Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers
Abstract
:1. Introduction
2. Materials and Methods
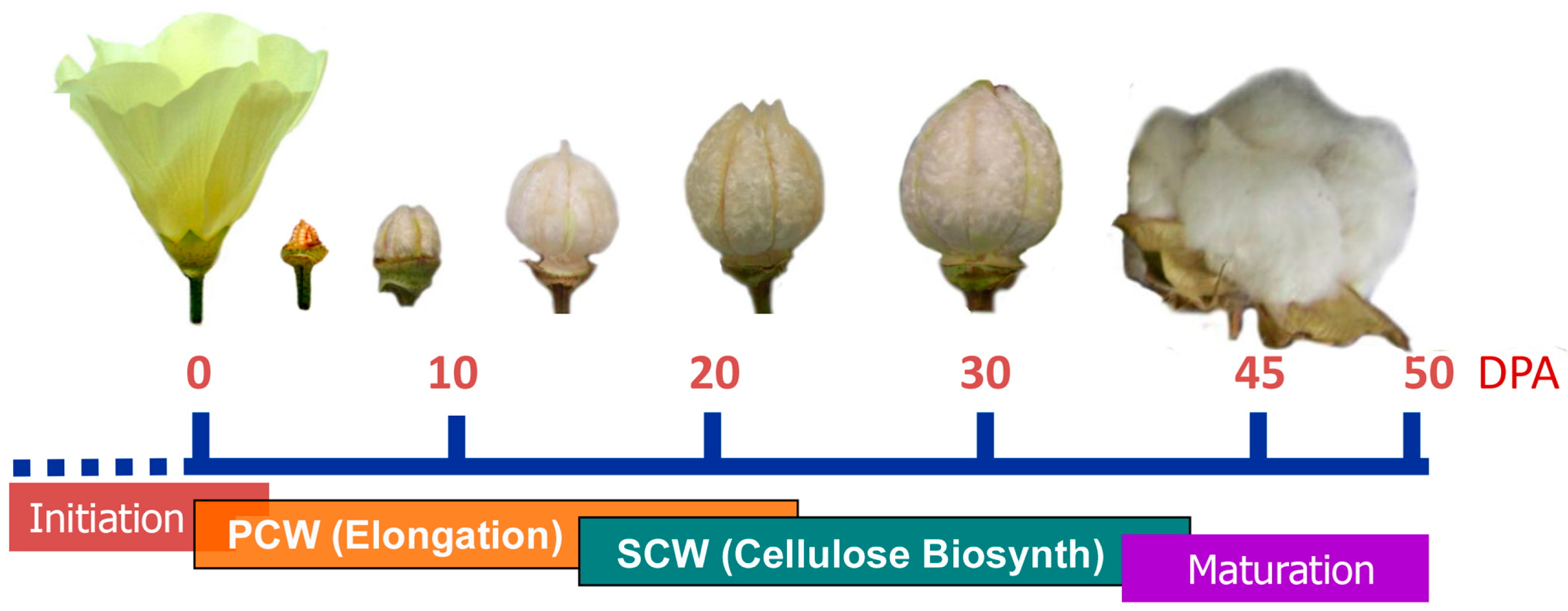
2.1. Texas Marker-1 (TM-1) and Immature Fiber (im) Mutant Fibers
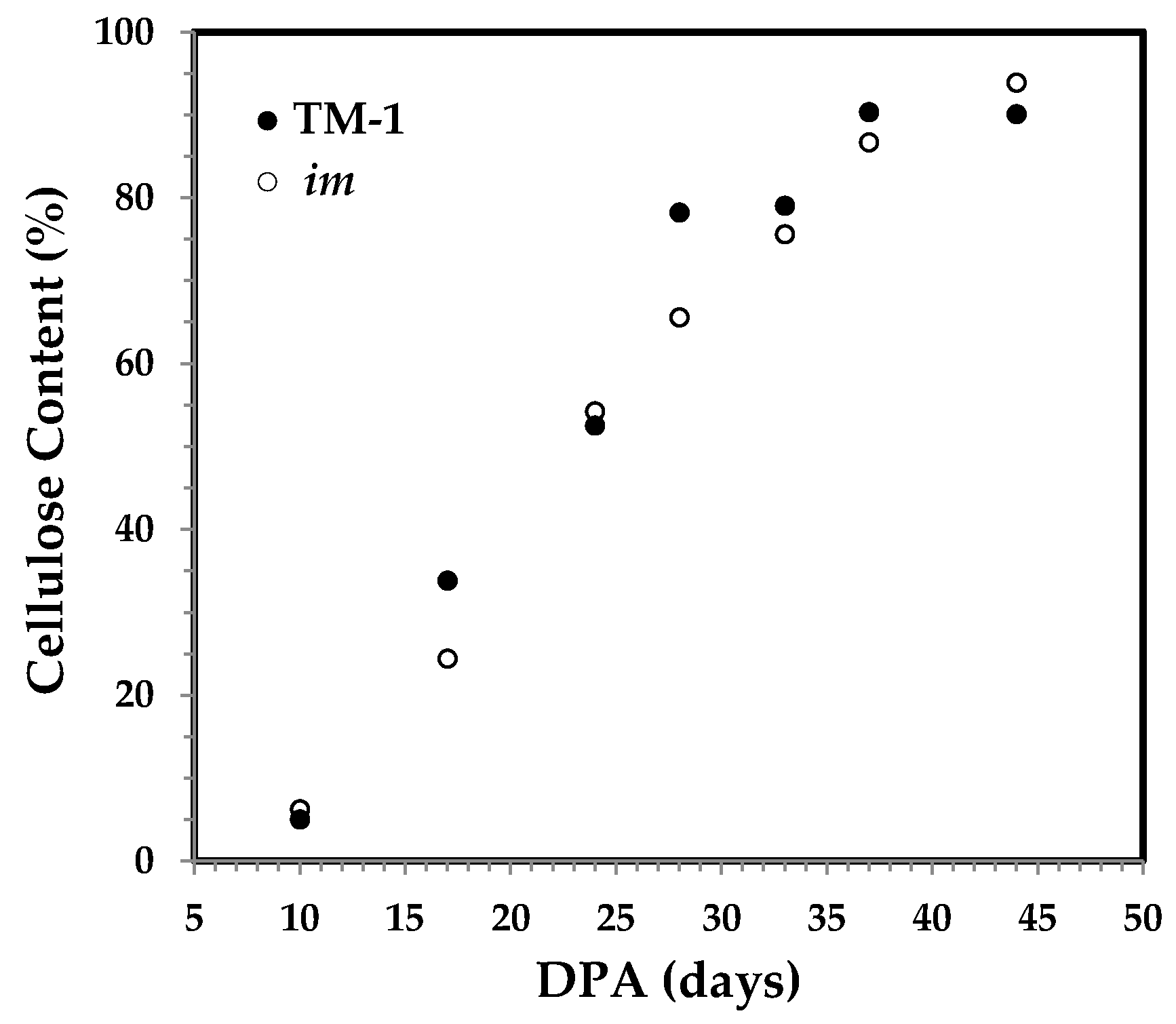
2.2. Cellulose Content
2.3. ATR FT-IR Spectral Collection and Data Analysis
3. Results and Discussion
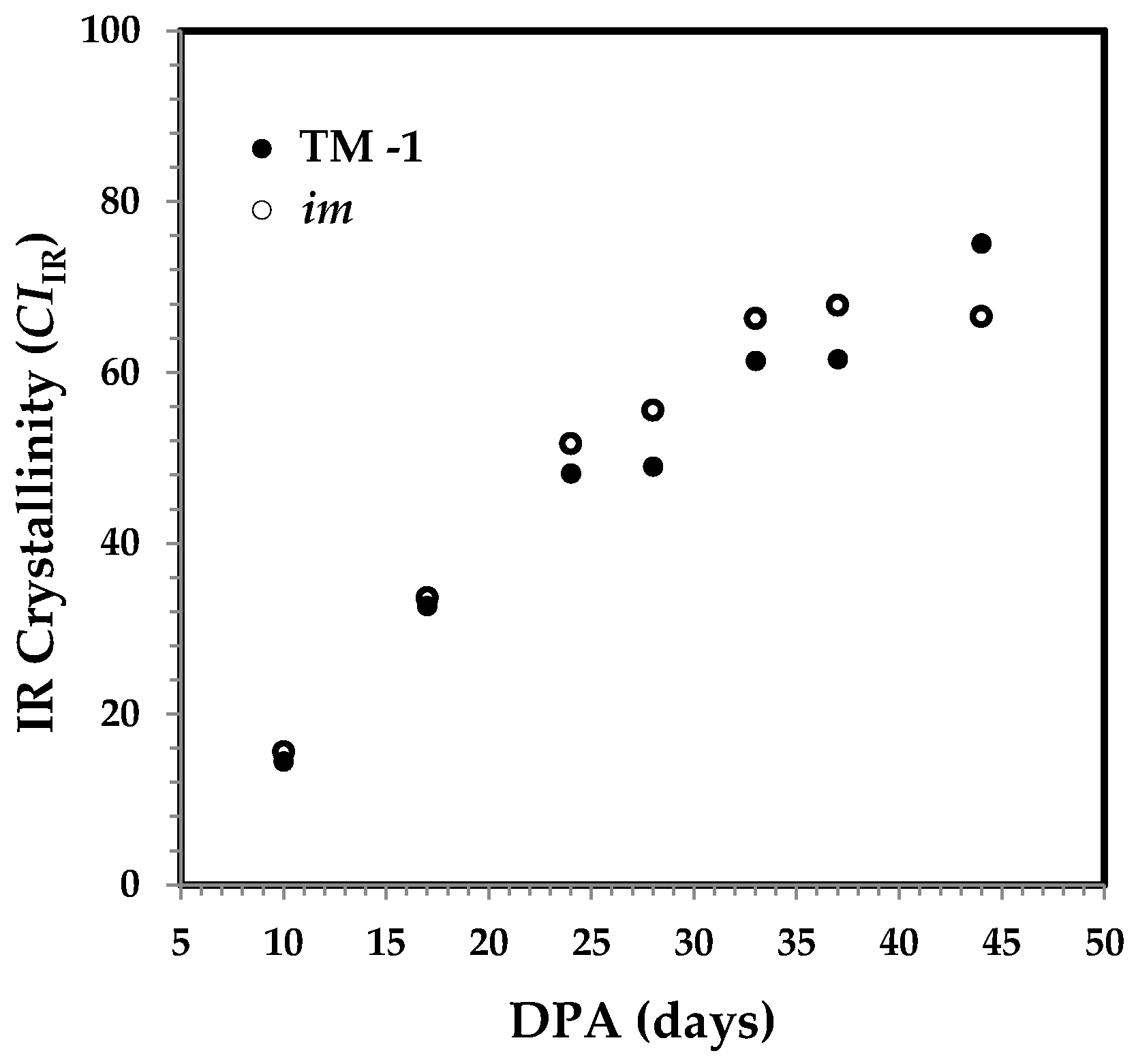
3.1. DPA-Dependent Cellulose Content of Developmental Fibers
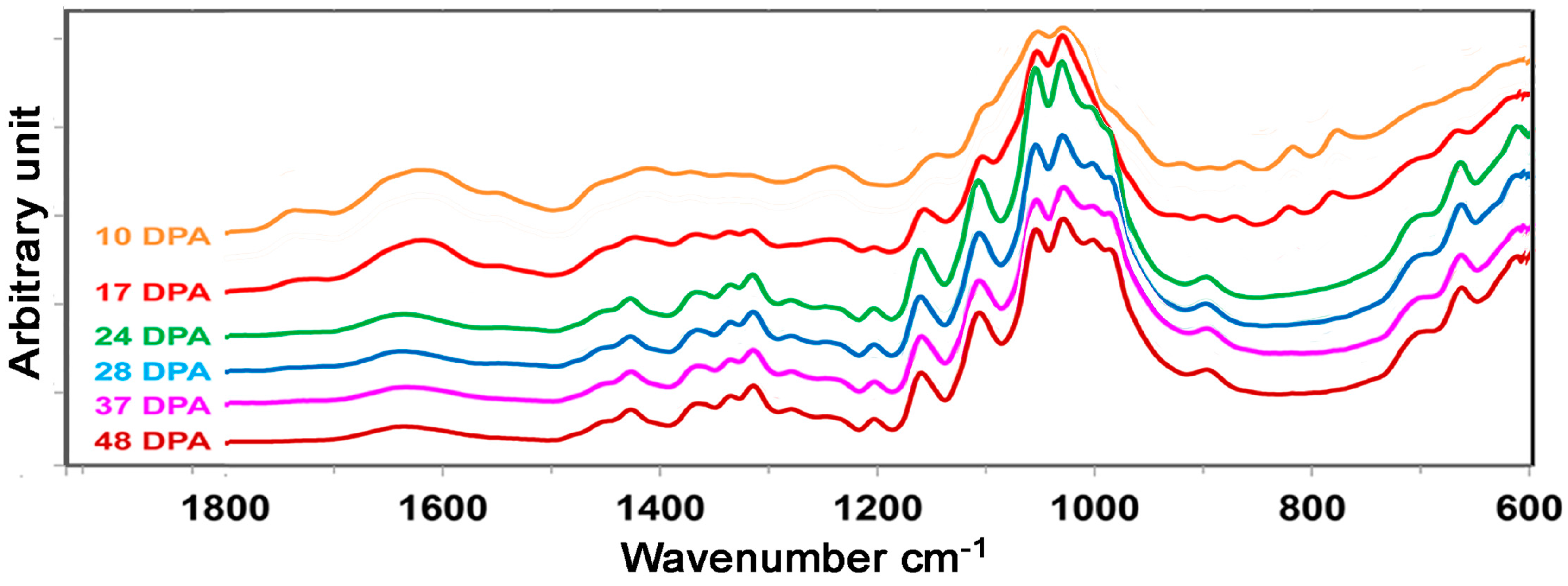
3.2. ATR FT-IR Spectral Characteristics of Developmental TM-1 Cotton Fibers
3.3. Correlation between Cellulose Content and ATR FT-IR Spectral Response of Developmental Fibers
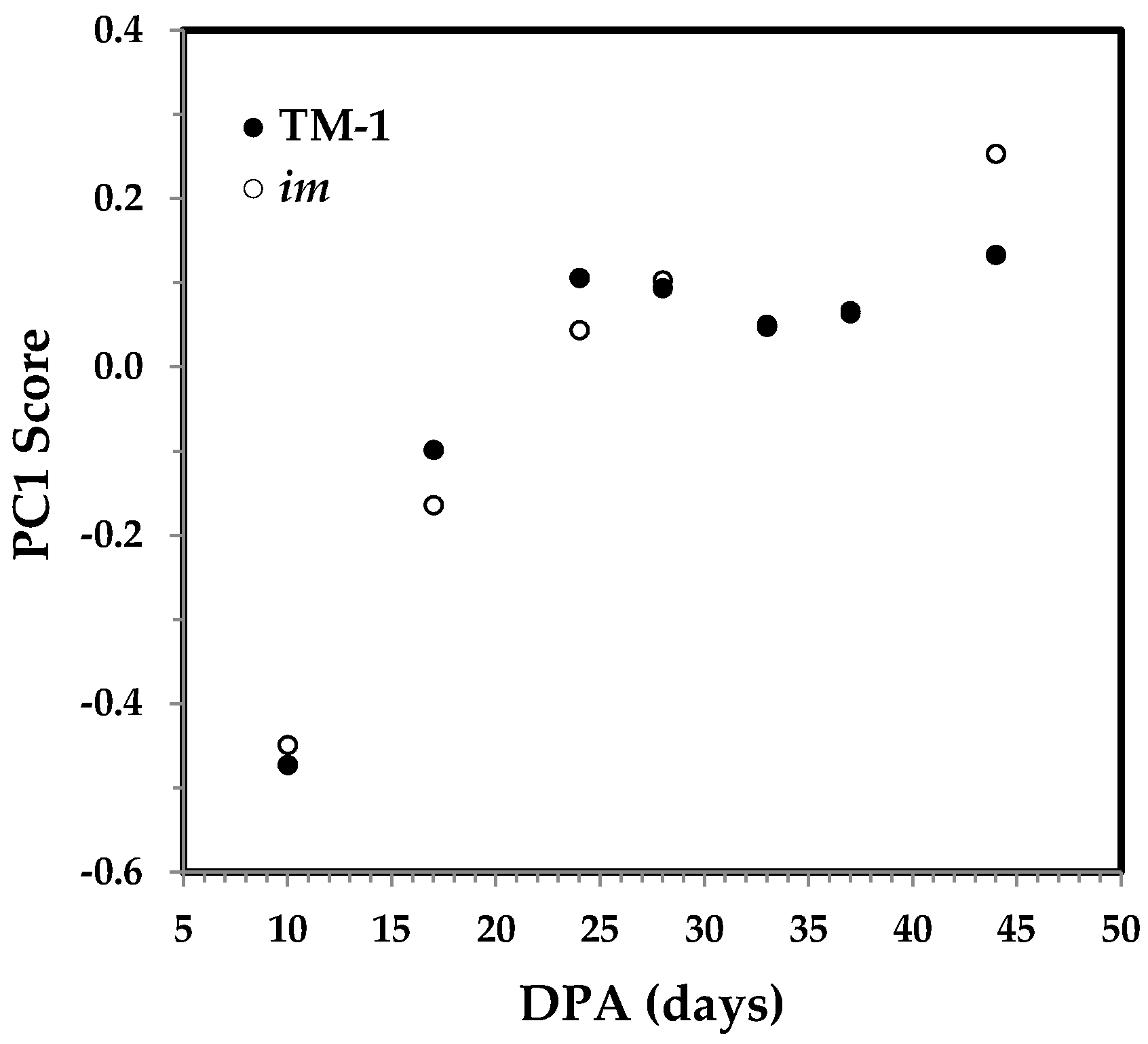
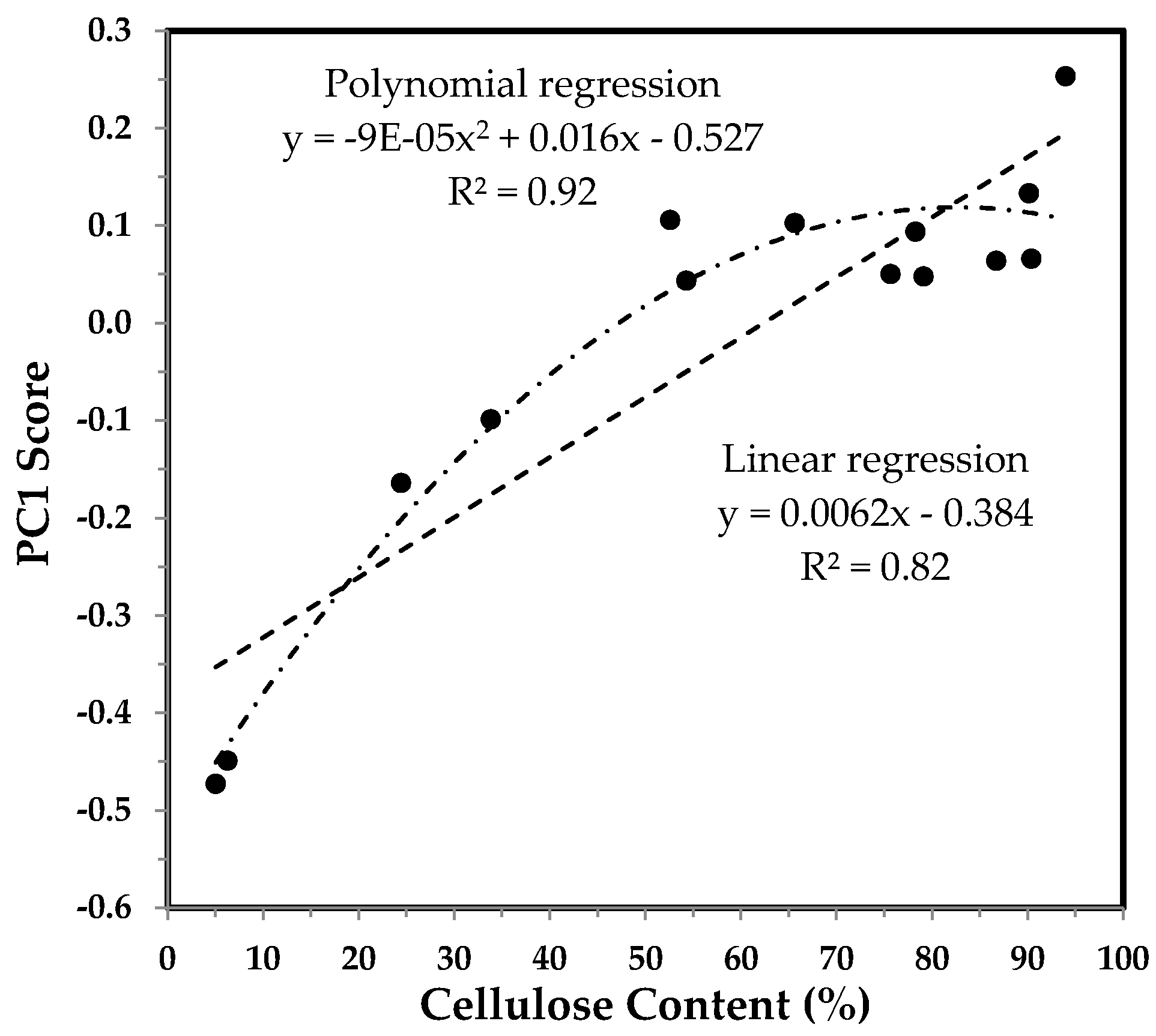
3.3.1. PC1 Score and the Correlation with Cellulose Content
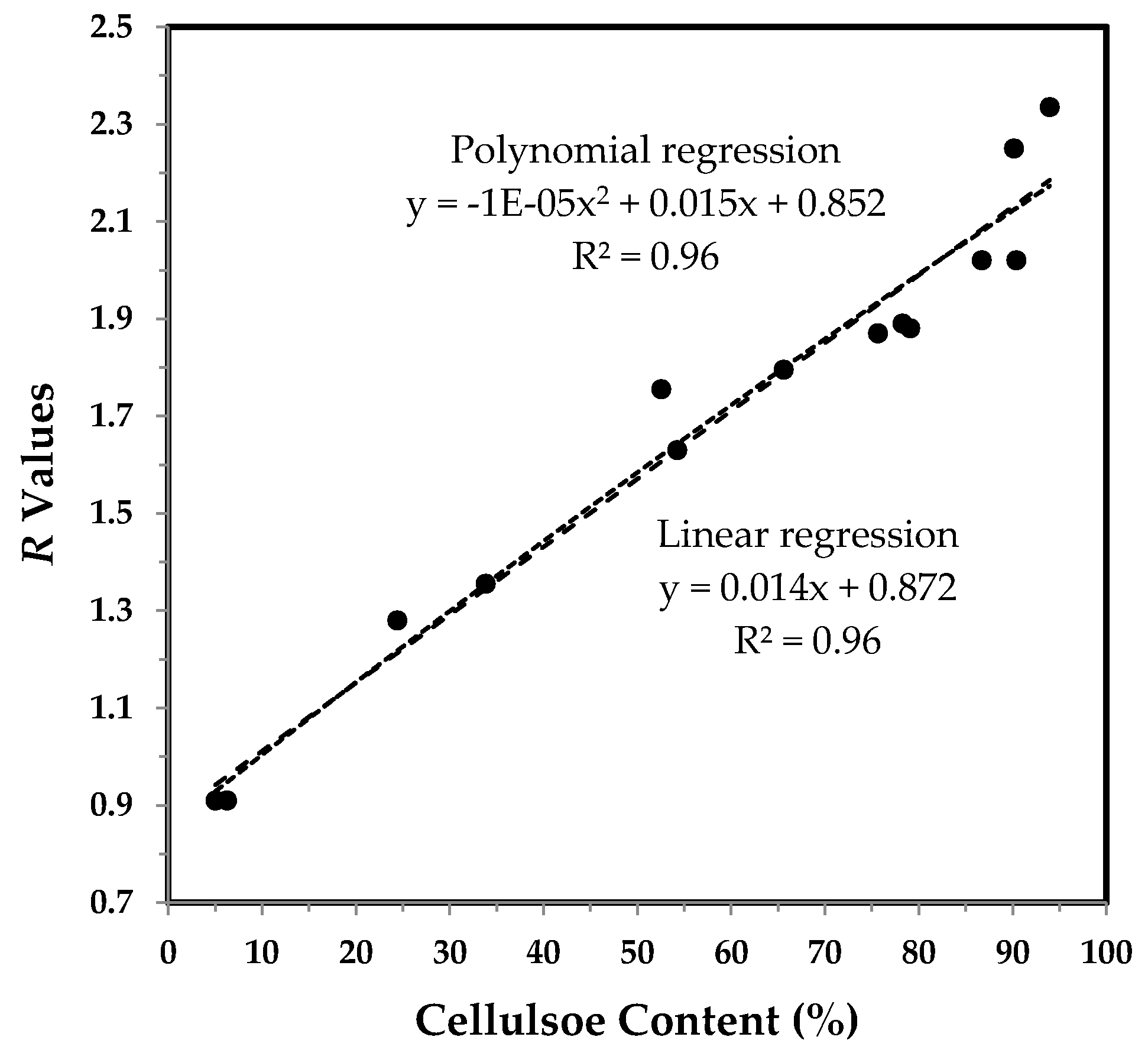
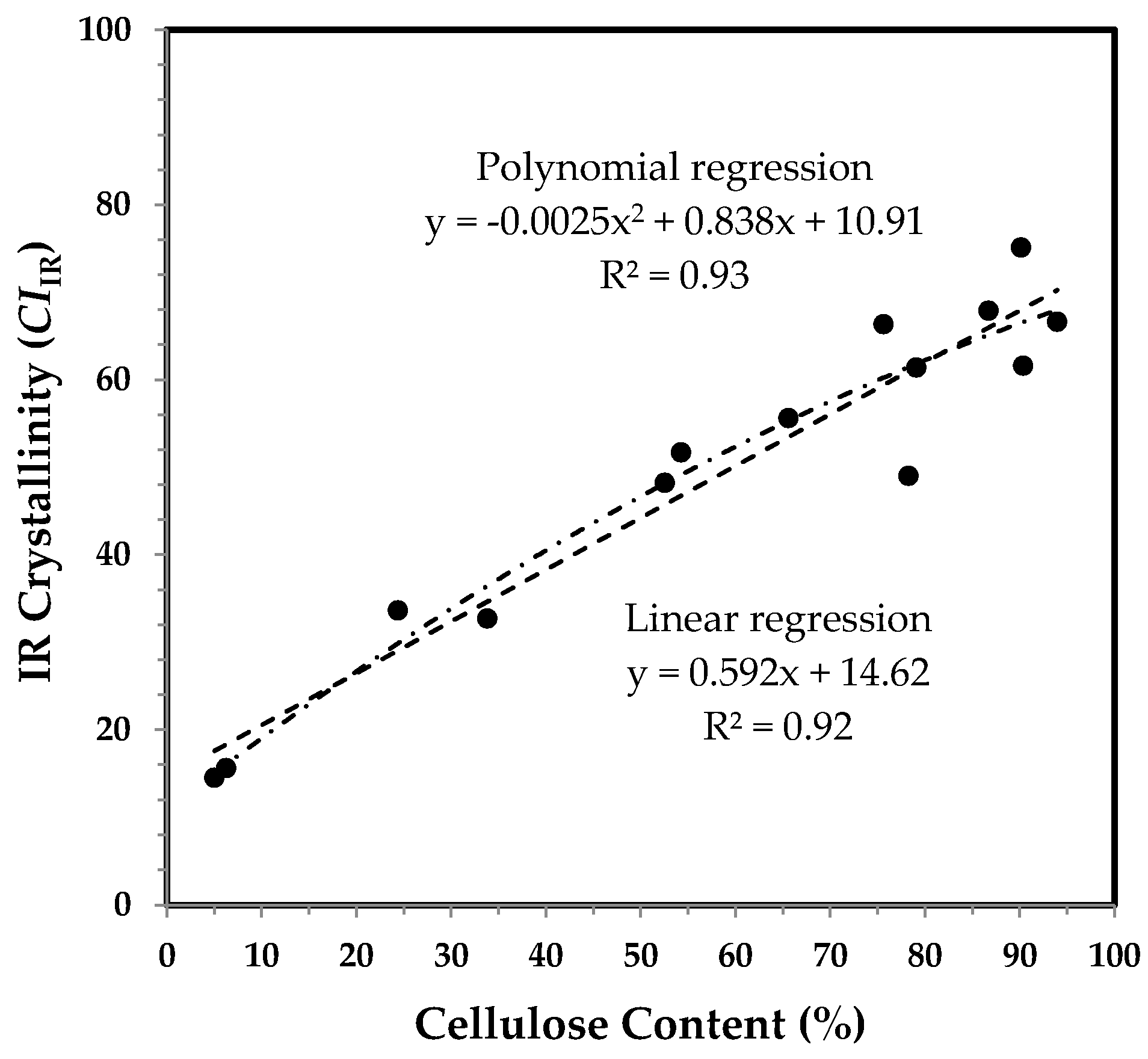
3.3.2. IR maturity and Crystallinity and the Correlation with Cellulose Content
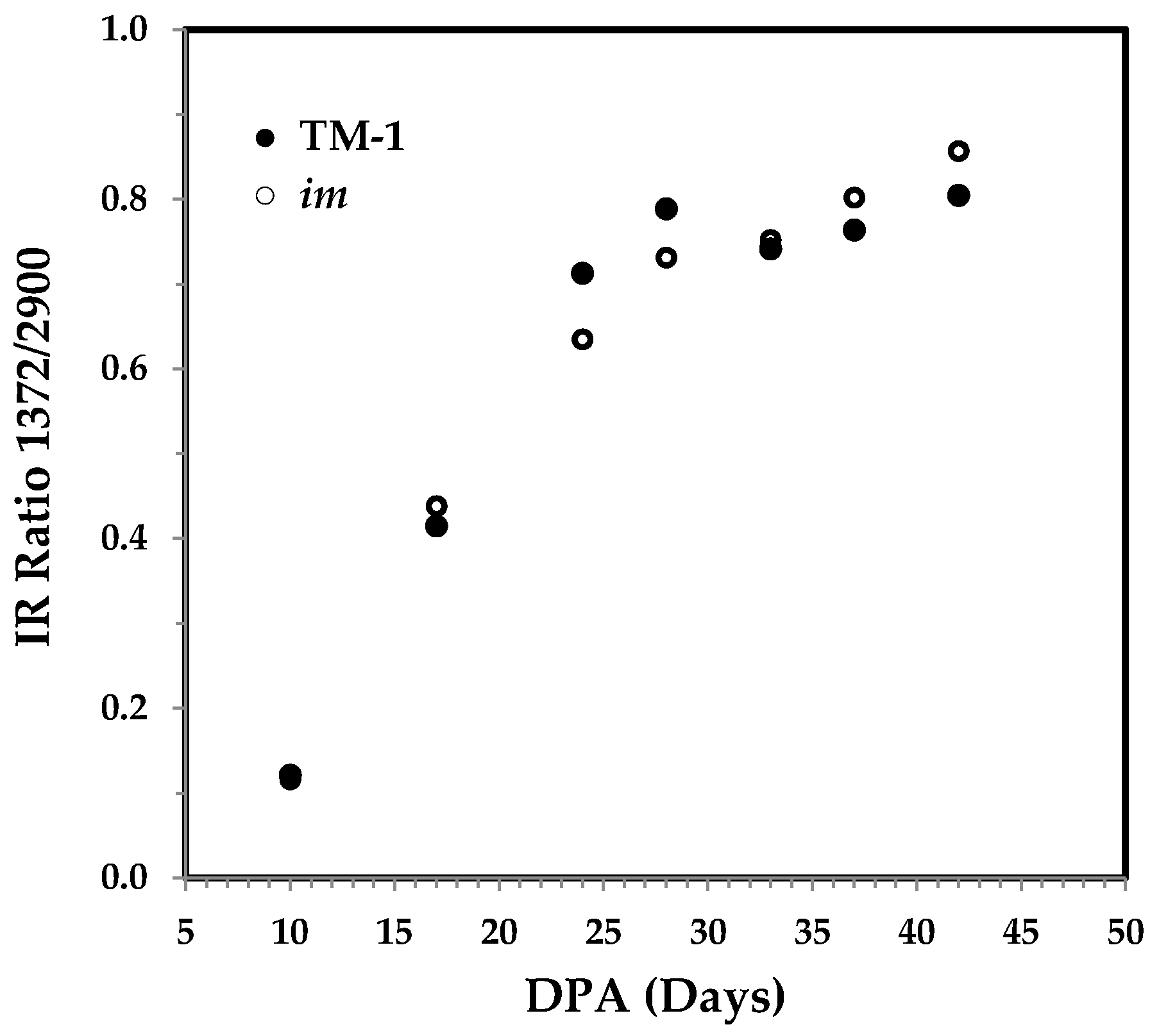
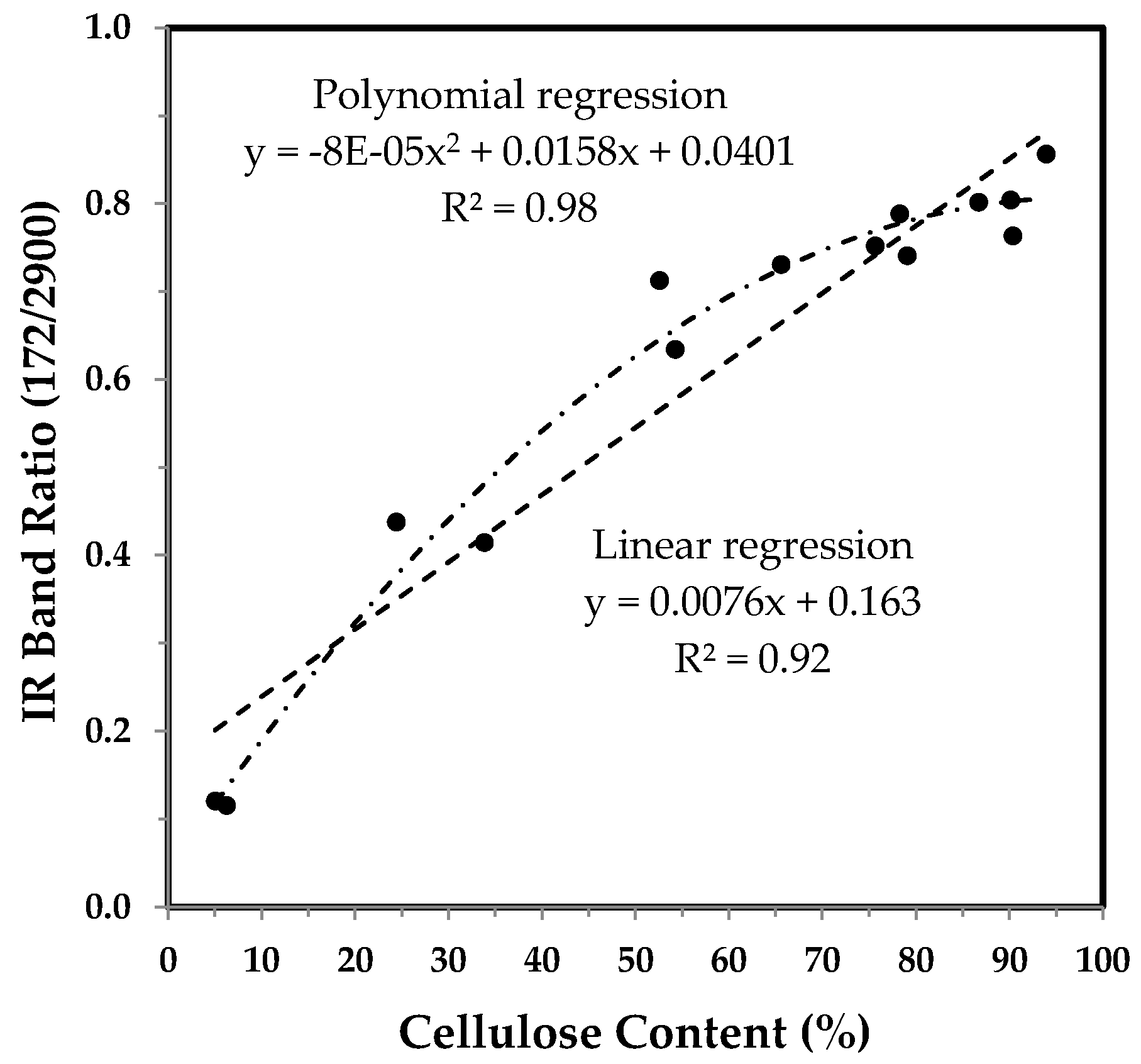
3.3.3. The 1372 and 2900 cm−1 Band Based IR Crystallinity and the Correlation with Cellulose Content
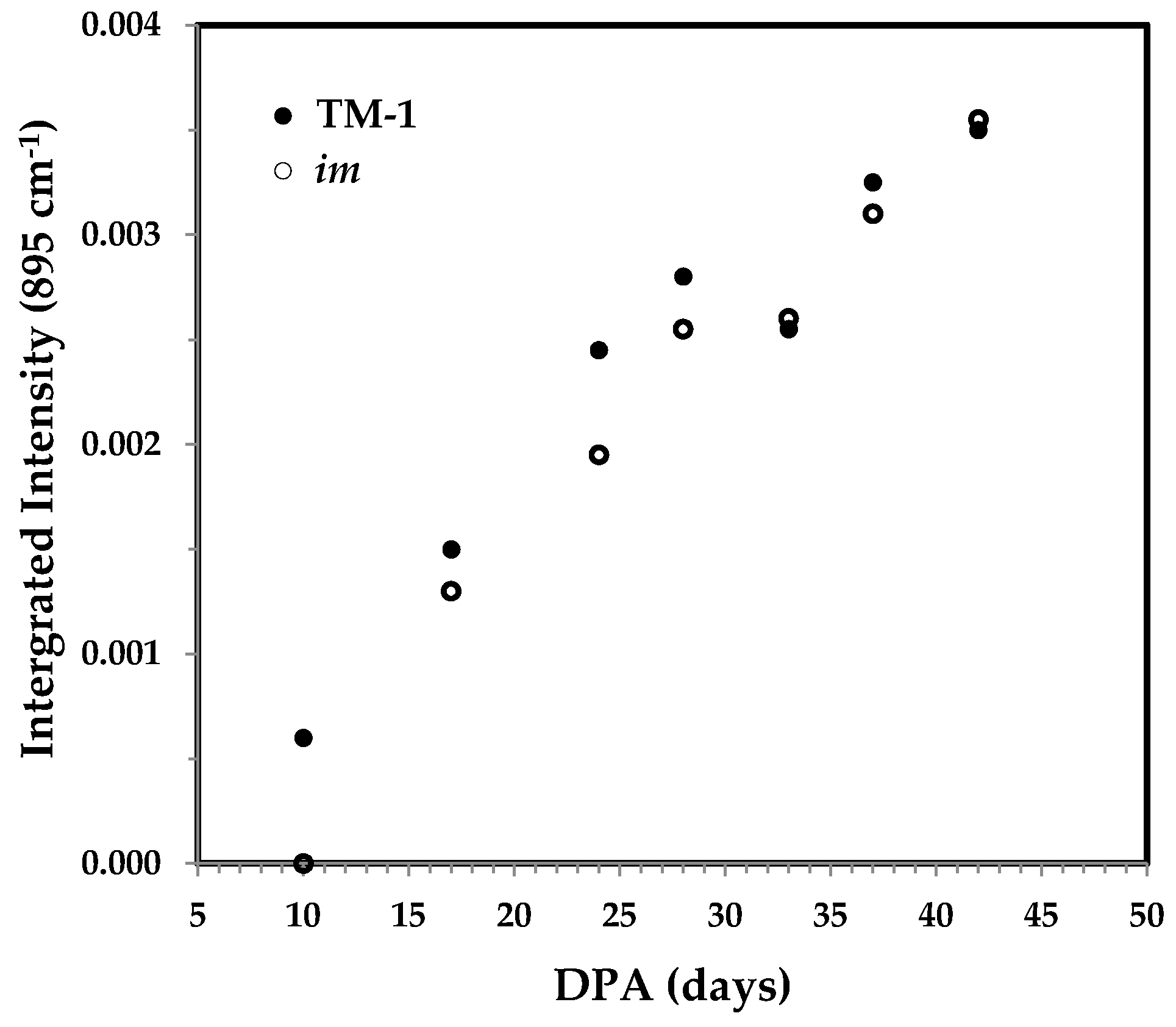
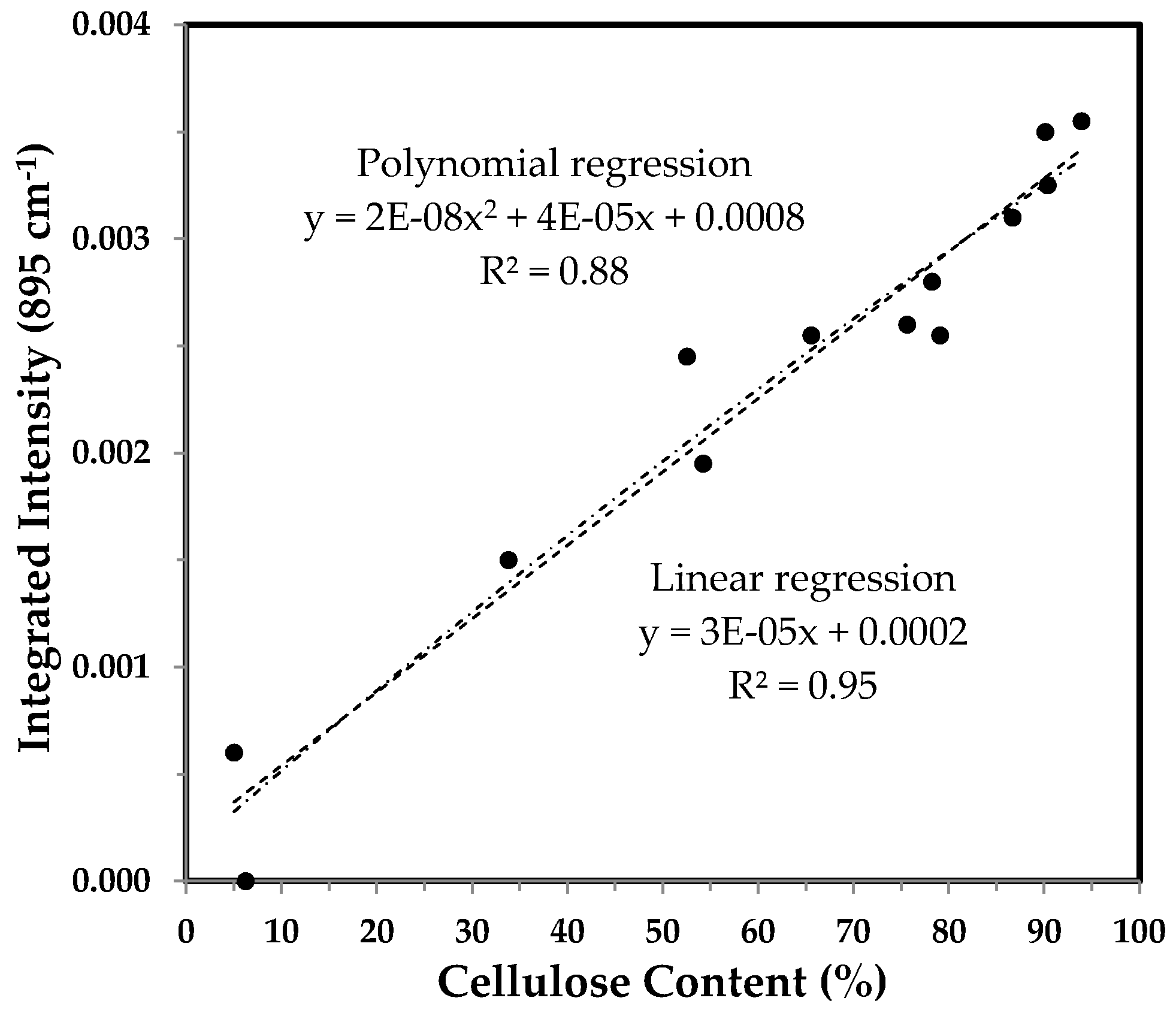
3.3.4. Integrated Intensity of the 895 cm−1 Band and the Correlation with Cellulose Content
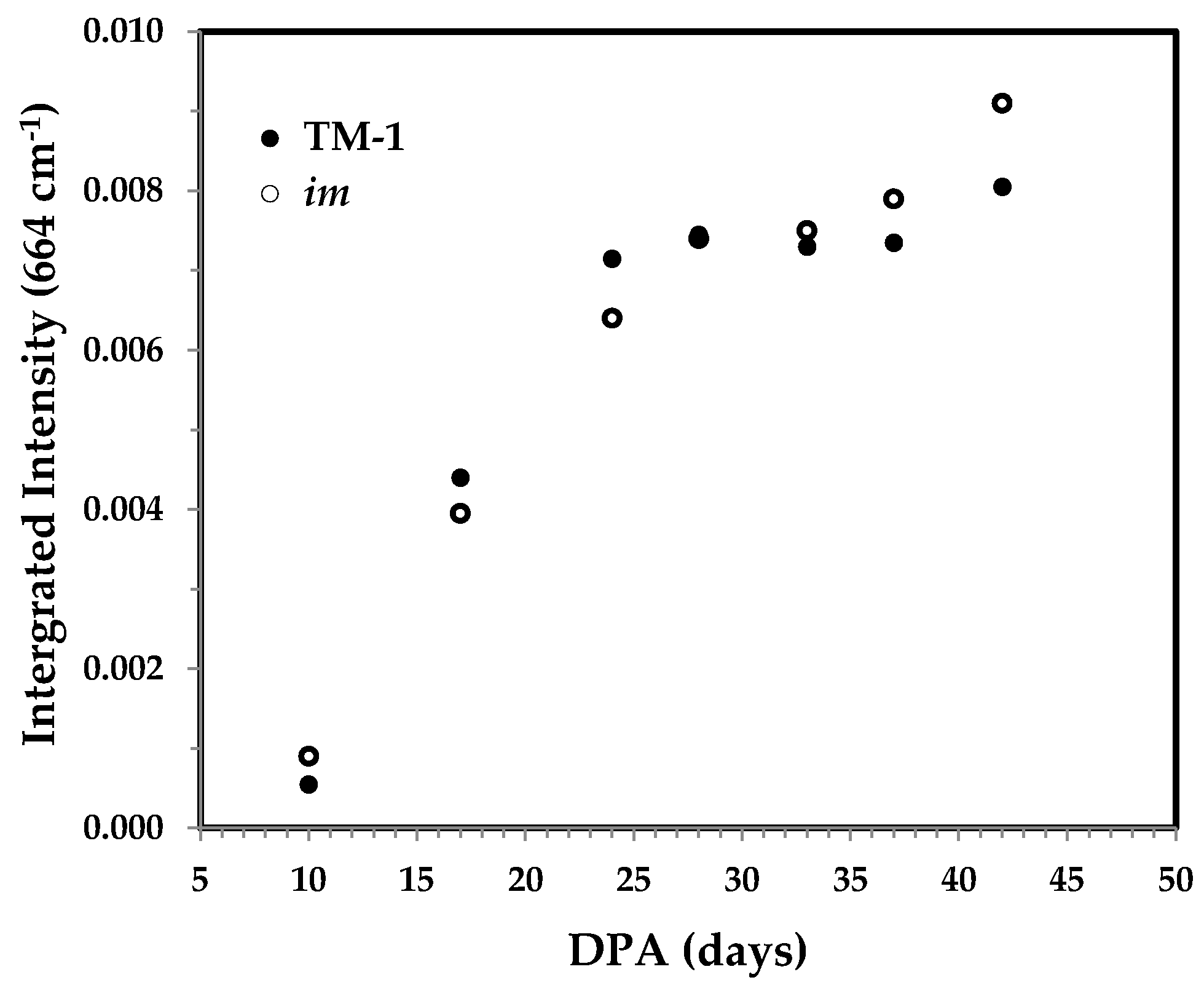
3.3.5. Integrated Intensity of the 664 cm−1 Band and the Correlation with Cellulose Content
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Robertson, W.C.; Roberts, B.A. Integrated Crop Management for Cotton Production in the 21st Century. In Cotton: Technology for the 21st Century; Wakelyn, P.J., Chaudhry, M.R., Eds.; International Cotton Advisory Committee: Washington, DC, USA, 2010; pp. 63–97. [Google Scholar]
- Wakelyn, P.J.; Bertoniere, N.R.; French, A.D.; Thibodeaux, D.P.; Triplett, B.A.; Rousselle, A.A.; Goynes, W.R., Jr.; Edwards, J.V.; Hunter, L.; Mcalister, D.D.; et al. (Eds.) Biosynthesis of Cotton. In Cotton Fiber Chemistry and Technology; CRC Press: Boca Raton, FL, USA, 2007; Chapter 2; pp. 11–14. [Google Scholar]
- Kim, H.J. Fiber Biology. In Cotton, 2nd ed.; Fang, D.D., Percy, R.G., Eds.; American Society of Agronomy, Inc.; Crop Science Society of America, Inc.; Soil Science Society of America, Inc.: Madison, WI, USA, 2015; pp. 97–127. [Google Scholar]
- Frydrych, I.; Thibodeaux, D.P. Fiber quality evaluation-current and future trends/intrinsic value of fiber quality in cotton. In Cotton: Technology for the 21st Century; Wakelyn, P.J., Chaudhry, M.R., Eds.; International Cotton Advisory Committee: Washington, DC, USA, 2010; pp. 251–295. [Google Scholar]
- Hsieh, Y.L. Chemical Structure and Properties of Cotton. In Cotton: Science and Technology; Gordon, S., Hsieh, Y.L., Eds.; Woodhead Publishing Limited: Cambridge, UK, 2007; pp. 3–34. [Google Scholar]
- Meinert, M.C.; Delmer, D.P. Changes in biochemical composition of the cell wall of the cotton fiber during development. Plant Physiol. 1977, 59, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Huwyler, H.R.; Franz, G.; Meier, H. Changes in the composition of cotton fiber cell walls during development. Planta 1979, 146, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Abidi, N.; Hequet, E.; Cabrales, L. Changes in sugar composition and cellulose content during the secondary cell wall biogenesis in cotton fibers. Cellulose 2010, 17, 153–160. [Google Scholar] [CrossRef]
- Wang, C.; Lv, Y.; Xu, W.; Zhang, T.; Guo, W. Aberrant phenotype and transcriptome expression during fiber cell wall thickening caused by the mutation of the Im gene in immature fiber (im) mutant in Gossypium hirsutum L. BMC Genom. 2014, 15, 94. [Google Scholar] [CrossRef] [PubMed]
- Abidi, N.; Hequet, E.; Cabrales, L.; Gannaway, J.; Wilkins, T.; Wells, L.W. Evaluating cell wall structure and composition of developing cotton fibers using Fourier transform infrared spectroscopy and thermogravimetric analysis. J. Appl. Phys. 2008, 107, 476–486. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Hequet, E. Fourier transform infrared spectroscopic approach to the study of the secondary cell wall development in cotton fiber. Cellulose 2010, 17, 309–320. [Google Scholar] [CrossRef]
- Hartzell-Lawson, M.M.; Hsieh, Y.-L. Characterizing the noncellulosics in developing cotton fibers. Text. Res. J. 2010, 70, 810–819. [Google Scholar] [CrossRef]
- Abidi, N.; Cabrales, L.; Haigler, C.H. Changes in the cell wall and cellulose content of developing cotton fibers investigated by FTIR spectroscopy. Carbohydr. Polym. 2014, 100, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Abidi, N.; Manike, M. X-ray diffraction and FTIR investigations of cellulose deposition during cotton fiber development. Text. Res. J. 2017. [Google Scholar] [CrossRef]
- Liu, Y.; Thibodeaux, D.; Gamble, G. Development of Fourier transform infrared spectroscopy in direct, non-destructive, and rapid determination of cotton fiber maturity. Text. Res. J. 2011, 81, 1559–1567. [Google Scholar]
- Liu, Y.; Thibodeaux, D.; Gamble, G.; Bauer, P.; VanDerveer, D. Comparative investigation of Fourier transform infrared (FT-IR) spectroscopy and X-ray diffraction (XRD) in the determination of cotton fiber crystallinity. Appl. Spectrosc. 2012, 66, 983–986. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, H.J. Use of attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy in direct, non-destructive, and rapid assessment of developmental cotton fibers grown in planta and in culture. Appl. Spectrosc. 2015, 69, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Kim, H.J. Characterization of developmental immature fiber (im) mutant and Texas Marker-1 (TM-1) cotton fibers by attenuated total reflection Fourier transform infrared (ATR FT-IR) spectroscopy. Appl. Spectrosc. 2017. [Google Scholar] [CrossRef] [PubMed]
- Santiago, C.M.; Hinchliffe, D.J. FT-IR examination of the development of secondary cell wall in cotton fibers. Fibers 2015, 3, 30–40. [Google Scholar]
- Lee, C.M.; Kafle, K.; Belias, D.W.; Park, Y.B.; Glick, R.E.; Haigler, C.H.; Kim, S.H. Comprehensive analysis of cellulose content, crystallinity, and lateral packing in Gossypium hirsutum and Gossypium barbadense cotton fibers using sum frequency generation, infrared and Raman spectroscopy, and X-ray diffraction. Cellulose 2015, 22, 971–989. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in cellulose I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Updegraff, D.M. Semimicro determination of cellulose in biological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]


| 10 DPA Fiber | 37 DPA Fiber | Band Assignment |
|---|---|---|
| 1740 (s) | 1740 (w) | C=O stretching |
| 1620 (s) | HOH bending of adsorbed water + amide I | |
| 1620 (w) | HOH bending of adsorbed water | |
| 1545 (s) | 1545 (w) | Amide II |
| 1425 (w) | 1425 (s) | CH2 scissoring |
| 1405 (s) | 1405 (w) | O–H deformation |
| 1365 (w) | 1365 (s) | C–H bending |
| 1335 (w) | 1335 (s) | CH2 wagging |
| 1315 (w) | 1315 (s) | CH2 wagging |
| 1236 (s) | 1236 (w) | O–H deformation or N–H deformation |
| 1200 (w) | 1200 (s) | C–O stretching |
| 1158 (w) | 1158 (s) | C–O–C stretching |
| 1104 (w) | 1104 (s) | C–O stretching |
| 1055 (w) | 1055 (s) | C–O stretching |
| 1028 (w) | 1028 (s) | C–O stretching |
| 985 (w) | 985 (s) | C–O stretching |
| 895 (w) | 895 (s) | β-glycosidic linkage |
| 662 (w) | 662 (s) | O–H out-of-plane bending |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Kim, H.-J. Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers. Sensors 2017, 17, 1469. https://doi.org/10.3390/s17071469
Liu Y, Kim H-J. Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers. Sensors. 2017; 17(7):1469. https://doi.org/10.3390/s17071469
Chicago/Turabian StyleLiu, Yongliang, and Hee-Jin Kim. 2017. "Fourier Transform Infrared Spectroscopy (FT-IR) and Simple Algorithm Analysis for Rapid and Non-Destructive Assessment of Developmental Cotton Fibers" Sensors 17, no. 7: 1469. https://doi.org/10.3390/s17071469




