Hybrid Optical Unobtrusive Blood Pressure Measurements
Abstract
:1. Introduction
2. Methods and Materials
2.1. Methods
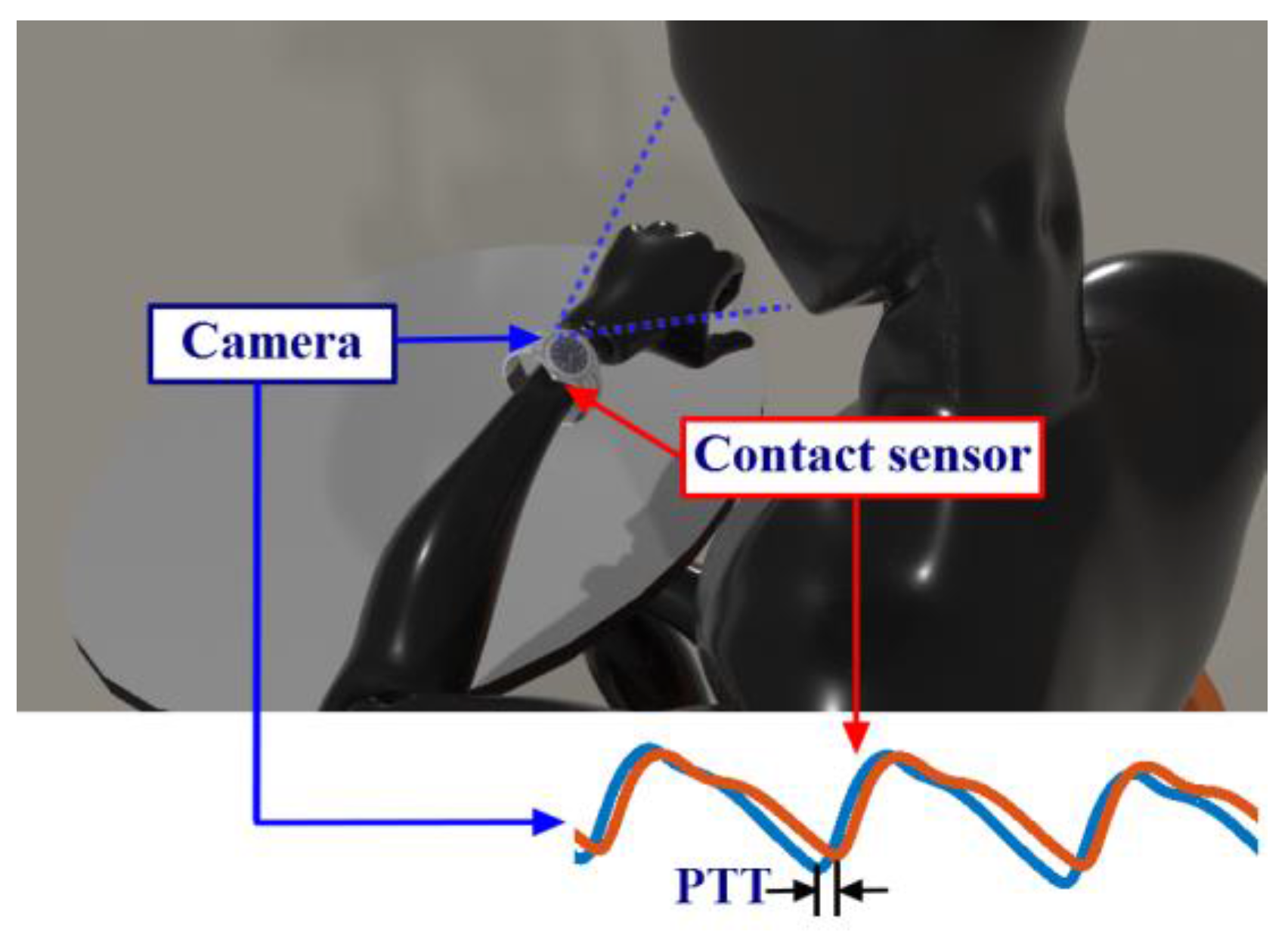
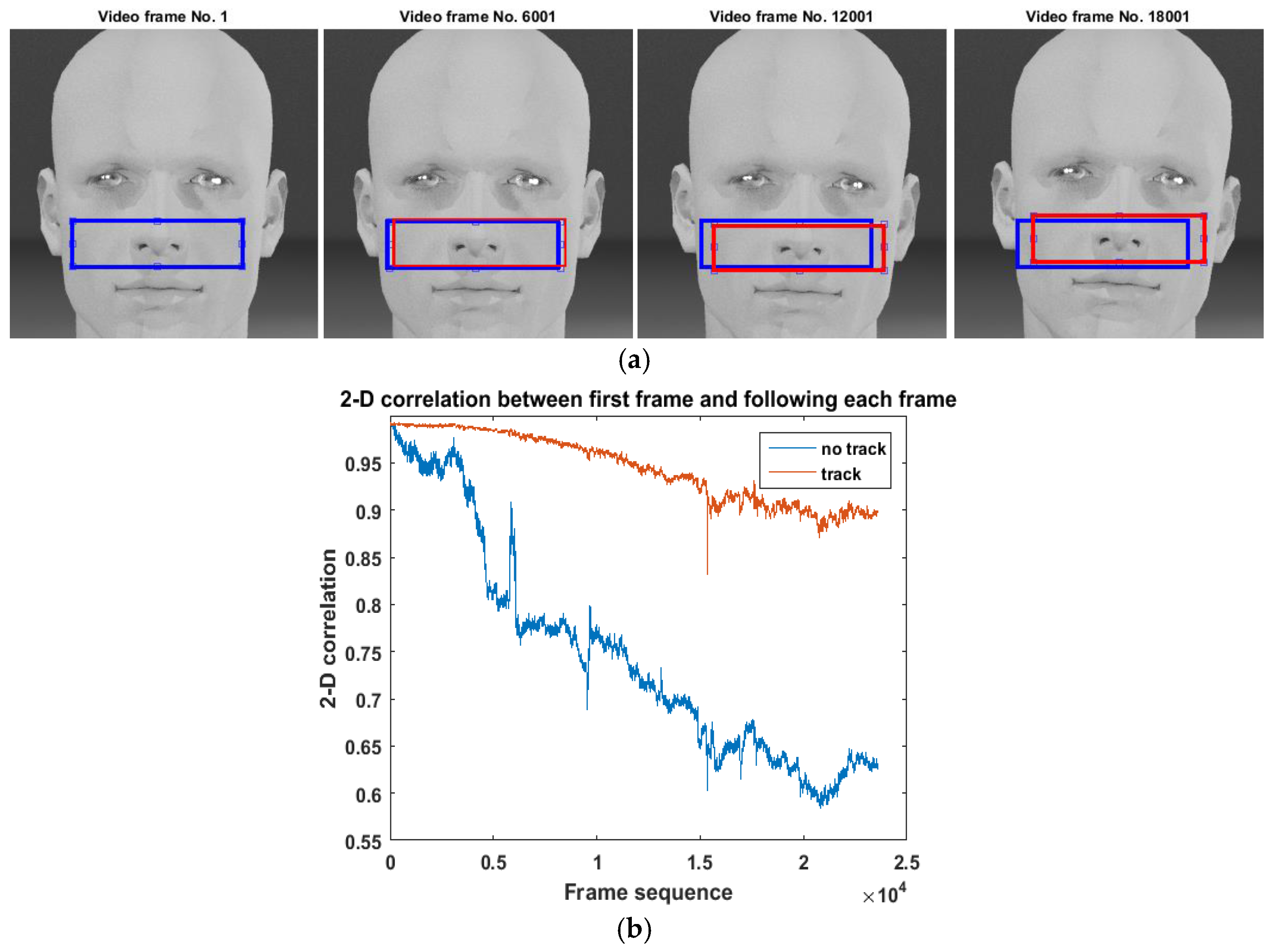
2.1.1. iPPG Signal Extraction
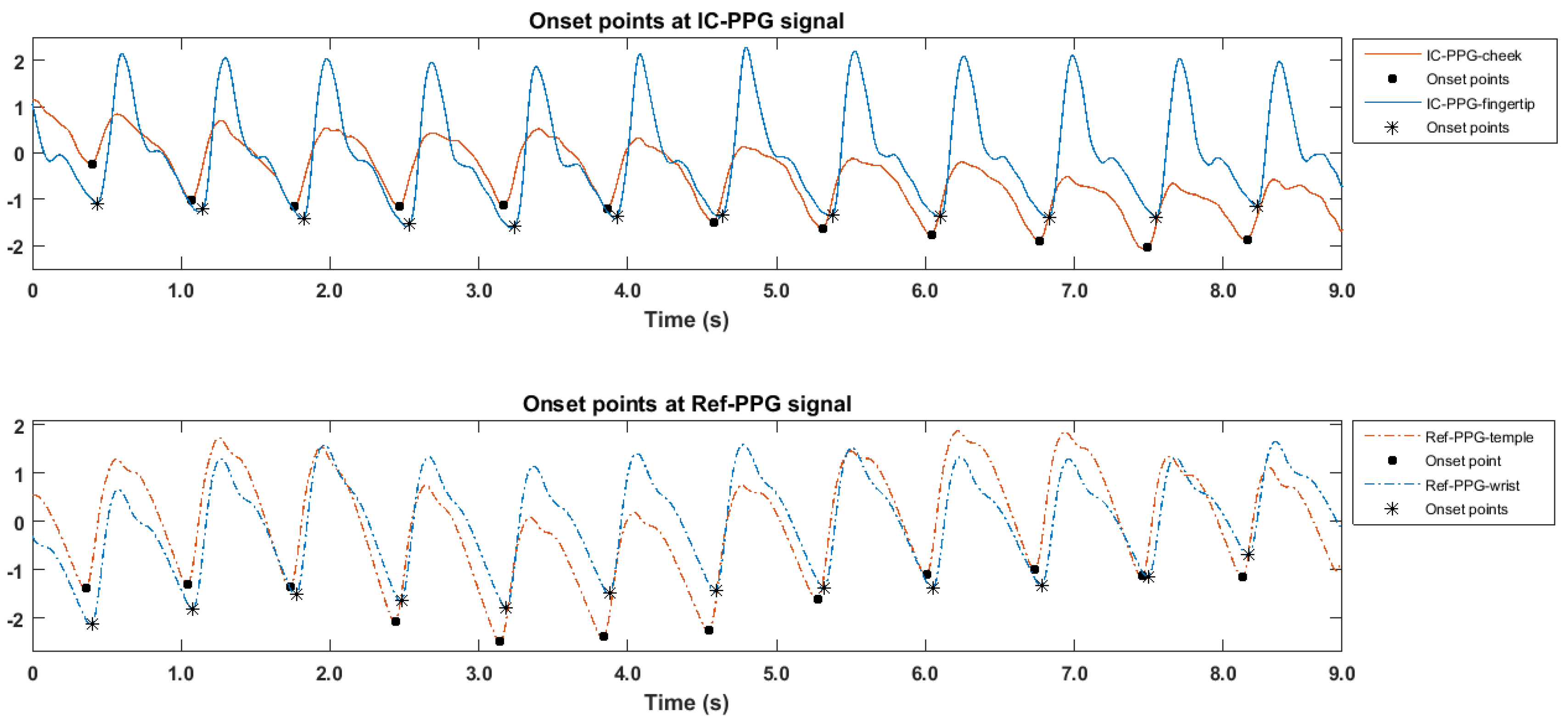
2.1.2. PTT Calculation
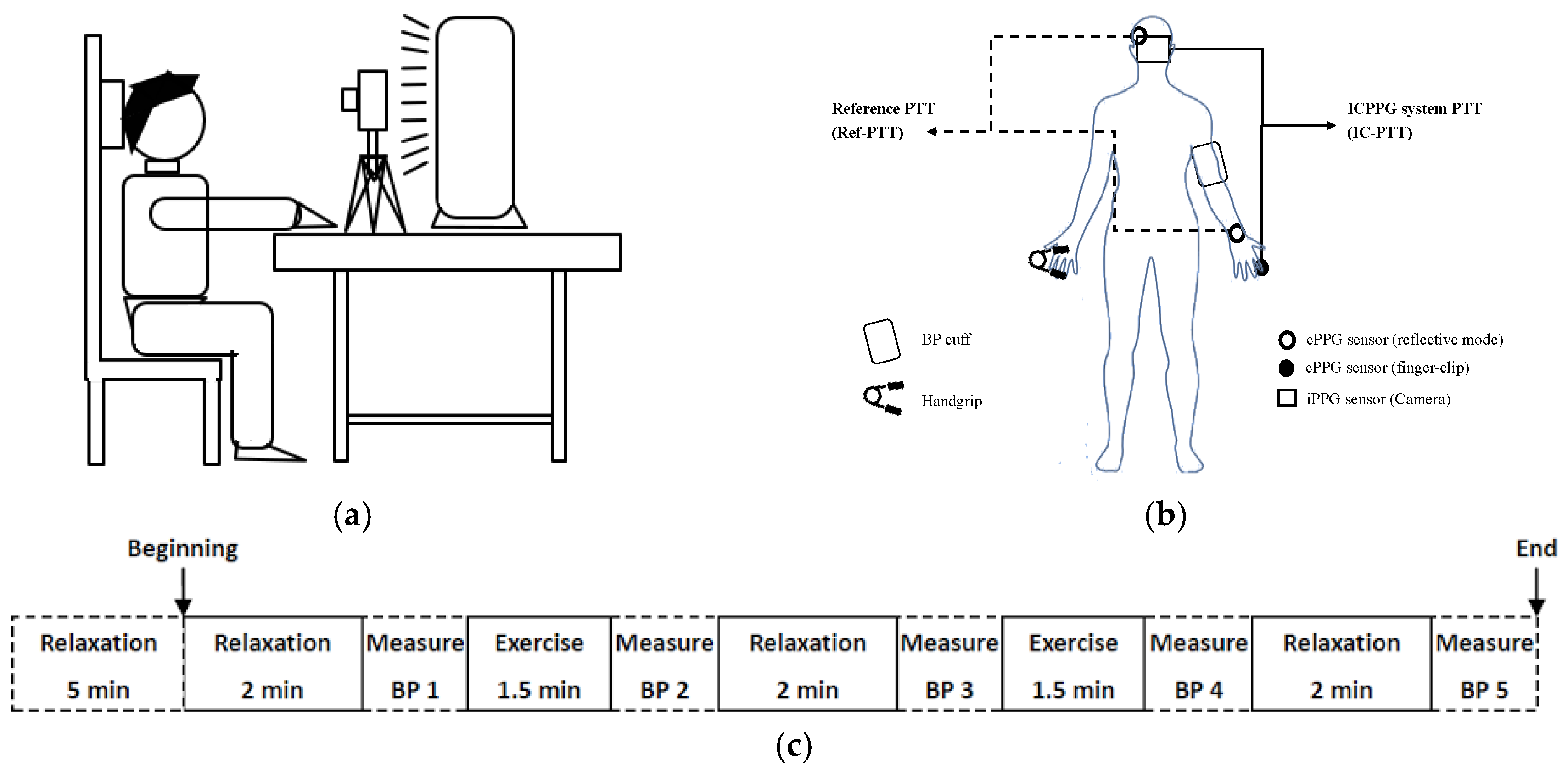
2.2. Experiment and Data
3. Results
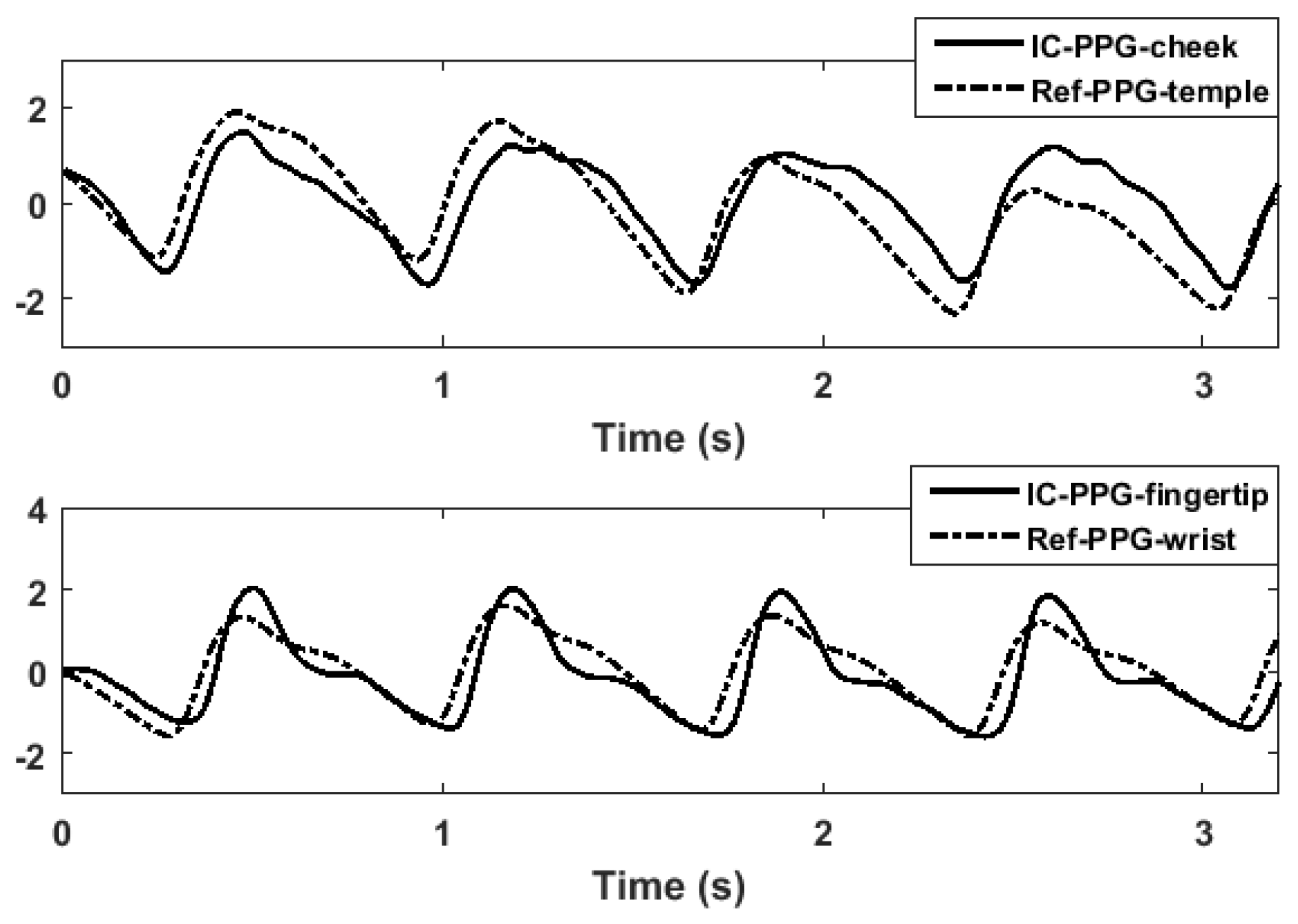
3.1. Morphologic Differences between PPG Signals
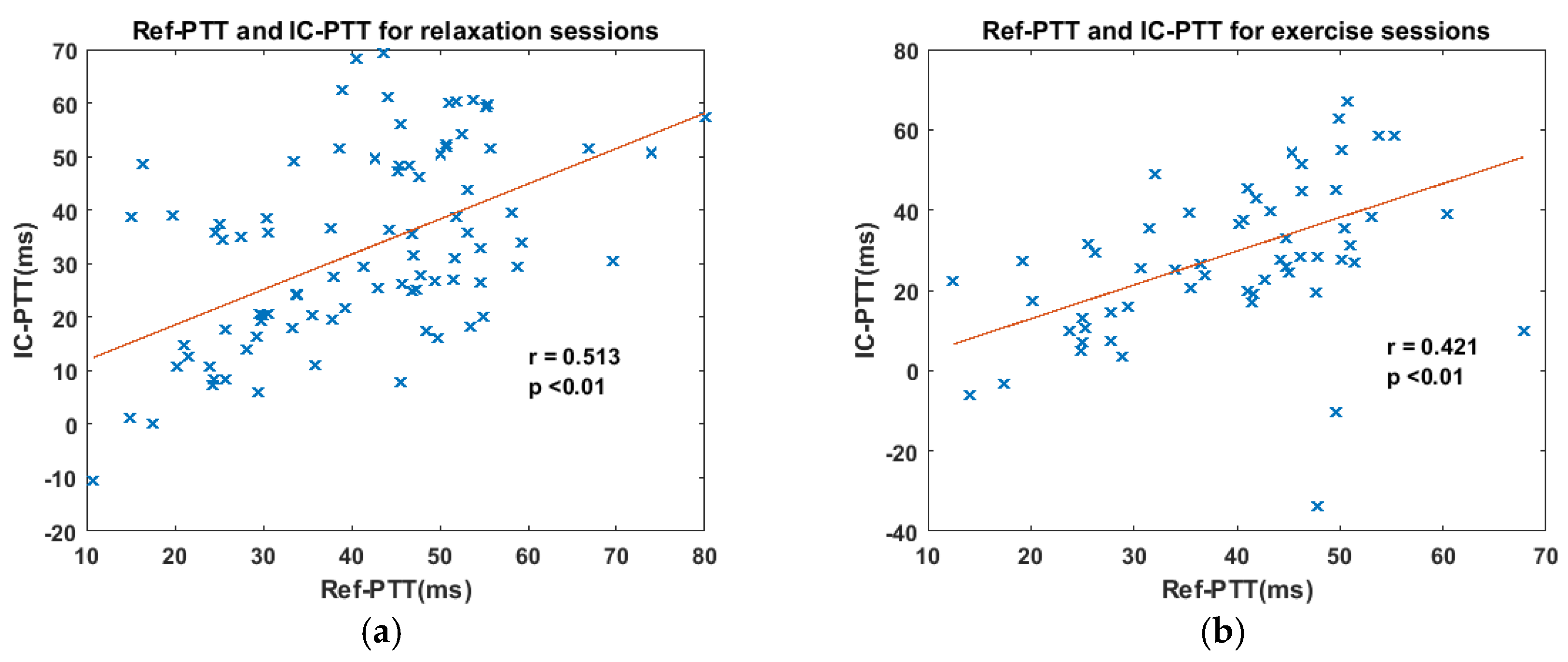
3.2. Consistency between IC-PTT and Its Reference
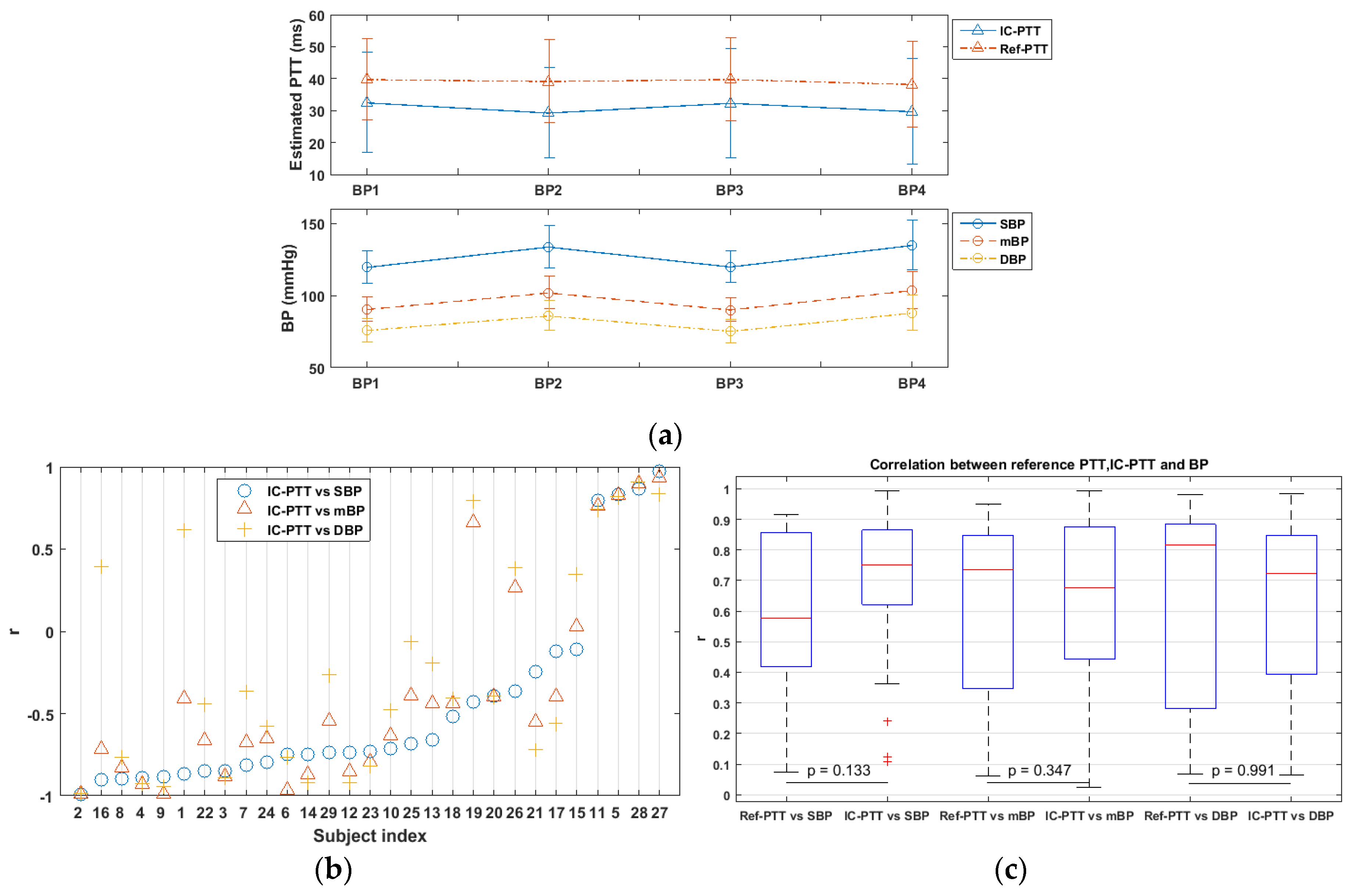
3.3. Variability of IC-PTT and Its Reference
3.4. IC-PTT vs. BP
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| Subject Index | Phase I | Phase II | Phase III | Phase IV | |
|---|---|---|---|---|---|
| 1 | Ref-PTT (ms) | 49.41 | 50.98 | 51.27 | 45.83 |
| IC-PTT (ms) | 28.28 | 29.81 | 28.84 | 31.76 | |
| SBP (mmHg) | 124.00 | 125.00 | 122.00 | 109.00 | |
| DBP (mmHg) | 81.00 | 86.00 | 81.00 | 84.00 | |
| 2 | Ref-PTT (ms) | 49.19 | 42.38 | 44.93 | 42.25 |
| IC-PTT (ms) | 55.54 | 39.33 | 50.39 | 42.26 | |
| SBP (mmHg) | 119.00 | 141.00 | 124.00 | 135.00 | |
| DBP (mmHg) | 79.00 | 101.00 | 82.00 | 98.00 | |
| 3 | Ref-PTT (ms) | 51.09 | 48.80 | 50.54 | 47.52 |
| IC-PTT (ms) | 52.80 | 46.87 | 59.04 | 49.55 | |
| SBP (mmHg) | 112.00 | 147.00 | 114.00 | 141.00 | |
| DBP (mmHg) | 69.00 | 87.00 | 64.00 | 90.00 | |
| 4 | Ref-PTT (ms) | 30.51 | 32.65 | 30.97 | 26.22 |
| IC-PTT (ms) | 23.67 | 25.51 | 24.19 | 11.00 | |
| SBP (mmHg) | 117.00 | 119.00 | 119.00 | 123.00 | |
| DBP (mmHg) | 78.00 | 81.00 | 76.00 | 92.00 | |
| 5 | Ref-PTT (ms) | 39.69 | 39.97 | 35.78 | 40.79 |
| IC-PTT (ms) | 20.27 | 22.58 | 20.41 | 22.18 | |
| SBP (mmHg) | 124.00 | 141.00 | 124.00 | 128.00 | |
| DBP (mmHg) | 75.00 | 90.00 | 77.00 | 79.00 | |
| 6 | Ref-PTT (ms) | 45.49 | 47.26 | 47.78 | 48.78 |
| IC-PTT (ms) | 40.35 | 28.38 | 31.66 | 32.38 | |
| SBP (mmHg) | 111.00 | 123.00 | 116.00 | 126.00 | |
| DBP (mmHg) | 68.00 | 75.00 | 72.00 | 68.00 | |
| 7 | Ref-PTT (ms) | 49.00 | 48.26 | 47.11 | 47.14 |
| IC-PTT (ms) | 10.43 | -0.25 | 8.33 | -10.79 | |
| SBP (mmHg) | 141.00 | 161.00 | 141.00 | 157.00 | |
| DBP (mmHg) | 91.00 | 95.00 | 87.00 | 91.00 | |
| 8 | Ref-PTT (ms) | 31.18 | 36.75 | 38.32 | 39.33 |
| IC-PTT (ms) | 24.27 | 17.70 | 22.77 | 23.34 | |
| SBP (mmHg) | 121.00 | 148.00 | 119.00 | 131.00 | |
| DBP (mmHg) | 78.00 | 92.00 | 75.00 | 86.00 | |
| 9 | Ref-PTT (ms) | 37.44 | 35.57 | 35.26 | 31.32 |
| IC-PTT (ms) | 24.81 | 21.61 | 24.42 | 21.89 | |
| SBP (mmHg) | 128.00 | 150.00 | 135.00 | 167.00 | |
| DBP (mmHg) | 89.00 | 108.00 | 86.00 | 100.00 | |
| 10 | Ref-PTT (ms) | 48.35 | 54.58 | 56.76 | 52.51 |
| IC-PTT (ms) | 66.13 | 56.59 | 65.55 | 61.24 | |
| SBP (mmHg) | 115.00 | 138.00 | 115.00 | 153.00 | |
| DBP (mmHg) | 81.00 | 83.00 | 77.00 | 90.00 | |
| 11 | Ref-PTT (ms) | 28.88 | 24.96 | 28.71 | 27.24 |
| IC-PTT (ms) | 9.03 | 12.41 | 3.97 | 10.62 | |
| SBP (mmHg) | 107.00 | 132.00 | 108.00 | 129.00 | |
| DBP (mmHg) | 71.00 | 92.00 | 73.00 | 94.00 | |
| 12 | Ref-PTT (ms) | 16.79 | 18.48 | 17.40 | 12.77 |
| IC-PTT (ms) | 41.71 | 34.92 | 49.41 | 32.93 | |
| SBP (mmHg) | 136.00 | 152.00 | 140.00 | 148.00 | |
| DBP (mmHg) | 92.00 | 101.00 | 91.00 | 100.00 | |
| 13 | Ref-PTT (ms) | 47.53 | 46.30 | 39.88 | 46.41 |
| IC-PTT (ms) | 53.66 | 45.93 | 53.54 | 54.61 | |
| SBP (mmHg) | 132.00 | 143.00 | 132.00 | 140.00 | |
| DBP (mmHg) | 84.00 | 84.00 | 77.00 | 85.00 | |
| 14 | Ref-PTT (ms) | 53.55 | 42.06 | 52.00 | 42.86 |
| IC-PTT (ms) | 49.18 | 39.92 | 47.99 | 41.33 | |
| SBP (mmHg) | 145.00 | 152.00 | 137.00 | 166.00 | |
| DBP (mmHg) | 69.00 | 85.00 | 67.00 | 92.00 | |
| 15 | Ref-PTT (ms) | 34.65 | 37.58 | 40.55 | 42.32 |
| IC-PTT (ms) | 45.24 | 42.99 | 55.40 | 50.73 | |
| SBP (mmHg) | 123.00 | 138.00 | 122.00 | 153.00 | |
| DBP (mmHg) | 84.00 | 86.00 | 86.00 | 91.00 | |
| 16 | Ref-PTT (ms) | 56.01 | 56.06 | 54.66 | 51.76 |
| IC-PTT (ms) | 59.52 | 56.24 | 62.40 | 59.33 | |
| SBP (mmHg) | 117.00 | 122.00 | 112.00 | 121.00 | |
| DBP (mmHg) | 67.00 | 68.00 | 69.00 | 69.00 | |
| 17 | Ref-PTT (ms) | 30.00 | 30.59 | 33.10 | 25.07 |
| IC-PTT (ms) | 16.62 | 17.53 | 18.54 | 16.52 | |
| SBP (mmHg) | 113.00 | 117.00 | 122.00 | 132.00 | |
| DBP (mmHg) | 72.00 | 77.00 | 71.00 | 83.00 | |
| 18 | Ref-PTT (ms) | 30.30 | 30.46 | 44.13 | 38.10 |
| IC-PTT (ms) | 30.55 | 20.38 | 29.53 | 28.06 | |
| SBP (mmHg) | 124.00 | 152.00 | 124.00 | 166.00 | |
| DBP (mmHg) | 76.00 | 99.00 | 75.00 | 119.00 | |
| 19 | Ref-PTT (ms) | 53.45 | 53.32 | 52.88 | 51.84 |
| IC-PTT (ms) | 43.53 | 40.99 | 34.82 | 49.12 | |
| SBP (mmHg) | 112.00 | 115.00 | 118.00 | 116.00 | |
| DBP (mmHg) | 71.00 | 75.00 | 68.00 | 76.00 | |
| 20 | Ref-PTT (ms) | 27.33 | 25.20 | 27.80 | 28.20 |
| IC-PTT (ms) | 33.99 | 31.20 | 33.60 | 28.33 | |
| SBP (mmHg) | 128.00 | 136.00 | 124.00 | 129.00 | |
| DBP (mmHg) | 72.00 | 81.00 | 71.00 | 74.00 | |
| 21 | Ref-PTT (ms) | 19.22 | 14.78 | 15.90 | 13.85 |
| IC-PTT (ms) | 16.82 | 0.33 | 3.07 | 1.00 | |
| SBP (mmHg) | 112.00 | 116.00 | 103.00 | 123.00 | |
| DBP (mmHg) | 72.00 | 79.00 | 75.00 | 86.00 | |
| 22 | Ref-PTT (ms) | 42.03 | 42.98 | 49.69 | 46.19 |
| IC-PTT (ms) | 22.39 | 14.65 | 36.30 | 22.52 | |
| SBP (mmHg) | 133.00 | 145.00 | 130.00 | 134.00 | |
| DBP (mmHg) | 91.00 | 99.00 | 94.00 | 93.00 | |
| 23 | Ref-PTT (ms) | 22.57 | 23.90 | 20.55 | 24.52 |
| IC-PTT (ms) | 12.40 | 8.78 | 8.44 | 6.14 | |
| SBP (mmHg) | 101.00 | 131.00 | 102.00 | 138.00 | |
| DBP (mmHg) | 66.00 | 93.00 | 71.00 | 109.00 | |
| 24 | Ref-PTT (ms) | 25.74 | 20.81 | 21.29 | −1.10 |
| IC-PTT (ms) | 13.55 | 14.14 | 13.63 | 12.59 | |
| SBP (mmHg) | 114.00 | 115.00 | 109.00 | 128.00 | |
| DBP (mmHg) | 68.00 | 77.00 | 66.00 | 86.00 | |
| 25 | Ref-PTT (ms) | 50.79 | 49.64 | 52.70 | 49.20 |
| IC-PTT (ms) | 36.63 | 31.62 | 22.39 | 22.69 | |
| SBP (mmHg) | 112.00 | 121.00 | 118.00 | 124.00 | |
| DBP (mmHg) | 69.00 | 73.00 | 68.00 | 73.00 | |
| 26 | Ref-PTT (ms) | 53.83 | 52.10 | 54.84 | 51.37 |
| IC-PTT (ms) | 30.49 | 27.02 | 26.15 | 31.87 | |
| SBP (mmHg) | 98.00 | 104.00 | 101.00 | 102.00 | |
| DBP (mmHg) | 62.00 | 70.00 | 61.00 | 72.00 | |
| 27 | Ref-PTT (ms) | 31.09 | 32.88 | 33.52 | 30.64 |
| IC-PTT (ms) | 30.37 | 37.09 | 26.94 | 34.98 | |
| SBP (mmHg) | 108.00 | 113.00 | 102.00 | 112.00 | |
| DBP (mmHg) | 67.00 | 76.00 | 69.00 | 78.00 | |
| 28 | Ref-PTT (ms) | 46.56 | 46.33 | 45.72 | 48.43 |
| IC-PTT (ms) | 28.03 | 29.05 | 26.81 | 29.53 | |
| SBP (mmHg) | 114.00 | 141.00 | 118.00 | 152.00 | |
| DBP (mmHg) | 76.00 | 93.00 | 75.00 | 105.00 | |
| 29 | Ref-PTT (ms) | 70.30 | 68.36 | 60.34 | 66.37 |
| IC-PTT (ms) | 37.80 | 23.09 | 28.64 | 34.28 | |
| SBP (mmHg) | 121.00 | 134.00 | 119.00 | 120.00 | |
| DBP (mmHg) | 78.00 | 83.00 | 75.00 | 82.00 | |
References
- McGhee, B.H.; Elizabeth, J.B. Monitoring arterial blood pressure: What you may not know. Crit. Care Nurse 2002, 22, 60–79. [Google Scholar] [PubMed]
- Perloff, D.; Grim, C.; Flack, J.; Frohlich, E.D.; Hill, M.; McDonald, M.; Morgenstern, B.Z. Human blood pressure determination by sphygmoman: Ometry. Circulation 1993, 88, 2460–2470. [Google Scholar] [CrossRef] [PubMed]
- Drzewiecki, G.; Hood, R.; Apple, H. Theory of the oscillometric maximum and the systolic and diastolic detection ratios. Ann. Biomed. Eng. 1994, 22, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Imholz, B.P.; Wieling, W.; van Montfrans, G.A.; Wesseling, K.H. Fifteen years experience with finger arterial pressure monitoring. Cardiovasc. Res. 1998, 38, 605–616. [Google Scholar] [CrossRef]
- Eckerle, J.S. Tonometry, arterial. In Encyclopedia of Medical Devices and Instrumentation; Wiley-Interscienc: Hoboken, NJ, USA, 2006. [Google Scholar]
- Lewis, O. Stephen Hales and the measurement of blood pressure. J. Hum. Hypertens. 1994, 8, 865–871. [Google Scholar] [PubMed]
- Quinney, D.A. Daniel Bernoulli and the making of the fluid equation. Keele University, Great Britain. Available online: http://pass.maths.org.uk/issue1/bern (accessed on 1 January 1997).
- Zheng, Y.L.; Ding, X.R.; Poon, C.C.Y.; Lo, B.P.L.; Zhang, H.; Zhou, X.L.; Yang, G.Z.; Zhao, N.; Zhang, Y.T. Unobtrusive sensing and wearable devices for health informatics. IEEE Trans. Biomed. Eng. 2014, 61, 1538–1554. [Google Scholar] [CrossRef] [PubMed]
- Gribbin, B.; Steptoe, A.; Sleight, P. Pulse wave velocity as a measure of blood pressure change. Psychophysiology 1976, 13, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, C.; Tao, G.; Bi, M.; Li, G. Continuous and noninvasive blood pressure measurement: A novel modeling methodology of the relationship between blood pressure and pulse wave velocity. Ann. Biomed. Eng. 2009, 37, 2222–2233. [Google Scholar] [CrossRef] [PubMed]
- Can, Y.; Kilic, H.; Akdemir, R.; Acar, B.; Edem, E.; Kocyigit, I.; Vatan, M.B.; Aksoy, M.; Can, N.; Gunduz, H. An investigation of pulse transit time as a blood pressure measurement method in patients undergoing carotid artery stenting. Blood Press. Monit. 2016, 21, 168–170. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.T. A blood pressure monitoring method for stroke management. BioMed Res. Int. 2014, 2014, 571623. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.M.; Vaughan, C.J.; O’Fljnn, B.; Mathewson, A.; Mathúna, C.Ó. An examination of calibration intervals required for accurately tracking blood pressure using pulse transit time algorithms. J. Hum. Hypertens. 2013, 27, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Bezemer, R.; Long, X.; Muehlsteff, J.; Aarts, R. Systolic blood pressure estimation using PPG and ECG during physical exercise. Physiol. Meas. 2016, 37, 2154–2169. [Google Scholar] [CrossRef] [PubMed]
- Remoissenet, M. Waves Called Solitons: Concepts and Experiments; Springer Science & Business Media: Berlin, Germany, 2013. [Google Scholar]
- Westerhof, N.; Stergiopulos, N.; Noble, M.I. Snapshots of Hemodynamics: An Aid for Clinical Research and Graduate Education; Springer Science & Business Media: Berlin, Germany, 2010. [Google Scholar]
- Sun, Y.; Nitish, T. Photoplethysmography revisited: From contact to noncontact, from point to imaging. IEEE Trans. Biomed. Eng. 2016, 63, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Verkruysse, W.; Svaasand, L.O.; Nelson, J.S. Remote plethysmographic imaging using ambient light. Opt. Express 2008, 16, 21434–21445. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Tarbox, E.; Cissel, M.; Moses, S.; Luthra, M.; Vaidya, M.; Tran, N.; Ikonomidou, V.N. Remotely detected differential pulse transit time as a stress indicator. In SPIE Sensing Technology+ Applications; International Society for Optics and Photonics: Bellingham, DC, USA, 2015. [Google Scholar]
- Takano, C.; Ohta, Y. Heart rate measurement based on a time-lapse image. Med. Eng. Phys. 2007, 29, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, S.; Azorin-Peris, V.; Kalawsky, R.; Greenwald, S. Noncontactimaging photoplethysmography to effectively access pulse rate variability. J. Biomed. Opt. 2013, 18, 061205. [Google Scholar] [CrossRef] [PubMed]
- Poh, M.Z.; McDuff, D.J.; Picard, R.W. Advancements in noncontact, multiparameter physiological measurements using a webcam. IEEE Trans. Biomed. Eng. 2011, 58, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Kyal, S.; Mestha, L.K.; Xu, B.; Couderc, J.P. A method to detect cardiac arrhythmias with a webcam. In Proceedings of the 2013 IEEE Signal Processing in Medicine and Biology Symposium (SPMB), Brooklyn, NY, USA, 7 December 2013. [Google Scholar]
- De Haan, G.; Jeanne, V. Robust pulse rate from chrominance-based rPPG. IEEE Trans. Biomed. Eng. 2013, 60, 2878–2886. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Stuijk, S.; De Haan, G. A novel algorithm for remote photoplethysmography: Spatial subspace rotation. IEEE Trans. Biomed. Eng. 2016, 63, 1974–1984. [Google Scholar] [CrossRef] [PubMed]
- Przybyło, J.; Kańtoch, E.; Jabłoński, M.; Augustyniak, P. Distant Measurement of Plethysmographic Signal in Various Lighting Conditions Using Configurable Frame-Rate Camera. Metrol. Meas. Syst. 2016, 23, 579–592. [Google Scholar] [CrossRef]
- Amelard, R.; Scharfenberger, C.; Kazemzadeh, F.; Pfisterer, K.J.; Lin, B.S.; Clausi, D.A.; Wong, A. Feasibility of long-distance heart rate monitoring using transmittance photoplethysmographic imaging (PPGI). Sci. Rep. 2015, 5, 14637. [Google Scholar] [CrossRef] [PubMed]
- Couderc, J.P.; Kyal, S.; Mestha, L.K.; Xu, B.; Peterson, D.R.; Xia, X.; Hall, B. Detection of atrial fibrillation using contactless facial video monitoring. Heart Rhythm 2015, 12, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Tsouri, G.R.; Li, Z. On the benefits of alternative color spaces for noncontact heart rate measurements using standard red-green-blue cameras. J. Biomed. Opt. 2015, 20, 048002. [Google Scholar] [CrossRef] [PubMed]
- Mestha, L.K.; Kyal, S.; Xu, B.; Lewis, L.E.; Kumar, V. Towards continuous monitoring of pulse rate in neonatal intensive care unit with a webcam. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Chicago, IL, USA, 26–30 August 2014; pp. 3817–3820. [Google Scholar]
- McCombie, D.B.; Reisner, A.T.; Asada, H.H. Motion based adaptive calibration of pulse transit time measurements to arterial blood pressure for an autonomous, wearable blood pressure monitor. In Proceedings of the 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society(EMBS), Vancouver, BC, Canada, 20–25 August 2008; pp. 989–992. [Google Scholar]
- Jeong, I.C.; Finkelstein, J. Introducing contactless blood pressure assessment using a high speed video camera. J. Med. Syst. 2016, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nakano, K.; Aoki, Y.; Satoh, R.; Hoshi, A.; Suzuki, H.; Nishidate, I. Non-contact measurement of pulse wave velocity using RGB cameras. In SPIE BiOS; International Society for Optics and Photonics: Bellingham, DC, USA, 2016; p. 971515. [Google Scholar]
- Tang, T. Pulse Transit Time Estimation on Remote Photoplethysmography; Technische Universiteit Eindhoven: Eindhoven, The Netherlands.
- Sun, Y.; Hu, S.; Azorin-Peris, V.; Greenwald, S.; Chambers, J.; Zhu, Y. Motion-compensated noncontact imaging photoplethysmography to monitor cardiorespiratory status during exercise. J. Biomed. Opt. 2011, 16, 077010. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Clifford, G.D. Dynamic time warping and machine learning for signal quality assessment of pulsatile signals. Physiol. Meas. 2012, 33, 1491–1501. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Heldt, T.; Moody, G.B.; Mark, R.G. An open-source algorithm to detect onset of arterial blood pressure pulses. In Proceedings of the Computers in Cardiology, Thessaloniki Chalkidiki, Greece, 21–24 September 2003; pp. 259–262. [Google Scholar]
- Park, J.H.; Jang, D.G.; Park, J.W.; Youm, S.K. Wearable sensing of in-ear pressure for heart rate monitoring with a piezoelectric sensor. Sensors 2015, 15, 23402–23417. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, H.P.; Carreiras, C.; Lourenço, A.; Fred, A.; das Neves, R.C.; Ferreira, R. Off-the-person electrocardiography: Performance assessment and clinical correlation. Health Technol. 2015, 4, 309–318. [Google Scholar] [CrossRef]
- Zhou, X.; Peng, R.; Ding, H.; Zhang, N.; Li, P. Validation of new and existing decision rules for the estimation of beat-to-beat pulse transit time. BioMed Res. Int. 2015, 2015, 306934. [Google Scholar] [CrossRef] [PubMed]
- Elgendi, M.; Norton, I.; Brearley, M.; Abbott, D.; Schuurmans, D. Systolic peak detection in acceleration photoplethysmograms measured from emergency responders in tropical conditions. PLoS ONE 2013, 8, e76585. [Google Scholar] [CrossRef] [PubMed]
- Heathers, J.A. Smartphone-enabled pulse rate variability: An alternative methodology for the collection of heart rate variability in psychophysiological research. Int. J. Psychophysiol. 2013, 89, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Asmar, R.; Benetos, A.; Topouchian, J.; Laurent, P.; Pannier, B.; Brisac, A.M.; Levy, B.I. Assessment of arterial distensibility by automatic pulse wave velocity measurement. Hypertension 1995, 26, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Li, H.; Tao, S.; Zhang, D.; Jiang, Z.; Cui, L.; Gou, D. Research on the main elements influencing blood pressure measurement by pulse wave velocity. Front. Med. Biol. Eng. 1991, 4, 189–199. [Google Scholar]
- Hermeling, E.; Hoeks, A.P.; Winkens, M.H.; Waltenberger, J.L.; Reneman, R.S.; Kroon, A.A.; Reesink, K.D. Noninvasive assessment of arterial stiffness should discriminate between systolic and diastolic pressure ranges. Hypertension 2010, 55, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Hermeling, E.; Vermeersch, S.J.; Rietzschel, E.R.; de Buyzere, M.L.; Gillebert, T.C.; van de Laar, R.J.; Segers, P. The change in arterial stiffness over the cardiac cycle rather than diastolic stiffness is independently associated with left ventricular mass index in healthy middle-aged individuals. J. Hypertens. 2012, 30, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Murray, A. Variability of photoplethysmography peripheral pulse measurements at the ears, thumbs and toes. IEE Proc. Sci. Meas. Technol. 2000, 147, 403–407. [Google Scholar] [CrossRef]
- Thomas, M.N. Essentials of Human Physiology; Micron Bio Systems: Bridgwater, UK, 1996. [Google Scholar]
- Mukkamala, R.; Hahn, J.O.; Inan, O.T.; Mestha, L.K.; Kim, C.S.; Töreyin, H.; Kyal, S. Toward ubiquitous blood pressure monitoring via pulse transit time: Theory and practice. IEEE Trans. Biomed. Eng. 2015, 62, 1879–1901. [Google Scholar] [CrossRef] [PubMed]
- Radha, M.; Zhang, G.; Gelissen, J.; de Groot, K.; Haakma, R.; Aarts, R.M. Arterial path selection to measure pulse wave velocity as a surrogate marker of blood pressure. Biomed. Phys. Eng. Express 2007, 3, 015022. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, G.; Shan, C.; Kirenko, I.; Long, X.; Aarts, R.M. Hybrid Optical Unobtrusive Blood Pressure Measurements. Sensors 2017, 17, 1541. https://doi.org/10.3390/s17071541
Zhang G, Shan C, Kirenko I, Long X, Aarts RM. Hybrid Optical Unobtrusive Blood Pressure Measurements. Sensors. 2017; 17(7):1541. https://doi.org/10.3390/s17071541
Chicago/Turabian StyleZhang, Guangfei, Caifeng Shan, Ihor Kirenko, Xi Long, and Ronald M. Aarts. 2017. "Hybrid Optical Unobtrusive Blood Pressure Measurements" Sensors 17, no. 7: 1541. https://doi.org/10.3390/s17071541





