Diazonium Salt-Based Surface-Enhanced Raman Spectroscopy Nanosensor: Detection and Quantitation of Aromatic Hydrocarbons in Water Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Diazonium Salt Chemical Synthesis
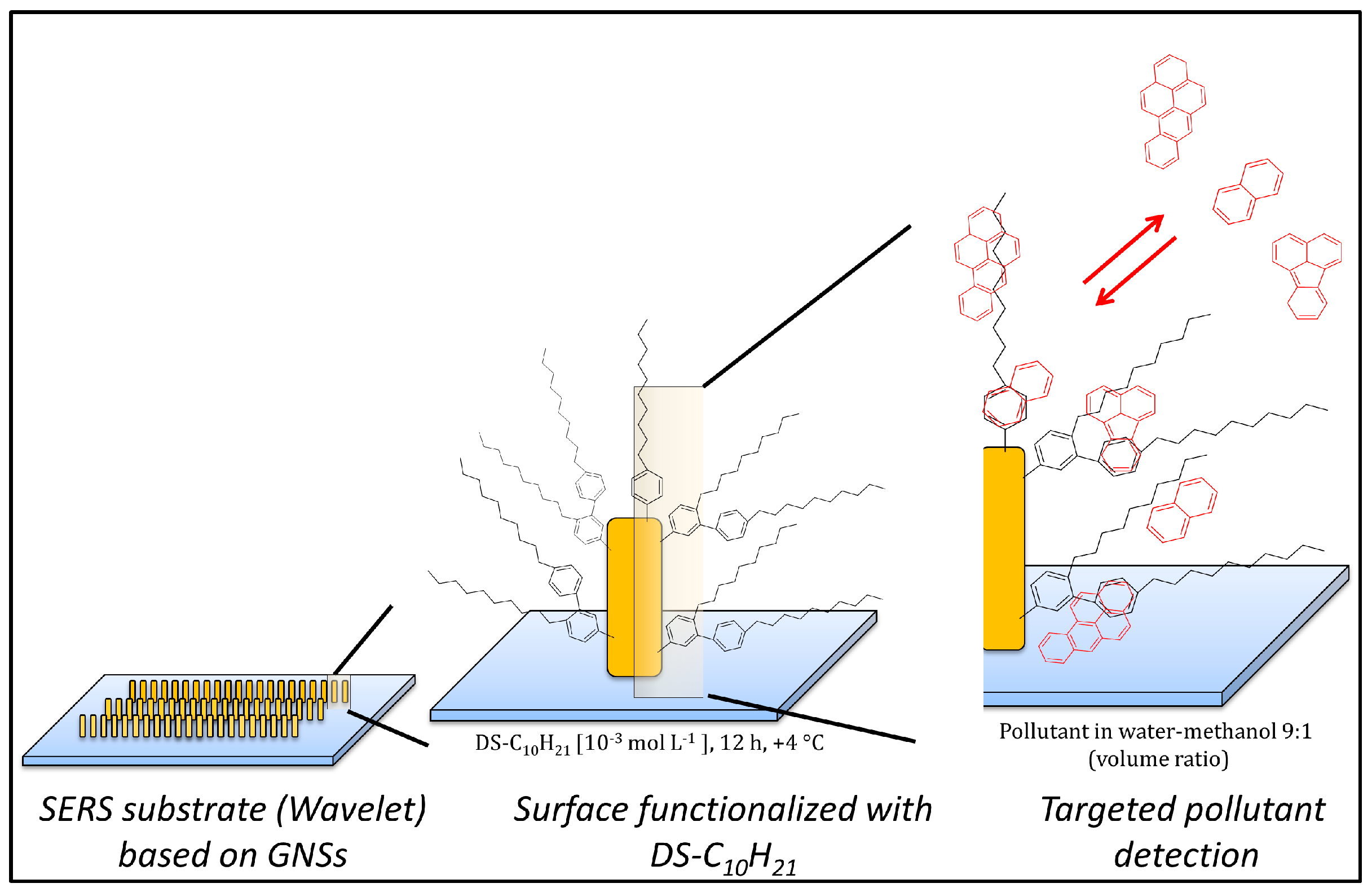
2.3. SERS Substrate
2.4. Surface Functionalization
2.5. Solutions of the Targeted Compounds
2.6. Nanosensor Surface Regeneration
2.7. Raman and SERS Measurements
2.8. Chemometrics
3. Results and Discussion
3.1. Diazonium Salt Based Surface Functionalization
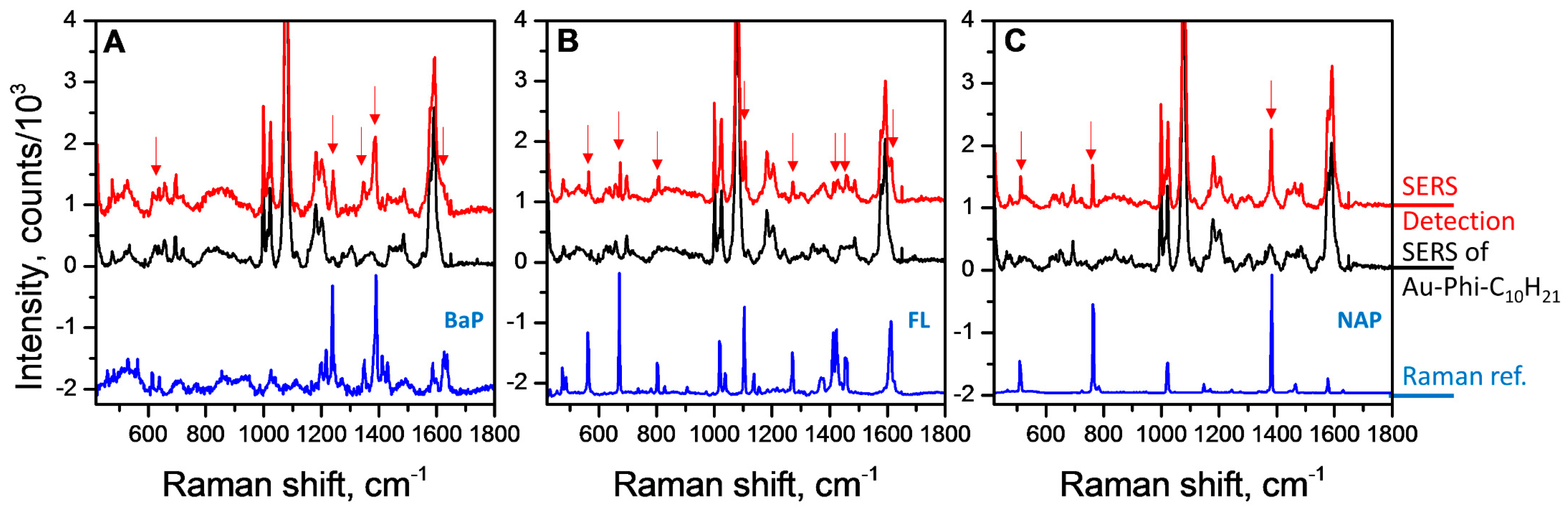
3.2. Detection of PAHs
3.3. Sensing Performance
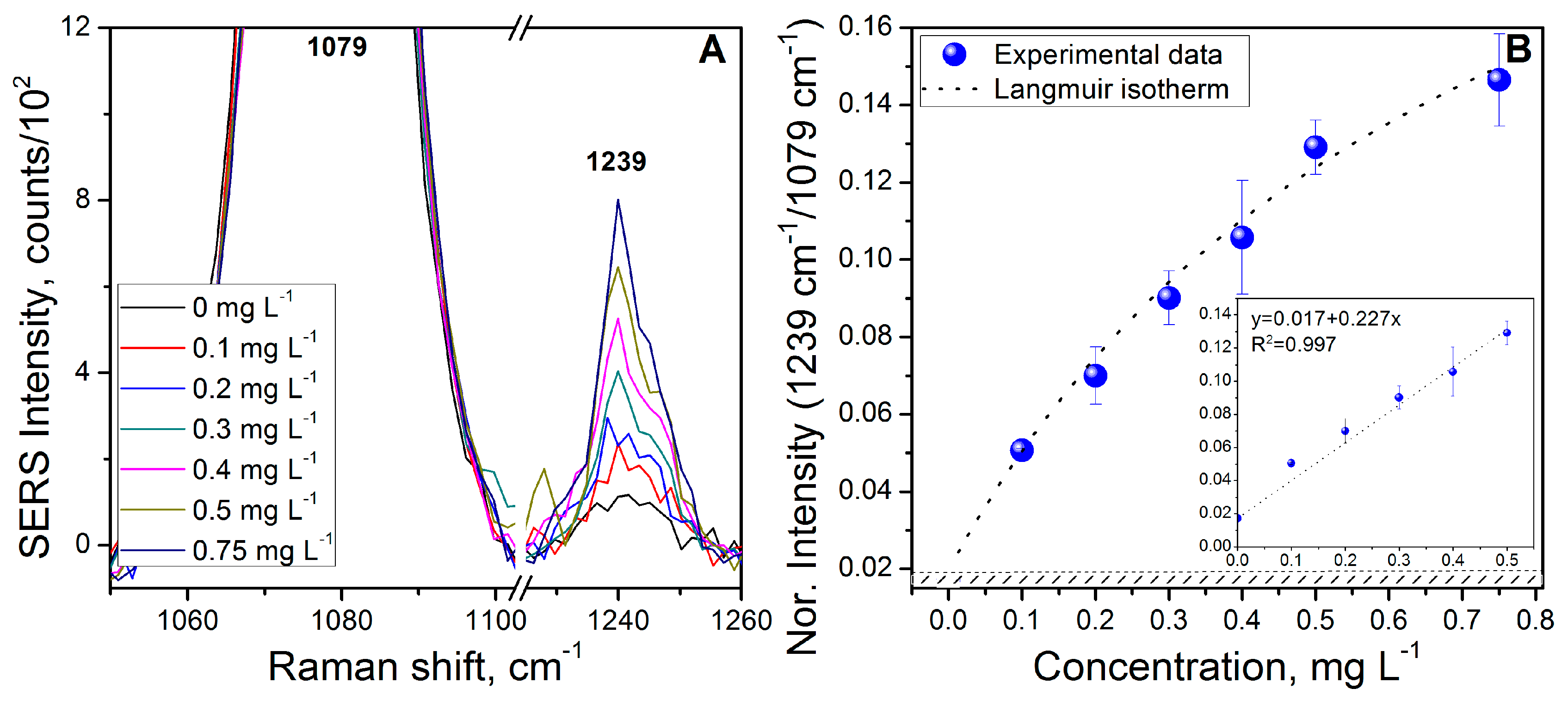
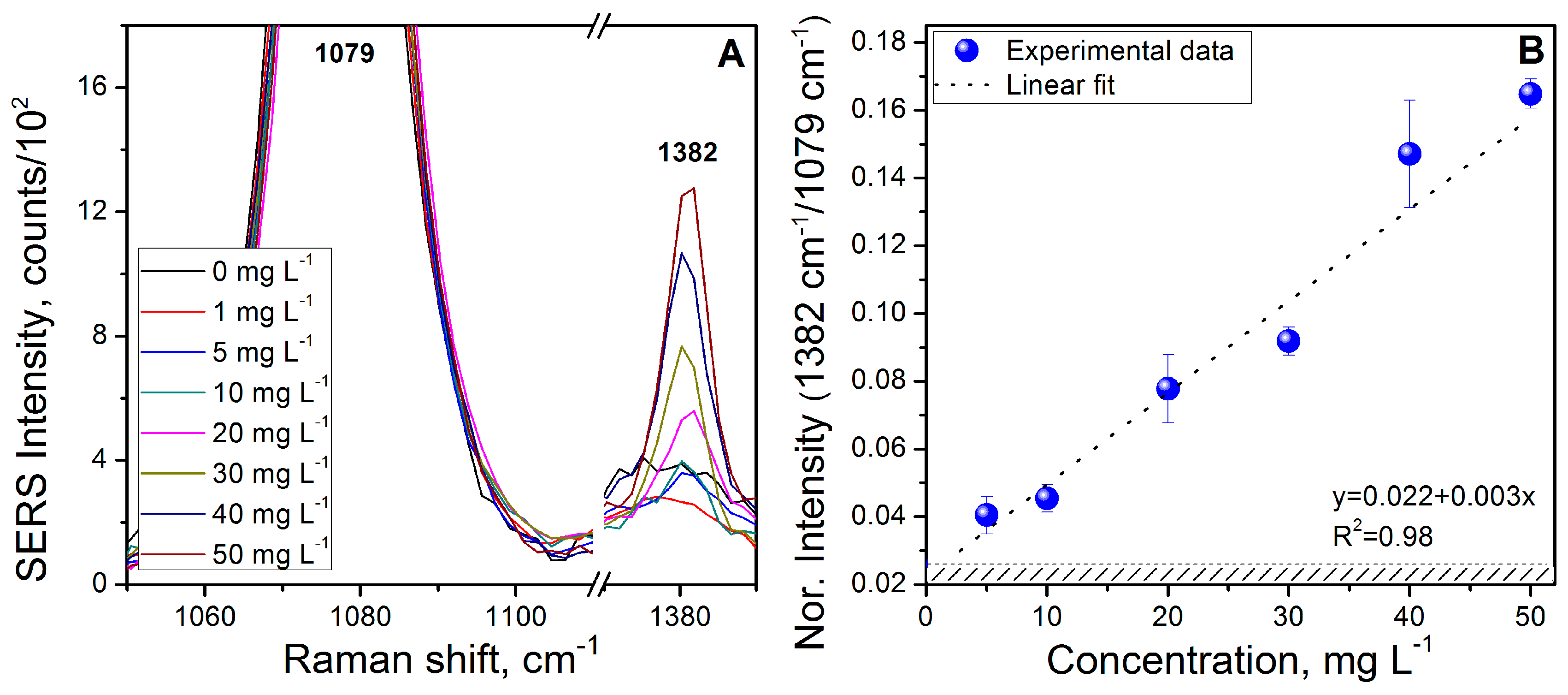
3.4. Benzo[a]pyrene (BaP) Calibration Curve
3.5. Fluoranthene (FL) Calibration Curve
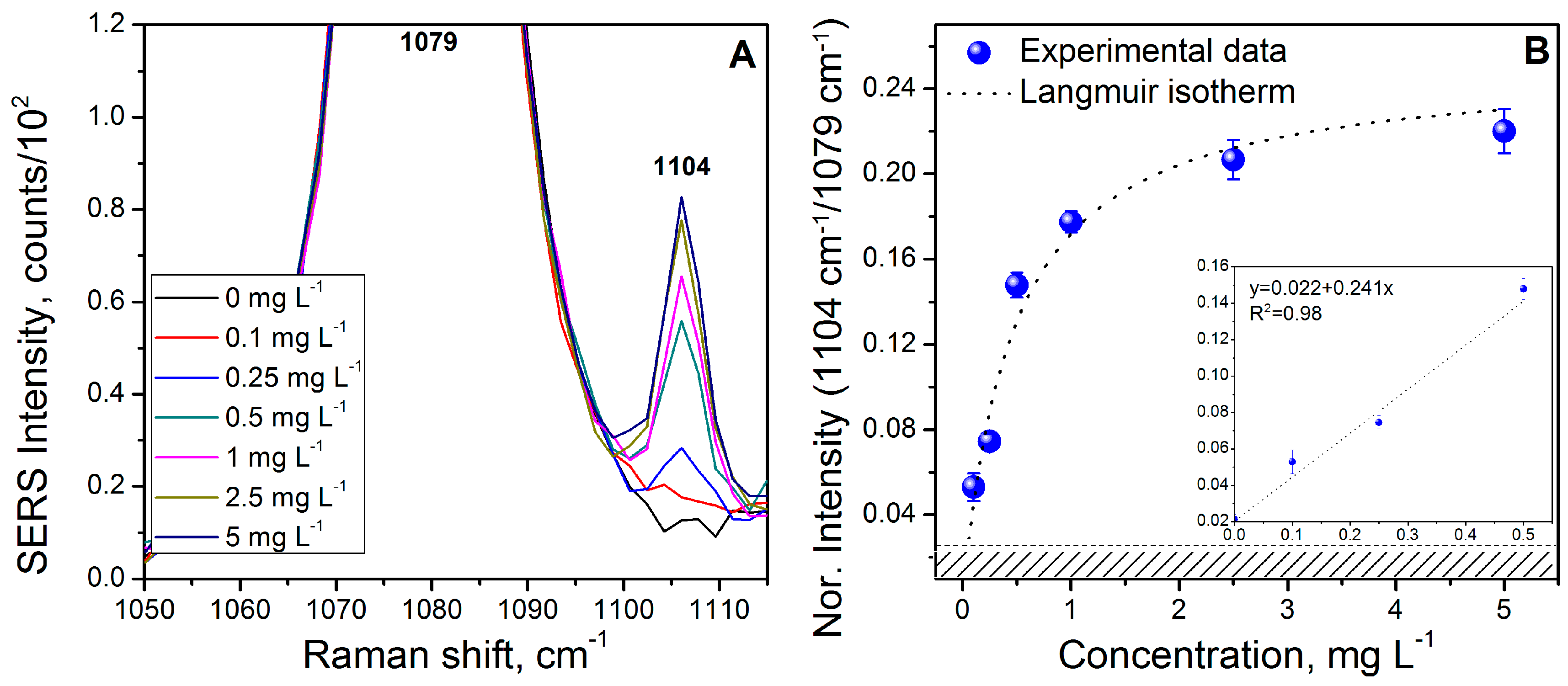
3.6. Naphthalene (NAP) Calibration Curve
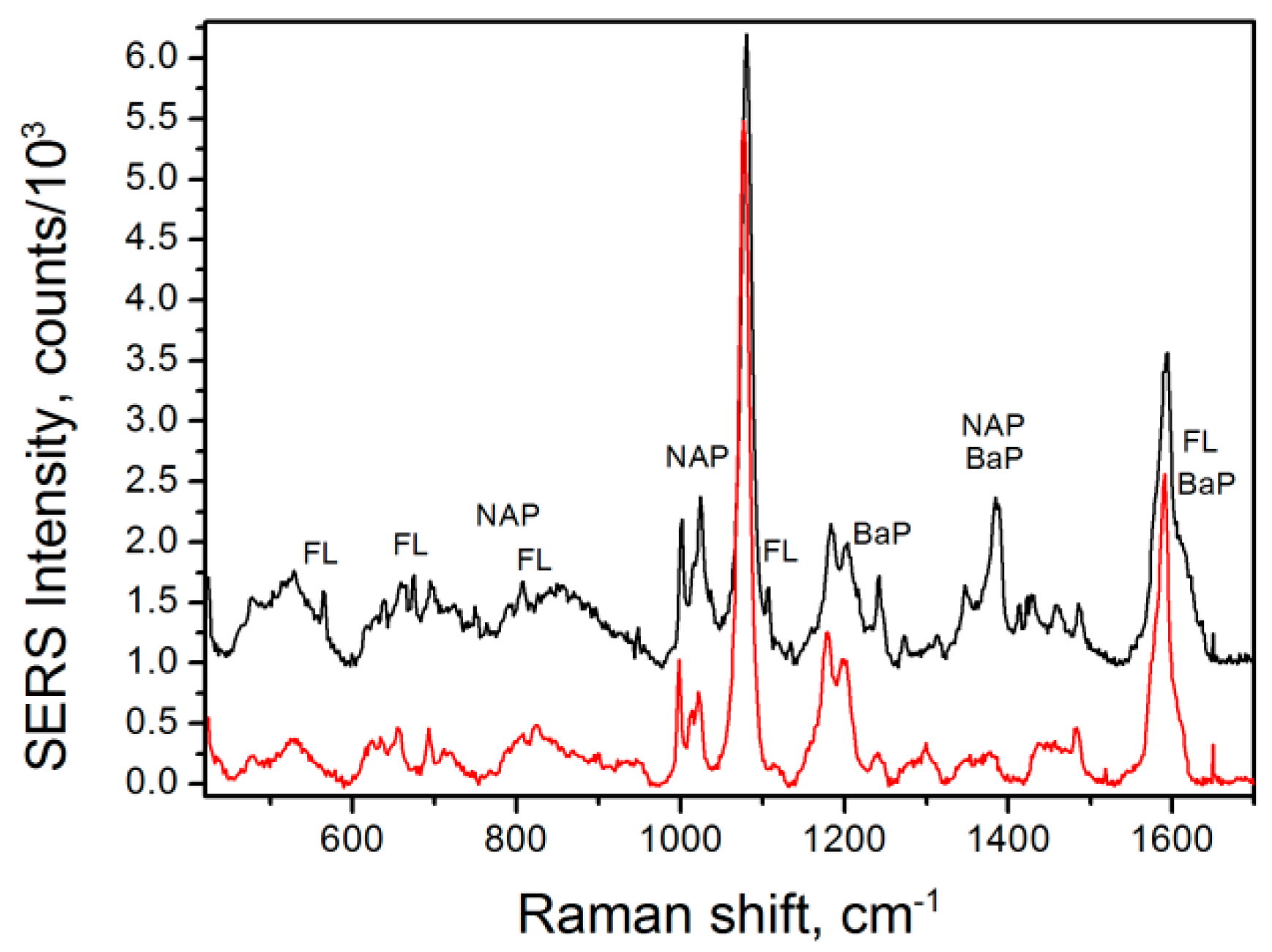
3.7. Analysis in a Mixture of Analytes
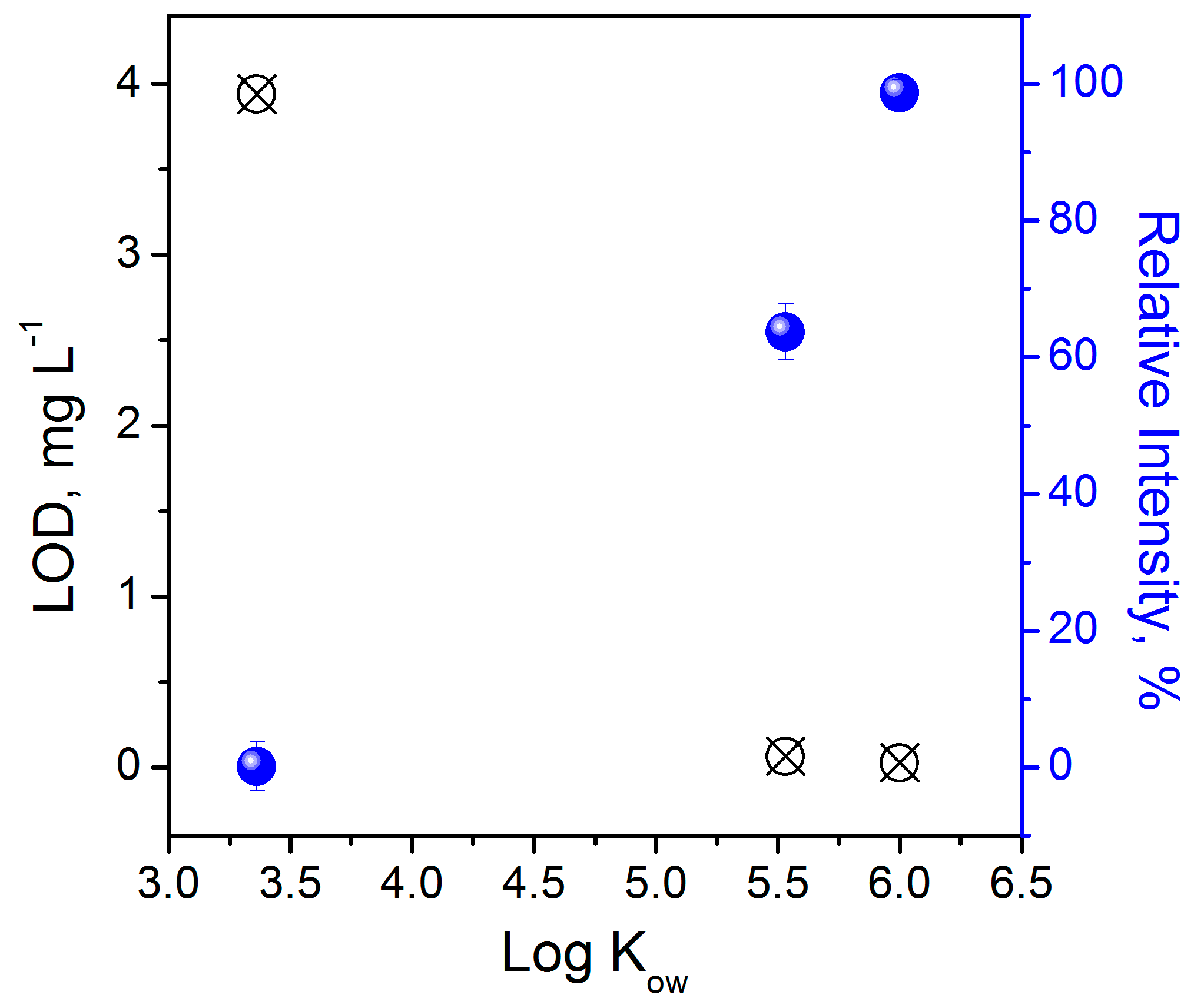
3.8. Feasibility of the Nanosensor for Sensing Polycyclic Aromatic Hydrocarbons
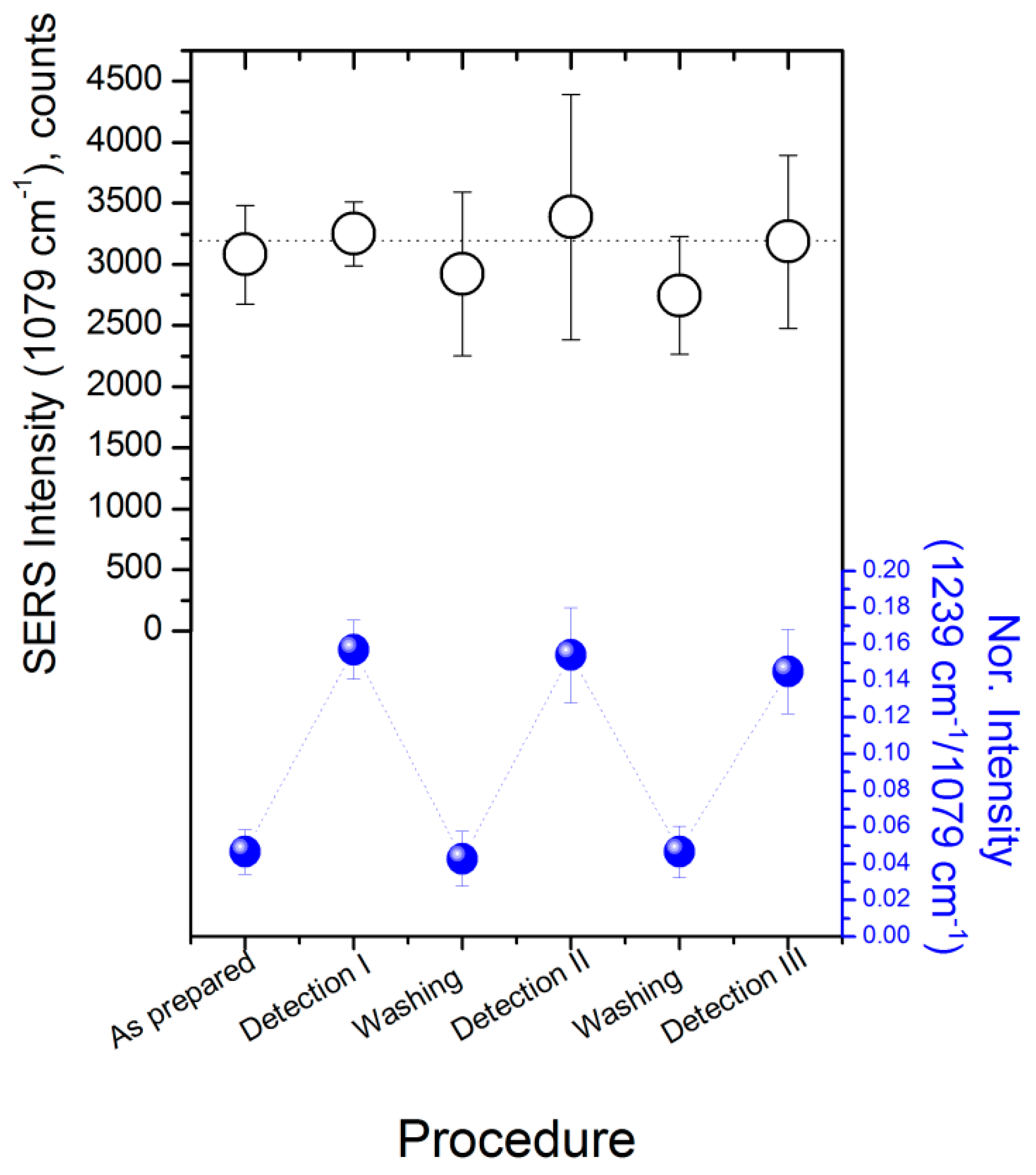
3.9. Surface Regeneration
3.10. Detection Reproducibility and Repeatability
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bessbousse, H.; Nandhakumar, I.; Decker, M.; Barsbay, M.; Cuscito, O.; Lairez, D.; Clochard, M.C.; Wade, T.L. Functionalized nanoporous track-etched β-pvdf membrane electrodes for lead (ii) determination by square wave anodic stripping voltammetry. Anal. Methods 2011, 3, 1351–1359. [Google Scholar] [CrossRef]
- Parat, C.; Authier, L.; Betelu, S.; Petrucciani, N.; Potin-Gautier, M. Determination of labile cadmium using a screen-printed electrode modified by a microwell. Electroanalysis 2007, 19, 403–406. [Google Scholar] [CrossRef]
- Betelu, S.; Parat, C.; Petrucciani, N.; Castetbon, A.; Authier, L.; Potin-Gautier, M. Semicontinuous monitoring of cadmium and lead with a screen-printed sensor modified by a membrane. Electroanalysis 2007, 19, 399–402. [Google Scholar] [CrossRef]
- Parat, C.; Betelu, S.; Authier, L.; Potin-Gautier, M. Determination of labile trace metals with screen-printed electrode modified by a crown-ether based membrane. Anal. Chim. Acta 2006, 573, 573–574. [Google Scholar] [CrossRef] [PubMed]
- Buffle, J.; Tercier-Waeber, M.L. Trends in analytical chemistry. Voltammetric environmental trace-metal analysis and speciation: From laboratory to in situ measurements. Trends Anal. Chem. 2005, 24, 172–191. [Google Scholar] [CrossRef]
- Finch, M.S.; Hydesa, D.J.; Claysona, C.H.; Weiglb, B.; Dakinc, J.; Gwilliama, P. A low power ultra violet spectrophotometer for measurement of nitrate in seawater: Introduction, calibration and initial sea trials. Anal. Chim. Acta 1998, 377, 167–177. [Google Scholar] [CrossRef]
- Mikkelsen, Ø.; Skogvold, S.M.; Schrøder, K.H. Continuous heavy metal monitoring system for application in river and seawater. Electroanalysis 2005, 17, 431–439. [Google Scholar] [CrossRef]
- Heglund, D.L.; Tilotta, D.C. Determination of volatile organic compounds in water by solid phase microextraction and infrared spectroscopy. Environ. Sci. Technol. 1996, 30, 1212–1219. [Google Scholar] [CrossRef]
- Potter, D.W.; Pawliszyn, J. Rapid determination of polyaromatic hydrocarbons and polychlorinated biphenyls in water using solid-phase. Environ. Sci. Technol. 1994, 28, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Wittkamp, B.L.; Tillota, D.C. Determination of btex compounds in water by solid-phase microextraction and raman spectroscopy. Anal. Chem. 1995, 67, 600–605. [Google Scholar] [CrossRef]
- Young, M.A.; Stuart, D.A.; Lyandres, O.; Glucksberg, M.R.; Van Duyne, R.P. Surface-enhanced raman spectroscopy with a laser pointer light source and miniature spectrometer. Can. J. Chem. 2004, 82, 1435–1441. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; De Witte, B.; Van Langenhove, H. Review: Sample preparation for the analysis of volatile organic compounds in air and water matrices. J. Chromatogr. A 2007, 1153, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Arthur, C.L.; Killiam, L.M.; Motlagh, S.; Lim, M.; Potter, D.W.; Pawliszyn, J. Analysis of substituted benzene compounds in groundwater using solid-phase microextraction. Environ. Sci. Technol. 1992, 26, 979–983. [Google Scholar] [CrossRef]
- Buchholz, K.D.; Pawliszyn, J. Determination of phenols by solid-phase microextraction and gas chromatographic analysis. Environ. Sci. Technol. 1993, 27, 2844–2848. [Google Scholar] [CrossRef]
- Zhang, Z.; Pawliszyn, J. Quantitative extraction using an internally cooled solid phase microextraction device. Anal. Chem. 1995, 67, 34–43. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Qiaoa, M.; Qia, W.; Liua, H.; Qua, J. Simultaneous determination of typical substituted and parent polycyclic aromatic hydrocarbons in water and solid matrix by gas chromatography–mass spectrometry. J. Chromatogr. A 2013, 1291, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Eisert, R.; Levsen, K. Determination of pesticides in aqueous samples by solid-phase microextraction in-line coupled to gas chromatography-mass spectrometry. J. Am. Soc. Mass. Spectrom. 1995, 6, 1119–1130. [Google Scholar] [CrossRef]
- Roy, G.; Vuillemin, R.; Guyomarch, J. On-site determination of polynuclear aromatic hydrocarbons in seawater by stir bar sorptive extraction (SBSE) and thermal desorption GC-MS. Talanta 2005, 66, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qiu, N.; Yuan, D.; Huang, B. A novel stir bar sorptive extraction coating based on monolithic material for apolar, polar organic compounds and heavy metal ions. Talanta 2009, 78, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Brunink, J.A.J.; Di Natale, C.; Bungaro, F.; Davide, F.A.M.; D’Amico, A.; Paolesse, R.; Boschi, T.; Faccio, M.; Ferri, G. The application of metalloporphyrins as coating material for quartz microbalance-based chemical sensors. Anal. Chim. Acta 1995, 325, 53–64. [Google Scholar] [CrossRef]
- Cygan, M.T.; Collins, G.E.; Dunbar, T.D.; Allara, D.L.; Gibbs, C.G.; Gutsche, C.D. Calixarene monolayers as quartz crystal microbalance sensing elements in aqueous solution. Anal. Chem. 1999, 71, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, M.; Erbahar, D.D.; Gürol, I.; Musluoglu, E.; Ahsen, V.; Öztürk, Z.Z. Phthalocyanines as sensitive coatings for qcm sensors operating in liquids for the detection of organic compounds. Sens. Actuators B 2010, 150, 346–354. [Google Scholar] [CrossRef]
- Lieberzeit, P.; Halikias, K.; Afzal, A.; Dickert, F. Polymers imprinted with pah mixtures-comparing fluorescence and qcm sensors. Anal. Bioanal. Chem. 2008, 392, 1405–1410. [Google Scholar] [CrossRef] [PubMed]
- Lucklum, R.; Rösler, S.; Hartmann, J.; Hauptmann, P. On-line detection of organic pollutants in water by thickness shear mode resonators. Sens. Actuators B 1996, 35–36, 103–111. [Google Scholar] [CrossRef]
- Menon, A.; Zhou, R.; Josse, F. Coated-quartz crystal resonator (qcr) sensors for on-line detection of organic contaminants in water. IEEE Trans. Ultrason. Ferroelect. Freq. Control 1998, 45, 1416–1426. [Google Scholar] [CrossRef]
- Patel, R.; Zhou, R.; Zinszer, K.; Josse, F. Real-time detection of organic compounds in liquid environments using polymer-coated thickness shear mode quartz resonator. Anal. Chem. 2000, 72, 4888–4898. [Google Scholar] [CrossRef] [PubMed]
- Pejcic, B.; Barton, C.; Crooke, E.; Eadington, P.; Jee, E.; Ross, A. Hydrocarbon sensing. Part 1: Some important aspects about sensitivity of a polymer-coated quartz crystal microbalance in the aqueous phase. Sens. Actuators B 2009, 135, 436–443. [Google Scholar] [CrossRef]
- Rösler, S.; Lucklum, R.; Borngräber, R.; Hartmann, J.; Hauptmann, P. Sensor system for the detection of organic pollutants in water by thickness shear mode resonators. Sens. Actuators B 1998, 48, 415–424. [Google Scholar] [CrossRef]
- Schierbaum, K.D.; Weimar, U.; Göpel, W. Comparison of ceramic, thick-film and thin-film chemical sensors based upon sno2. Sens. Actuators B 1992, 7, 709–716. [Google Scholar] [CrossRef]
- Zhou, R.; Haimbodi, M.; Everhart, D.; Josse, F. Polymer-coated QRC sensors for the detection of organic solvents in water. Sens. Actuators B 1996, 35, 176–182. [Google Scholar] [CrossRef]
- Dickert, F.L.; Lieberzeit, P.; Miarecka, S.G.; Mann, K.J.; Hayden, O.; Palfinger, C. Synthetic receptors for chemical sensors-subnano- and micrometre patterning by imprinting techniques. Biosens. Bioelectron. 2004, 20, 1040–1044. [Google Scholar] [CrossRef] [PubMed]
- Colas, F.J.; Cottat, M.; Gillibert, R.; Guillot, N.; Djaker, N.; Lidgi-Guigui, N.; Toury, T.; Barchiesi, D.; Toma, A.; Di Fabrizio, E.; et al. Red-shift effects in surface enhanced raman spectroscopy: Spectral or intensity dependence of the near-field? J. Phys. Chem. C 2016, 120, 13675–13683. [Google Scholar] [CrossRef]
- Grand, J.; de la Chapelle, M.L.; Bijeon, J.L.; Adam, P.M.; Vial, A.; Royer, P. Role of localized surface plasmons in surface-enhanced raman scattering of shape-controlled metallic particles in regular arrays. Phys. Rev. B 2005, 72, 033407. [Google Scholar] [CrossRef]
- Schatz, G.C.; Van Duyne, R.P. Electromagnetic mechanism of surface enhanced raman spectroscopy. In Handbook of Vibrational Spectroscopy; Chalmers, J.M., Griffiths, P.R., Eds.; Wiley: Hoboken, NJ, USA, 2002; Volume 1, p. 759. [Google Scholar]
- Wokaun, A. Surface-enhanced electromagnetic processes. Solid State Phys. 1984, 38, 223–294. [Google Scholar]
- Guillot, N.; de la Chapelle, M.L. The electromagnetic effect in surface enhanced raman scattering: Enhancement optimization using precisely controlled nanostructures. J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 2321–2333. [Google Scholar] [CrossRef]
- Cottat, M.; Lidgi-Guigui, N.; Hamouda, F.; Bartenlian, B.; Venkataraman, D.; Marks, R.S.; Steele, T.W.S.; Lamy de la Chapelle, M. Highly sensitive detection of paclitaxel by surface-enhanced raman scattering. J. Opt. 2015, 17, 114019. [Google Scholar] [CrossRef]
- Cottat, M.; D’Andrea, C.; Yasukuni, R.; Malashikhina, N.; Grinyte, R.; Lidgi-Guigui, N.; Fazio, B.; Sutton, A.; Oudar, O.; Charnaux, N.; et al. High sensitivity, high selectivity sers detection of mnsod using optical nanoantennas functionalized with aptamers. J. Phys. Chem. C 2015, 119, 15532–15540. [Google Scholar] [CrossRef]
- Albrecht, M.G.; Creighton, J.A. Intense raman spectra of pyridine at a silver electrode. J. Am. Chem. Soc. 1977, 99, 5215–5217. [Google Scholar] [CrossRef]
- Fleischmann, M.; Hendra, P.J.; McQuillan, A.J. Raman spectra of pyridine adsorbed at a silver electrode. Chem. Phys. Lett. 1974, 26, 163–166. [Google Scholar] [CrossRef]
- Jeanmaire, D.L.; Van Duyne, R.P. Surface raman spectroelectrochemistry: Part I. Heterocyclic, aromatic, and aliphatic amines adsorbed on the anodized silver electrode. J. Electroanal. Chem. Interfacial Electrochem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Kambhampati, P.; Child, C.M.; Foster, M.C.; Campion, A. On the chemical mechanism of surface enhanced raman scattering: Experiment and theory. J. Chem. Phys. 1998, 108, 5013–5026. [Google Scholar] [CrossRef]
- Guillot, N.; Lamy de la Chapelle, M. Lithographied nanostructures as nanosensors. J. Nanophotonics 2012, 6, 064506–064528. [Google Scholar] [CrossRef]
- Kneipp, K.; Wang, Y.; Kneipp, H.; Perelman, L.T.; Itzkan, I.; Dasari, R.R.; Feld, M.S. Single molecule detection using surface-enhanced raman scattering (sers). Phys. Rev. Lett. 1997, 78, 1667–1670. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Bich Ha, N.; Pfannkuche, J.; Amann, H.; Kronfeldt, H.-D.; Kowalewska, G. Detection of pahs in seawater using surface-enhanced raman scattering (sers). Mar. Pollut. Bull. 2004, 49, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Wang, X.; Han, X.; Song, W.; Ruan, W.; Liu, J.; Zhao, B.; Ozaki, Y. Selective sers detection of each polycyclic aromatic hydrocarbon (PAH) in a mixture of five kinds of pahs. J. Raman Spectrosc. 2011, 42, 945–950. [Google Scholar] [CrossRef]
- Otto, A.; Mrozek, I.; Grabhorn, H.; Akemann, W. Surface-enhanced raman scattering. J. Phys. Condens. Matter 1992, 4, 1143. [Google Scholar] [CrossRef]
- Peron, O.; Rinnert, E.; Lehaitre, M.; Crassous, P.; Compere, C. Detection of polycyclic aromatic hydrocarbon (pah) compounds in artificial sea-water using surface-enhanced raman scattering (sers). Talanta 2009, 79, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Allan, I.J.; Vrana, B.; Greenwood, R.; Mills, G.A.; Roig, B.; Gonzales, C. A “toolbox” for biological and chemical monitoring requirements for the european union’s water framework directive. Talanta 2006, 69, 302–322. [Google Scholar] [CrossRef] [PubMed]
- Peron, O.; Rinnert, E.; Florent, C.; Lehaitre, M.; Compere, C. First steps of in situ surface-enhanced raman scattering during shipboard experiments. Appl. Spectrosc. 2010, 64, 1086–1093. [Google Scholar] [CrossRef] [PubMed]
- Peron, O.; Rinnert, E.; Toury, T.; Lamy de la Chapelle, M.; Compère, C. Quantitative sers sensors for environmental analysis of naphthalene. Analyst 2011, 136, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Prien, R.D. The future of chemical in situ sensors. Mar. Chem. 2007, 107, 422–432. [Google Scholar] [CrossRef]
- Rogers, J.A.; Jackman, R.J.; Whitesides, G.M. Constructing single- and multiple-helical microcoils and characterizing their performance as components of microinductors and microelectromagnets. J. Microelectromech. Syst. 1997, 6, 184–192. [Google Scholar] [CrossRef]
- Ulman, A. Formation and structure of self-assembled monolayers. Chem. Rev. 1996, 96, 1533–1554. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, O.; Busch, J.A.; Cembella, A.D.; Daly, K.L.; Engelbrektsson, J.; Hannides, A.K.; Schmidt, H. Detecting marine hazardous substances and organisms: Sensors for pollutants, toxins, and pathogens. Ocean Sci. Discuss. 2009, 6, 953–1005. [Google Scholar] [CrossRef]
- Chow, E.; Hibbert, D.B.; Gooding, J.J. Voltammetric detection of cadmium ions at glutathione-modified gold electrodes. Analyst 2005, 130, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Delamarche, E.; Michel, B.; Kang, H.; Gerber, C. Thermal stability of self-assembled monolayers. Langmuir 1994, 10, 4103–4108. [Google Scholar] [CrossRef]
- Horn, A.B.; Russell, D.A.; Shorthouse, L.J.; Simpson, T.R.E. Ageing of alkanethiol self-assembled monolayers. J. Chem. Soc. 1996, 92, 4759–4762. [Google Scholar] [CrossRef]
- Betelu, S.; Tijunelyte, I.; Boubekeur-Lecaque, L.; Ignatiadis, I.; Ibrahim, J.; Gaboreau, S.; Berho, C.; Toury, T.; Guenin, E.; Lidgi-Guigui, N.; et al. Evidence of the grafting mechanisms of diazonium salts on gold nanostructures. J. Phys. Chem. C 2016, 120, 18158–18166. [Google Scholar] [CrossRef]
- Betelu, S.; Vautrin-Ul, C.; Chaussé, A. Novel 4-carboxyphenyl-grafted screen-printed electrode for trace cu(ii) determination. Electrochem. Commun. 2009, 11, 383–386. [Google Scholar] [CrossRef]
- Betelu, S.; Vautrin-Ul, C.; Ly, J.; Chaussé, A. Screen-printed electrografted electrode for trace uranium analysis. Talanta 2009, 80, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Delamar, M.; Hitmi, R.; Pison, J.; Savéan, J. Covalent modification of carbon surfaces by grafting of functionalized aryl radicals produced from electrochemical reduction of diazonium salts. J. Am. Chem. Soc. 1992, 114, 5883–5884. [Google Scholar] [CrossRef]
- Adenier, A.; Cabet-Deliry, E.; Chaussé, A.; Griveau, S.; Mercier, F.; Pinson, J.; Vautrin-Ul, C. Grafting of nitrophenyl groups on carbon without electrochemical induction. Chem. Mater. 2005, 17, 491–501. [Google Scholar] [CrossRef]
- Bekyarova, E.; Itkis, M.E.; Ramesh, P.; Berger, C.; Sprinkle, M.; de Heer, W.A.; Haddon, R.C. Chemical modification of epitaxial graphene: Spontaneous grafting of aryl groups. J. Am. Chem. Soc. 2009, 131, 1336–1337. [Google Scholar] [CrossRef] [PubMed]
- Combellas, C.M.; Delamar, F.; Kanoufi, F.; Pinson, J.; Podvorica, F.I. Spontaneous grafting of iron surfaces by reduction of aryldiazonium salts in acidic or neutral aqueous solution. Application to the protection of iron against corrosion. Chem. Mater. 2005, 17, 3968–3975. [Google Scholar] [CrossRef]
- Laurentius, L.; Stoyanov, S.R.; Gusarov, S.; Kovalenko, A.; Du, R.; Lopinski, G.P.; McDermott, M.T. Diazonium-derived aryl films on gold nanoparticles: Evidence for a carbon–gold covalent bond. ACS Nano 2011, 5, 4219–4227. [Google Scholar] [CrossRef] [PubMed]
- Griveau, S.; Mercier, D.; Vautrin-Ul, C.; Chaussé, A. Electrochemical grafting by reduction of 4-aminoethylbenzenediazonium salt: Application to the immobilization of (bio)molecules. Electrochem. Commun. 2007, 9, 2768–2773. [Google Scholar] [CrossRef]
- Manoli, E.; Samara, C. Polycyclic aromatic hydrocarbons in natural waters: Sources, occurrence and analysis. Trends Anal. Chem. 1999, 18, 417–428. [Google Scholar] [CrossRef]
- Eilers, P.H.C. A perfect smoother. Anal. Chem. 2003, 75, 3631–3636. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.; Rinnert, E. Fast identification and quantification of btex coupling by raman spectrometry and chemometrics. Analyst 2015, 140, 3535–3542. [Google Scholar] [CrossRef] [PubMed]
- Busson, M.; Berisha, A.; Combellas, C.; Kanoufi, F.; Pinson, J. Photochemical grafting of diazonium salts on metals. Chem. Commun. 2011, 47, 12631–12633. [Google Scholar] [CrossRef] [PubMed]
- Bouriga, M.; Chehimi, M.M.; Combellas, C.; Decorse, P.; Kanoufi, F.; Deronzier, A.; Pinson, J. Sensitized photografting of diazonium salts by visible light. Chem. Mater. 2013, 25, 90–97. [Google Scholar] [CrossRef]
- Abiman, P.; Wildgoose, G.G.; Compton, R.G. Investigating the mechanism for the covalent chemical modification of multiwalled carbon nanotubes using aryl diazonium salts. Int. J. Electrochem. Sci. 2008, 3, 104–117. [Google Scholar]
- Ahmad, R.; Boubekeur-Lecaque, L.; Nguyen, M.; Lau-Truong, S.; Lamouri, A.; Decorse, P.; Galtayries, A.; Pinson, J.; Felidj, N.; Mangeney, C. Tailoring the surface chemistry of gold nanorods through au–c/ag–c covalent bonds using aryl diazonium salts. J. Phys. Chem. C 2014, 118, 19098–19105. [Google Scholar] [CrossRef]
- Qu, L.-L.; Li, Y.-T.; Li, D.-W.; Xue, J.-Q.; Fossey, J.S.; Long, Y.-T. Humic acids-based one-step fabrication of sers substrates for detection of polycyclic aromatic hydrocarbons. Analyst 2013, 138, 1523–1528. [Google Scholar] [CrossRef] [PubMed]
- Tijunelyte, I.; Dupont, N.; Milosevic, I.; Barbey, C.; Rinnert, E.; Lidgi-Guigui, N.; Guenin, E.; de la Chapelle, M. Investigation of aromatic hydrocarbon inclusion into cyclodextrins by raman spectroscopy and thermal analysis. Environ. Sci. Pollut. Res. 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- International Union of Pure and Applied Chemistry. Compendium of Chemical Terminology; Blackwell Science: Oxford, UK, 1997. [Google Scholar]
- Guideline, I.H.T. Validation of Analytical Procedures: Text and Methodology. Available online: https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Quality/Q2_R1/Step4/Q2_R1__Guideline.pdf (accessed on 24 May 2017).
- Na, W.; Hai-Feng, Y.; Xuan, Z.; Rui, Z.; Yao, W.; Guan-Feng, H.; Zong-Rang, Z. Synthesis of anti-aggregation silver nanoparticles based on inositol hexakisphosphoric micelles for a stable surface enhanced raman scattering substrate. Nanotechnology 2009, 20, 315603. [Google Scholar]
- Fu, S.; Guo, X.; Wang, H.; Yang, T.; Wen, Y.; Yang, H. Functionalized au nanoparticles for label-free raman determination of ppb level benzopyrene in edible oil. Sens. Actuators B 2015, 212, 200–206. [Google Scholar] [CrossRef]
- Zheng, Y.; Thai, T.; Reineck, P.; Qiu, L.; Guo, Y.; Bach, U. DNA-directed self-assembly of core-satellite plasmonic nanostructures: A highly sensitive and reproducible near-ir sers sensor. Adv. Funct. Mater. 2013, 23, 1519–1526. [Google Scholar] [CrossRef]
| Polycyclic Aromatic Hydrocarbon | Structure | Solubility in Water (mg L−1) | Kow |
|---|---|---|---|
| Benzo[a]pyrene (BaP) | 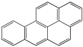 | 0.0038 at 25 °C | 106 |
| Fluoranthene (FL) | 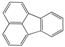 | 0.26 at 25 °C | 3.4 × 105 |
| Naphthalene (NAP) | 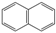 | 32 | 2.3 × 103 |
| Benzo[a]Pyrene (BaP) | Fluoranthene (FL) | Naphthalene (NAP) | |||
|---|---|---|---|---|---|
| Raman | SERS | Raman | SERS | Raman | SERS |
| 615 | 615 | 562 | 562 | 509 | 511 |
| 1215 | 1215 | 671 | 672 | 762 | 762 |
| 1236 | 1239 | 803 | 803 | 1021 | 1021 |
| 1345 | 1345 | 1018 | 1018 | 1382 | 1382 |
| 1389 | 1385 | 1104 | 1104 | 1577 | 1577 |
| 1623 | 1623 | 1269 | 1270 | ||
| 1612 | 1612 | ||||
| Polycyclic Aromatic Hydrocarbon | LOD | LOQ | ||
|---|---|---|---|---|
| (mg L−1) | (mol L−1) | (mg L−1) | (mol L−1) | |
| Benzo[a]pyrene (BaP) | 0.026 | 10−7 | 0.087 | 3.4 × 10−7 |
| Fluoranthene (FL) | 0.064 | 3.2 × 10−7 | 0.214 | 1.1 × 10−6 |
| Naphthalene (NAP) | 3.9 | 3 .0× 10−5 | 13 | 1.0 × 10−4 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tijunelyte, I.; Betelu, S.; Moreau, J.; Ignatiadis, I.; Berho, C.; Lidgi-Guigui, N.; Guénin, E.; David, C.; Vergnole, S.; Rinnert, E.; et al. Diazonium Salt-Based Surface-Enhanced Raman Spectroscopy Nanosensor: Detection and Quantitation of Aromatic Hydrocarbons in Water Samples. Sensors 2017, 17, 1198. https://doi.org/10.3390/s17061198
Tijunelyte I, Betelu S, Moreau J, Ignatiadis I, Berho C, Lidgi-Guigui N, Guénin E, David C, Vergnole S, Rinnert E, et al. Diazonium Salt-Based Surface-Enhanced Raman Spectroscopy Nanosensor: Detection and Quantitation of Aromatic Hydrocarbons in Water Samples. Sensors. 2017; 17(6):1198. https://doi.org/10.3390/s17061198
Chicago/Turabian StyleTijunelyte, Inga, Stéphanie Betelu, Jonathan Moreau, Ioannis Ignatiadis, Catherine Berho, Nathalie Lidgi-Guigui, Erwann Guénin, Catalina David, Sébastien Vergnole, Emmanuel Rinnert, and et al. 2017. "Diazonium Salt-Based Surface-Enhanced Raman Spectroscopy Nanosensor: Detection and Quantitation of Aromatic Hydrocarbons in Water Samples" Sensors 17, no. 6: 1198. https://doi.org/10.3390/s17061198






