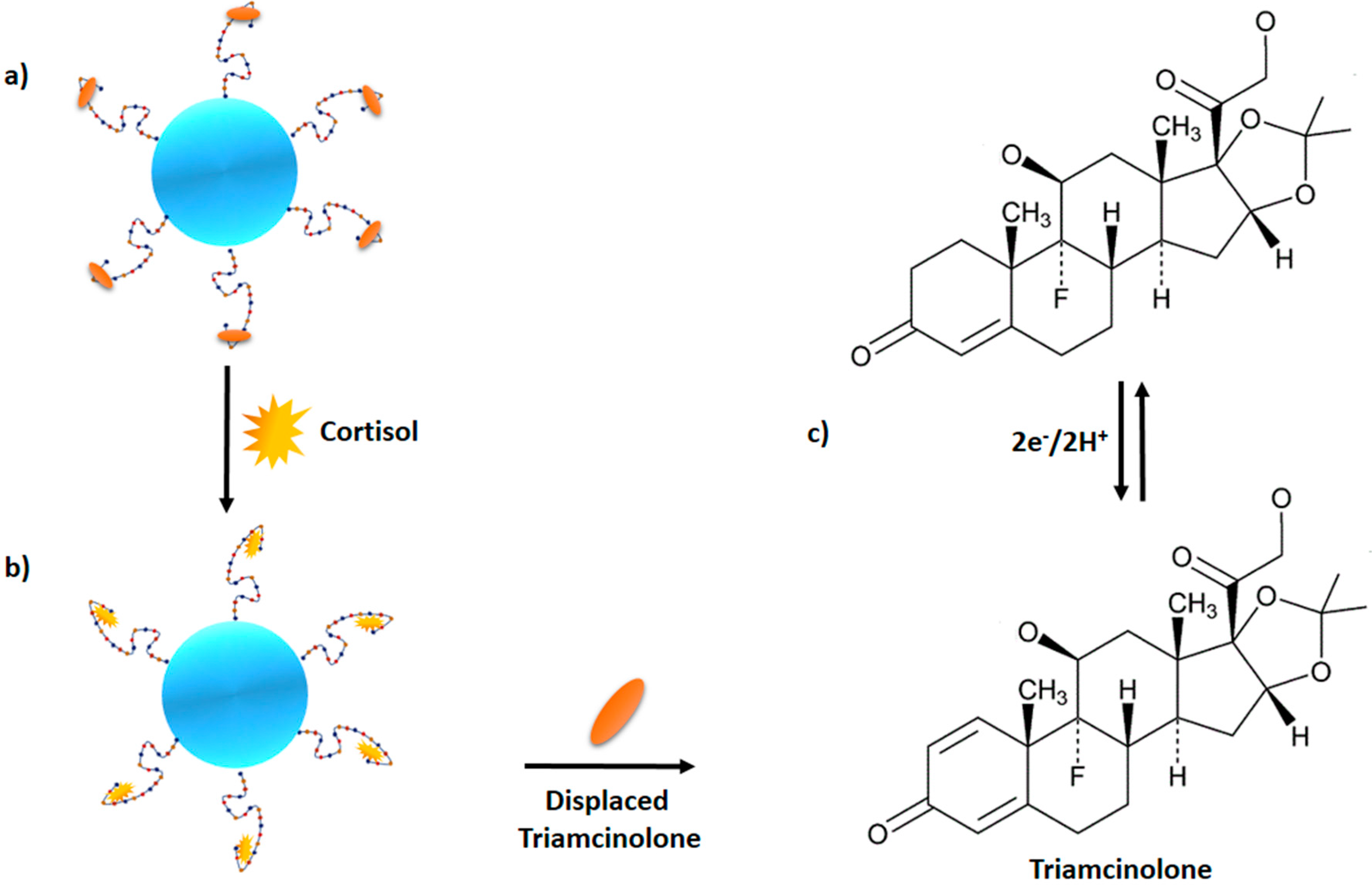
5.2. Surface Immobilization-Free Electrochemical Detection of Cortisol
6. Prophesying Aptamer-Based Lateral Flow Assay (LFA) for Cortisol Detection
7. Limitation of Aptamer-Based Sensor
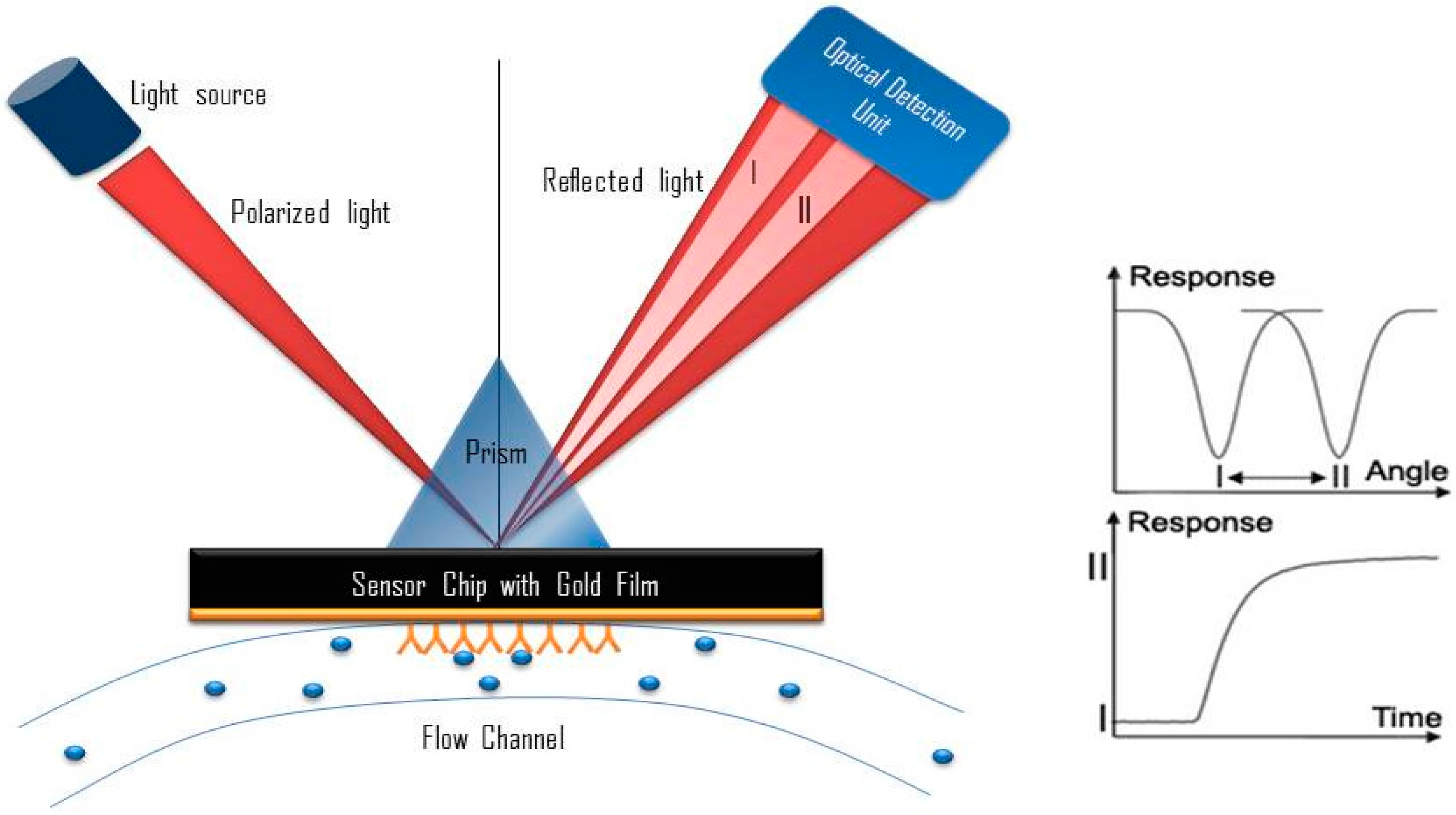
8. Potential Development of Surface Plasmon Resonance (SPR)-Based Aptasensor
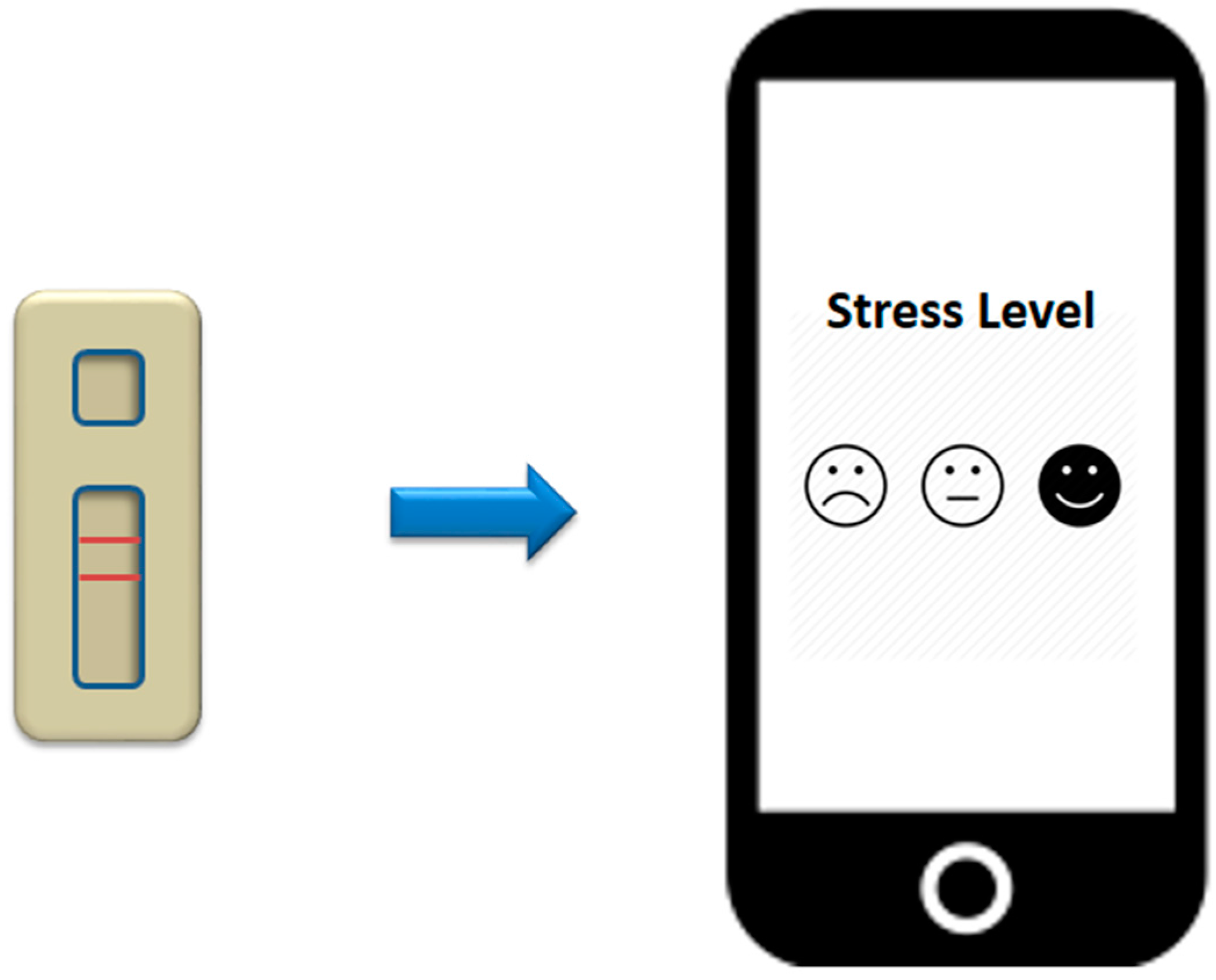
9. Smartphone-Based Aptasensor
10. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaplan, S. The restorative benefits of nature: Toward an integrative framework. J. Environ. Psychol. 1995, 15, 169–182. [Google Scholar] [CrossRef]
- Cacioppo, J.T.; Tassinary, L.G.; Berntson, G. The Handbook of Psychophysiology; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Holleman, M.; Vreeburg, S.A.; Dekker, J.J.M.; Penninx, B.W.J.H. The relationships of working conditions, recent stressors and childhood trauma with salivary cortisol levels. Psychoneuroendocrinology 2012, 37, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Landys, M.M.; Ramenofsky, M.; Wingfield, J.C. Actions of glucocorticoids at a seasonal baseline as compared to stress-related levels in the regulation of periodic life processes. Gen. Comp. Endocrinol. 2006, 148, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Crespi, E.J.; Williams, T.D.; Jessop, T.S.; Delehanty, B. Life history and the ecology of stress: How do glucocorticoid hormones in fl uence life-history variation in animals? Funct. Ecol. 2013, 27, 93–106. [Google Scholar] [CrossRef]
- Wingfield, J.C. Ecological processes and the ecology of stress: The impacts of abiotic environmental factors. Funct. Ecol. 2013, 27, 37–44. [Google Scholar] [CrossRef]
- Varghese, P.; Brown, S. The Hypothalamic-Pituitary Adrenal Axis in Major Depressive Disorder: A Brief Primer for Primary Care Physicians Femina. Prim. Care Companion J. Clin. Psychiatry 2001, 3, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Delahanty, D.L.; Raimonde, A.J.; Spoonster, E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol. Psychiatry 2000, 48, 940–947. [Google Scholar] [CrossRef]
- McEwen, B.S. Editorial: Cortisol, Cushing’s syndrome, and a shrinking brain—New evidence for reversibility. J. Clin. Endocrinol. Metab. 2002, 87, 1947–1948. [Google Scholar] [PubMed]
- Morgan, C.A.; Wang, S.; Rasmusson, A.; Hazlett, G.; Anderson, G.; Charney, D.S. Relationship among plasma cortisol, catecholamines, neuropeptide Y, and human performance during exposure to uncontrollable stress. Psychosom. Med. 2001, 63, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Halligan, S.L.; Bierer, L.M. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J. Psychiatr. Res. 2001, 35, 261–270. [Google Scholar] [CrossRef]
- Stalder, T.; Steudte, S.; Alexander, N.; Miller, R.; Gao, W.; Dettenborn, L.; Kirschbaum, C. Cortisol in hair, body mass index and stress-related measures. Biol. Psychol. 2012, 90, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair cortisol as a biological marker of chronic stress: Current status, future directions and unanswered questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, Y. Hair analysis for abused and therapeutic drugs. J. Chromatogr. B Biomed. Sci. Appl. 1999, 733, 161–180. [Google Scholar] [CrossRef]
- Cirimele, V.; Kintz, P.; Dumestre, V.; Goulle, J.P.; Ludes, B. Identification of ten corticosteroids in human hair by liquid chromatography-ion spray mass spectrometry. Forensic Sci. Int. 2000, 1–3, 381–388. [Google Scholar] [CrossRef]
- Raul, J.S.; Cirimele, V.; Ludes, B.; Kintz, P. Detection of physiological concentrations of cortisol and cortisone in human hair. Clin. Biochem. 2004, 37, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Davenport, M.D.; Tiefenbacher, S.; Lutz, C.K.; Novak, M.A.; Meyer, J.S. Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. Gen. Comp. Endocrinol. 2006, 147, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Kirschbaum, C.; Tietze, A.; Skoluda, N.; Dettenborn, L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009, 34, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, H.R.; Koelewijn, R.; Hofwegen, H.; Gilis, H.; Wetsteyn, J.C.F.M.; Wismans, P.J.; Sarfati, C.; Vervoort, T.; Van Gool, T. Use of enzyme-linked immunosorbent assay and dipstick assay for detection of Strongyloides stercoralis infection in humans. J. Clin. Microbiol. 2007, 45, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Gozansky, W.; Lynn, J.; Laudenslager, M.; Kohrt, W. Salivary cortisol determined by enzyme immunoassay is preferable to serum total cortisol for assessment of dynamic hypothalamic-pituitary-adrenal axis activity. Clin. Endocrinol. 2005, 63, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Sink, T.D.; Lochmann, R.T.; Fecteau, K.A. Validation, use, and disadvantages of enzyme-linked immunosorbent assay kits for detection of cortisol in channel catfish, largemouth bass, red pacu, and golden shiners. Fish Physiol. Biochem. 2008, 34, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Posthuma-Trumpie, G.A.; Korf, J.; van Amerongen, A. Lateral flow (immuno)assay: Its strengths, weaknesses, opportunities and threats. A literature survey. Anal. Bioanal. Chem. 2009, 393, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Tlili, C.; Myung, N.V.; Shetty, V.; Mulchandani, A. Label-free, chemiresistor immunosensor for stress biomarker cortisol in saliva. Biosens. Bioelectron. 2011, 26, 4382–4386. [Google Scholar] [CrossRef] [PubMed]
- Ito, T.; Aoki, N.; Kaneko, S.; Suzuki, K. Highly sensitive and rapid sequential cortisol detection using twin sensor QCM. Anal. Methods 2014, 6, 7469. [Google Scholar] [CrossRef]
- Frasconi, M.; Mazzarino, M.; Botrè, F.; Mazzei, F. Surface plasmon resonance immunosensor for cortisol and cortisone determination. Anal. Bioanal. Chem. 2009, 394, 2151–2159. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Chornokur, G.; Venugopal, M.; Bhansali, S. Antibody functionalized interdigitated μ-electrode (IDμE) based impedimetric cortisol biosensor. Analyst 2010, 135, 1941–1946. [Google Scholar] [CrossRef] [PubMed]
- Arya, S.K.; Dey, A.; Bhansali, S. Polyaniline protected gold nanoparticles based mediator and label free electrochemical cortisol biosensor. Biosens. Bioelectron. 2011, 28, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, M.J.; Dantzer, B.; Delehanty, B.; Palme, R.; Boonstra, R. Measuring stress in wildlife: Techniques for quantifying glucocorticoids. Oecologia 2011, 166, 869–887. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, C.K. Aptasensors—The future of biosensing? Anal. Bioanal. Chem. 2002, 372, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Eldin, P.; Pauza, M.E.; Hieda, Y.; Lin, G.; Murtaugh, M.P.; Pentel, P.R.; Pennell, C.A. High-level secretion of two antibody single chain Fv fragments by Pichia pastoris. J. Immunol. Methods 1997, 201, 67–75. [Google Scholar] [CrossRef]
- Mairal, T.; Ozalp, V.C.; Lozano Sánchez, P.; Mir, M.; Katakis, I.; O′Sullivan, C.K. Aptamers: Molecular tools for analytical applications. Anal. Bioanal. Chem. 2008, 390, 989–1007. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Medley, C.D.; Tang, Z.; Shangguan, D.; Lofton, C.; Tan, W. Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Anal. Chem. 2007, 79, 3075–3082. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Chávez, J.L.; Chushak, Y.; Chapleau, R.R.; Hagen, J.; Kelley-Loughnane, N. Tunable stringency aptamer selection and gold nanoparticle assay for detection of cortisol. Anal. Bioanal. Chem. 2014, 406, 4637–4647. [Google Scholar] [CrossRef] [PubMed]
- Nutiu, R.; Li, Y. Structure-switching signaling aptamers. J. Am. Chem. Soc. 2003, 125, 4771–4778. [Google Scholar] [CrossRef] [PubMed]
- Vanschoenbeek, K.; Vanbrabant, J.; Hosseinkhani, B.; Vermeeren, V.; Michiels, L. Aptamers targeting different functional groups of 17β-estradiol. J. Steroid Biochem. Mol. Biol. 2015, 147, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Stoltenburg, R.; Nikolaus, N.; Strehlitz, B. Capture-SELEX: Selection of DNA aptamers for aminoglycoside antibiotics. J. Anal. Methods Chem. 2012, 2012, 415697. [Google Scholar] [CrossRef] [PubMed]
- Nikolaus, N.; Strehlitz, B. DNA-aptamers binding aminoglycoside antibiotics. Sensors 2014, 14, 3737–3755. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.B.; Sun, H.; Meng, X.B.; Hu, J.; Zhang, Q.; Liu, B.; Wang, M.; Xu, H.B.; Sun, X.B. Aconitine-induced Ca2+ overload causes arrhythmia and triggers apoptosis through p38 MAPK signaling pathway in rats. Toxicol. Appl. Pharmacol. 2014, 279, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Lee, E.J.; Lee, G.H.; Hah, S.S. Aptamer selection for fishing of palladium ion using graphene oxide-adsorbed nanoparticles. Bioorg. Med. Chem. Lett. 2015, 25, 5536–5539. [Google Scholar] [CrossRef] [PubMed]
- McKeague, M.; Derosa, M.C. Challenges and opportunities for small molecule aptamer development. J. Nucleic Acids 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.; Han, J.; Choi, J.W.; Ahn, C.H. Point-of-care testing (POCT) diagnostic systems using microfluidic lab-on-a-chip technologies. Microelectron. Eng. 2014, 132, 46–57. [Google Scholar] [CrossRef]
- Jönsson, C.; Aronsson, M.; Rundström, G.; Pettersson, C.; Mendel-Hartvig, I.; Bakker, J.; Martinsson, E.; Liedberg, B.; MacCraith, B.; Ohman, O.; et al. Silane-dextran chemistry on lateral flow polymer chips for immunoassays. Lab Chip 2008, 8, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ahmad, H.; Ma, C.; Shi, Q.; Vermesh, O.; Vermesh, U.; Heath, J. A self-powered, one-step chip for rapid, quantitative and multiplexed detection of proteins from pinpricks of whole blood. Lab Chip 2010, 10, 3157–3162. [Google Scholar] [CrossRef] [PubMed]
- Soh, J.H.; Lin, Y.; Rana, S.; Ying, J.Y.; Stevens, M.M. Colorimetric Detection of Small Molecules in Complex Matrixes via Target-Mediated Growth of Aptamer-Functionalized Gold Nanoparticles. Anal. Chem. 2015, 87, 7644–7652. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Liu, X.; Liang, Z.; Song, S.; Li, W.; Li, G.; Fan, C. A gold nanoparticle-based aptamer target binding readout for ATP assay. Adv. Mater. 2007, 19, 3943–3946. [Google Scholar] [CrossRef]
- Slavkovic, S.; Altunisik, M.; Reinstein, O.; Johnson, P.E. Structure-affinity relationship of the cocaine-binding aptamer with quinine derivatives. Bioorg. Med. Chem. 2015, 23, 2593–2597. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Walton, S.P. Application and analysis of structure-switching aptamers for small molecule quantification. Anal. Chim. Acta 2009, 638, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.A.; Smith, J.E.; Warren, M.; Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N. A method for selecting structure-switching aptamers applied to a colorimetric gold nanoparticle assay. J. Vis. Exp. 2015, 96, e52545. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.E.; Griffin, D.K.; Leny, J.K.; Hagen, J.A.; Chávez, J.L.; Kelley-Loughnane, N. Colorimetric detection with aptamer-gold nanoparticle conjugates coupled to an android-based color analysis application for use in the field. Talanta 2014, 121, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, B.J.; Moore, J.A.; Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N.; Chou, C.F.; Swami, N.S. Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens. Bioelectron. 2016, 78, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Yang, S. Replacing antibodies with aptamers in lateral flow immunoassay. Biosens. Bioelectron. 2015, 71, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Kong, W.; Dou, X.; Zhao, M.; Ouyang, Z.; Yang, M. An aptamer based lateral flow strip for on-site rapid detection of ochratoxin A in Astragalus membranaceus. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1022, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Nat. 2013, 5, 34–43. [Google Scholar]
- Lioubashevski, O.; Chegel, V.I.; Patolsky, F.; Katz, E.; Willner, I. Enzyme-catalyzed bio-pumping of electrons into Au-nanoparticles: A surface plasmon resonance and electrochemical study. J. Am. Chem. Soc. 2004, 126, 7133–7143. [Google Scholar] [CrossRef] [PubMed]
- Tokareva, I.; Minko, S.; Fendler, J.; Hutter, E. Polymer Brushes and Gold Nanoparticle Enhanced Transmission Surface Plasmon. J. Am. Chem. Soc. 2004, 126, 15950–15951. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Electromagnetic Theory of Surface Plasmons. In Surface Plasmon Resonnance Based Sensors; Springer: Berlin/Heidelberg, Germany, 2006; pp. 3–44. [Google Scholar]
- Cooper, M.A. Optical biosensors in drug discovery. Nat. Rev. Drug Discov. 2002, 1, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Giannetti, A.M.; Koch, B.D.; Browner, M.F. Surface plasmon resonance based assay for the detection and characterization of promiscuous inhibitors. J. Med. Chem. 2008, 51, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Lokate, A.M.C.; Beusink, J.B.; Besselink, G.A.J.; Pruijn, G.J.M.; Schasfoort, R.B.M. Biomolecular interaction monitoring of autoantibodies by scanning surface plasmon resonance microarray imaging. J. Am. Chem. Soc. 2007, 129, 14013–14018. [Google Scholar] [CrossRef] [PubMed]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Goertz, J.P.; White, I.M. Smartphone-enabled filterless fluorescence assay utilizing the pyrene excimer. In Proceedings of the Optics and Biophotonics in Low-Resource Settings, San Francisco, CA, USA, 7–8 Frebruary 2015; Volume 9314, pp. 1–6. [Google Scholar]

| Method | Probe | Lowest LOD of Cortisol | Advantage | Limitation | References |
|---|---|---|---|---|---|
| ELISA | Monoclonal antibody | - |
|
| [21] |
| Lateral Flow Immunoassays | Colloidal gold-labelled primary antibody | 3.5 μg/L |
|
| [22] |
| Chemiresistor Immunosensor | Monoclonal antibody | 1 pg/mL |
|
| [23] |
| Quartz Crystal Microbalance | Monoclonal antibody | 11 pg/mL |
|
| [24] |
| Surface Plasmon Resonance (SPR) | Monoclonal antibody | 10 μg/L |
|
| [25] |
| Interdigitated µ-Electrode | Monoclonal antibody | 1 pM |
|
| [26] |
| Polyaniline protected gold nanoparticles (PPAuNPs) | Monoclonal antibody | 1 pM |
|
| [27] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zainol Abidin, A.S.; Rahim, R.A.; Md Arshad, M.K.; Fatin Nabilah, M.F.; Voon, C.H.; Tang, T.-H.; Citartan, M. Current and Potential Developments of Cortisol Aptasensing towards Point-of-Care Diagnostics (POTC). Sensors 2017, 17, 1180. https://doi.org/10.3390/s17051180
Zainol Abidin AS, Rahim RA, Md Arshad MK, Fatin Nabilah MF, Voon CH, Tang T-H, Citartan M. Current and Potential Developments of Cortisol Aptasensing towards Point-of-Care Diagnostics (POTC). Sensors. 2017; 17(5):1180. https://doi.org/10.3390/s17051180
Chicago/Turabian StyleZainol Abidin, Azrul Syafiq, Ruslinda A. Rahim, Mohd Khairuddin Md Arshad, Mohd Faudzi Fatin Nabilah, Chun Hong Voon, Thean-Hock Tang, and Marimuthu Citartan. 2017. "Current and Potential Developments of Cortisol Aptasensing towards Point-of-Care Diagnostics (POTC)" Sensors 17, no. 5: 1180. https://doi.org/10.3390/s17051180






