A Noncontact Dibutyl Phthalate Sensor Based on a Wireless-Electrodeless QCM-D Modified with Nano-Structured Nickel Hydroxide
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
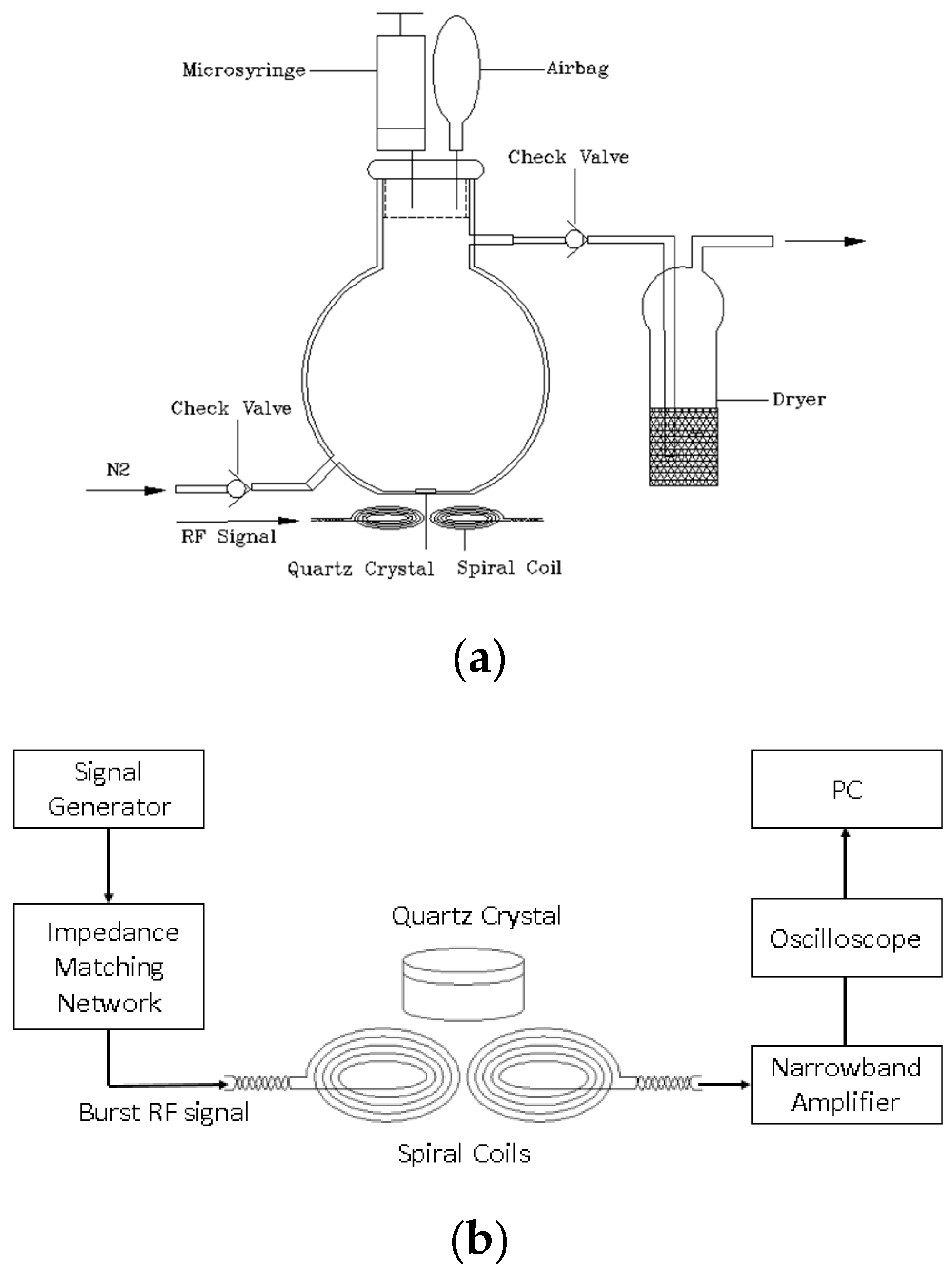
2.2. The Wireless-Electrodeless QCM-D System
2.3. Fabrication of a QCM Gas Sensor
2.4. Preparation of Measured Vapors
2.5. Gas Sensing Experiments
3. Results and Discussion
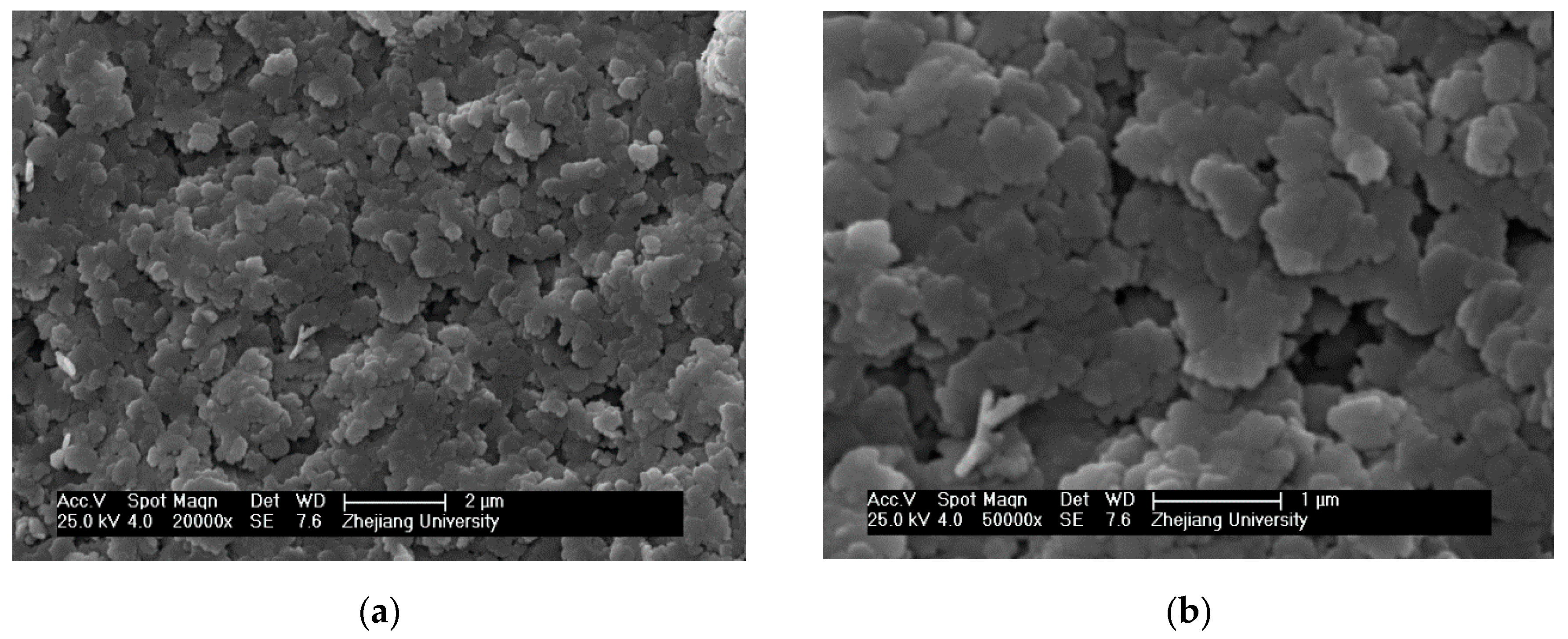
3.1. SEM Morphology
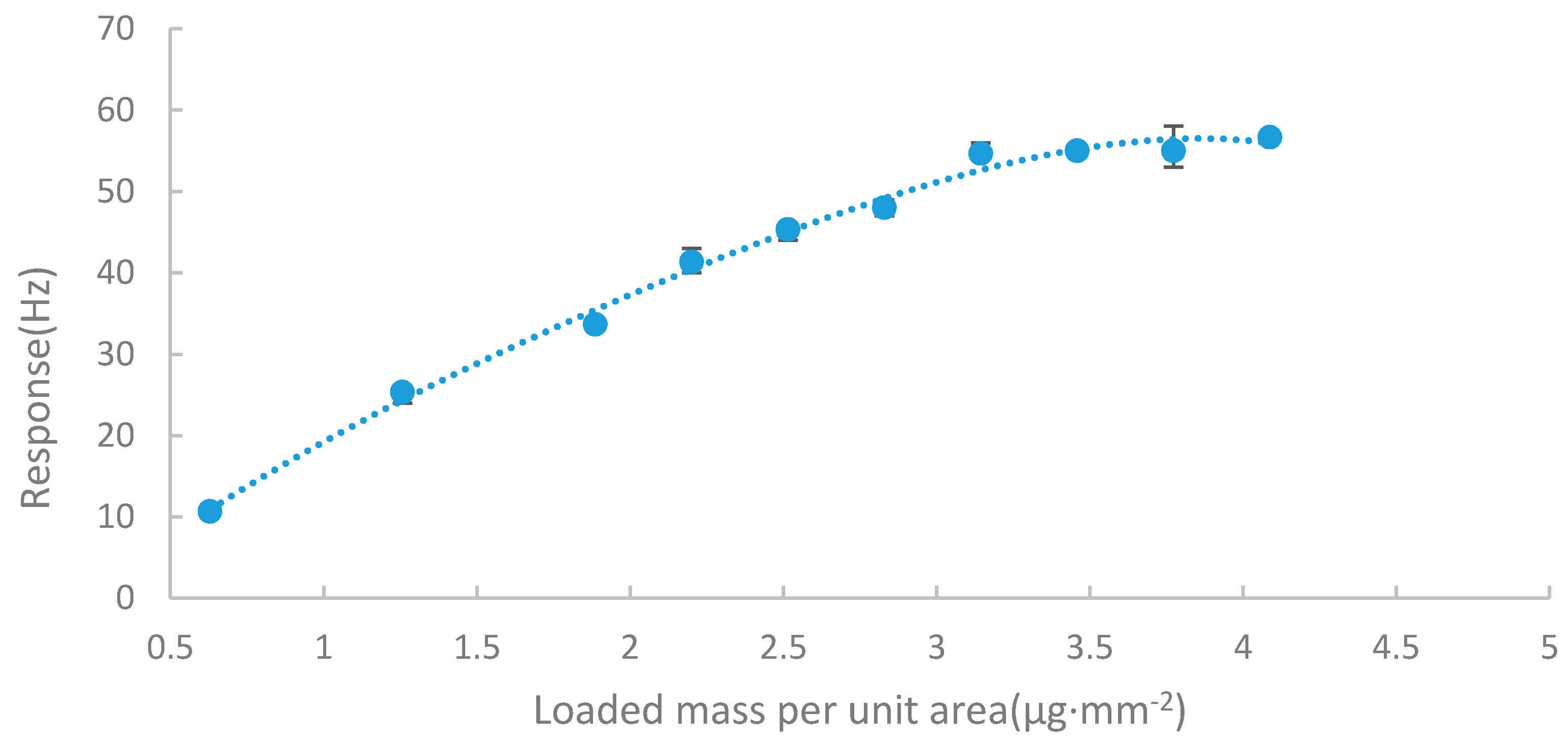
3.2. Optimization of the Thickness of the Sensing FILM
3.3. Selectivity
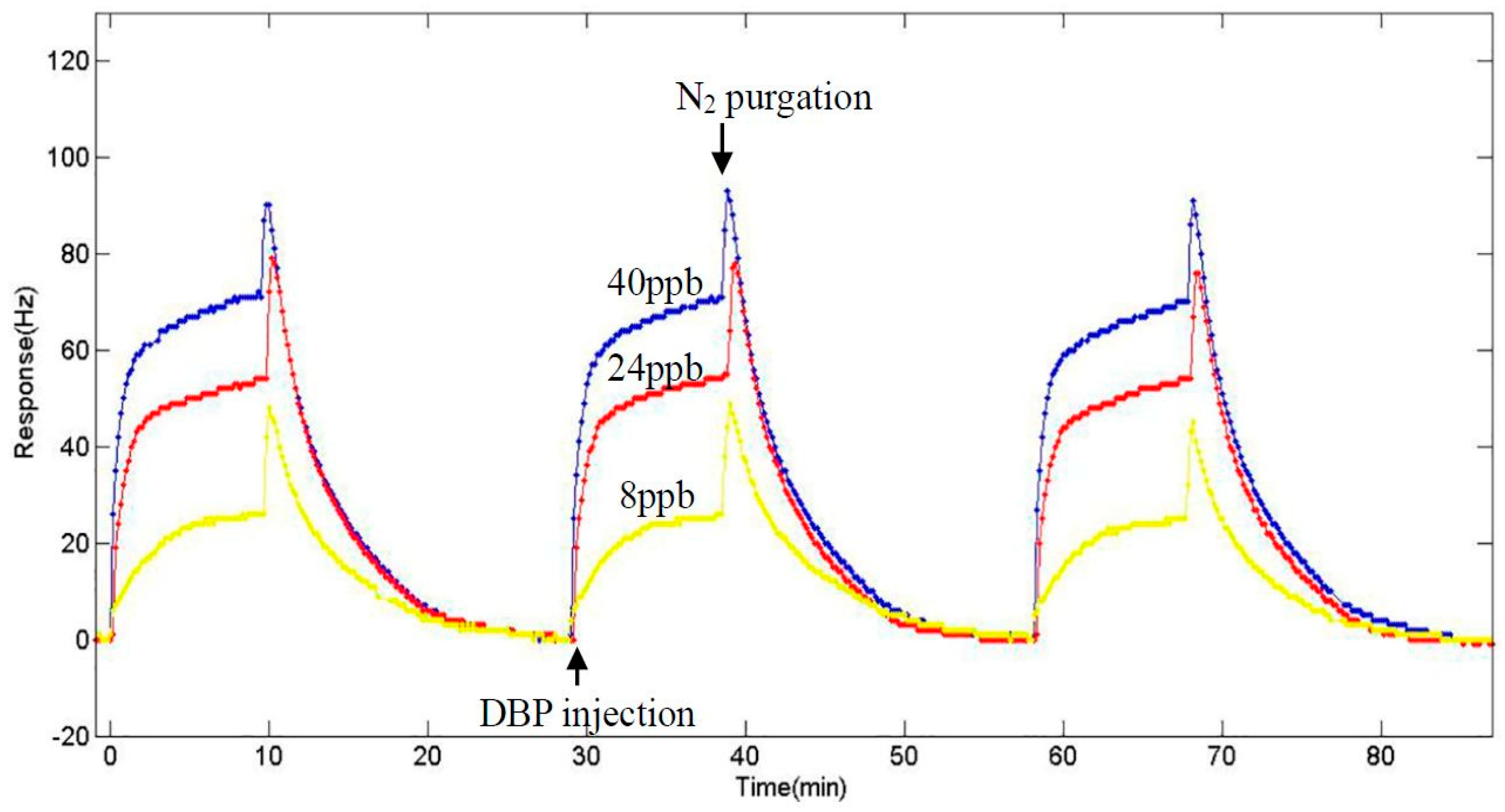
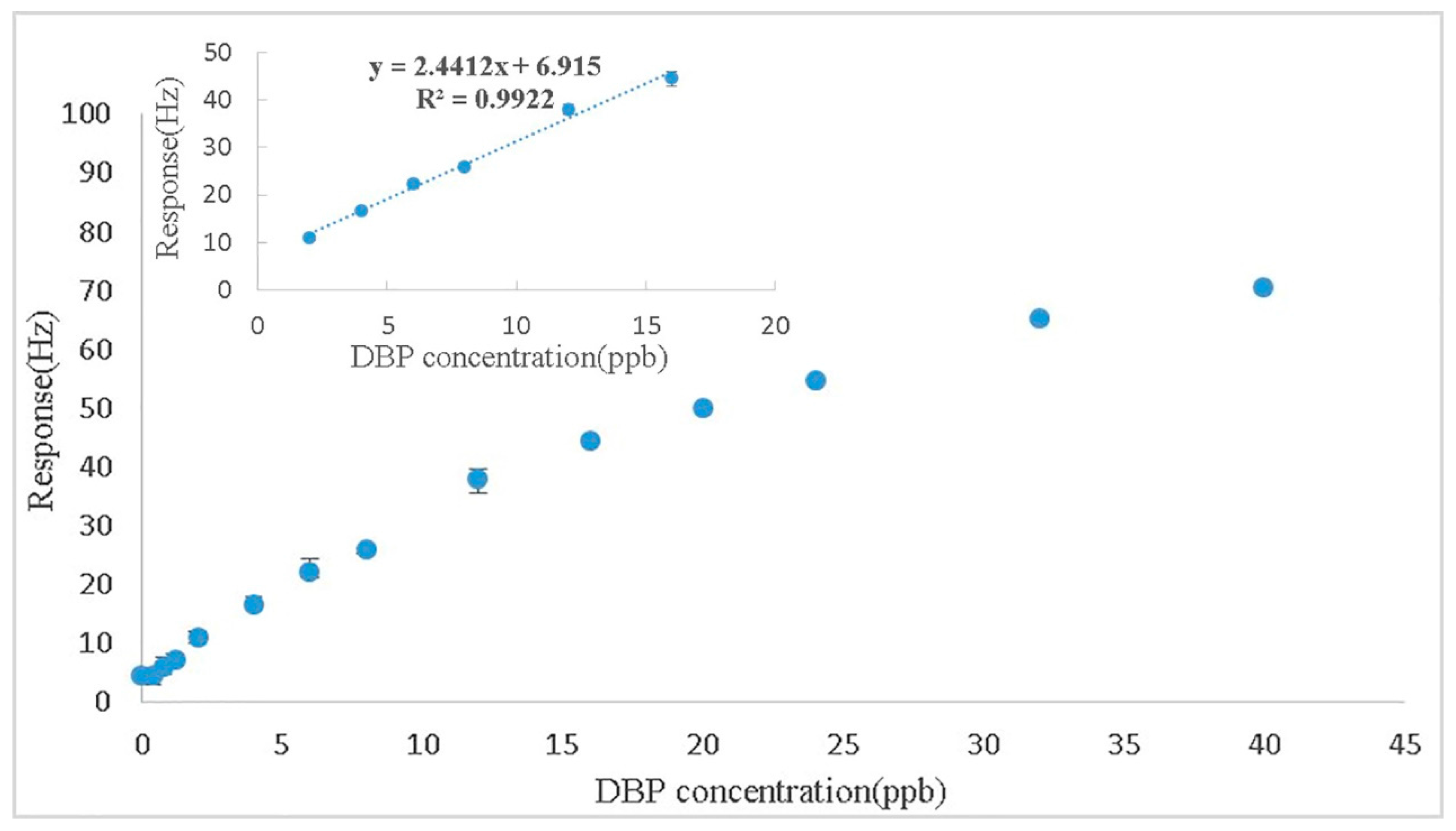
3.4. Sensitivity at the Fundamental Frequency
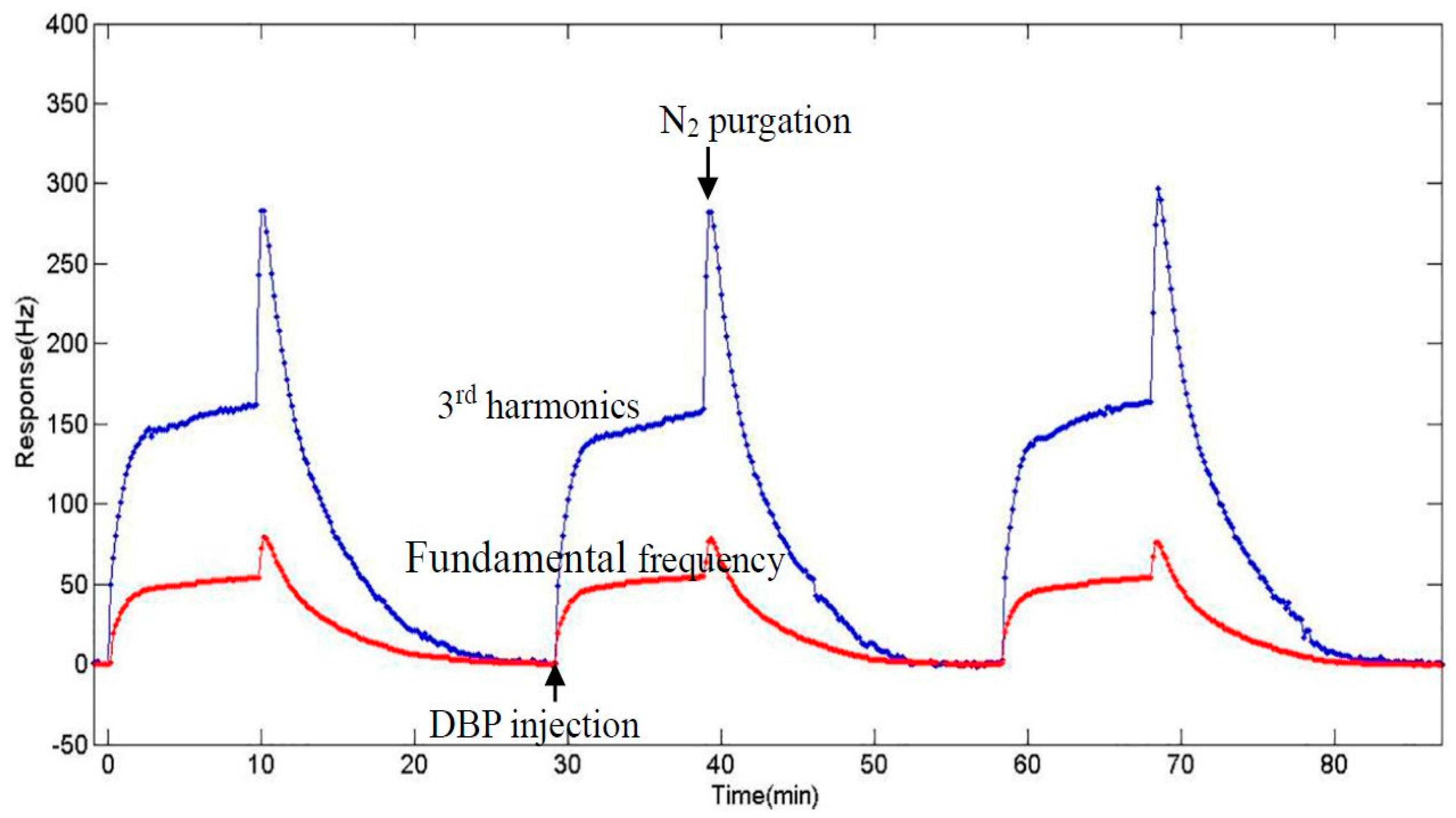
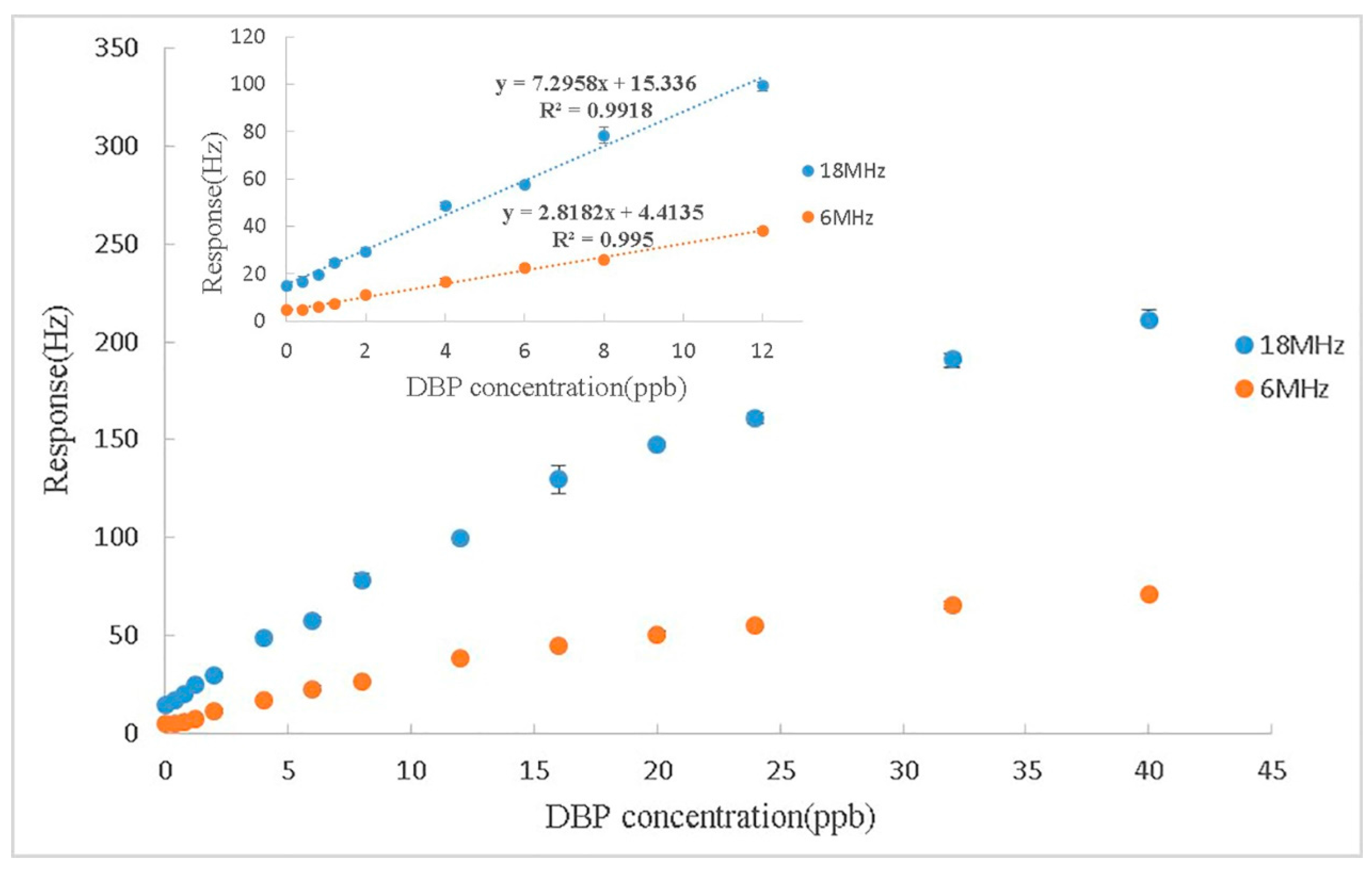
3.5. Experiment at 3rd Harmonics
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Al-Natsheh, M.; Alawi, M.; Fayyad, M.; Tarawneh, I. Simultaneous GC-MS determination of eight phthalates in total and migrated portions of plasticized polymeric toys and childcare articles. J. Chromatogr. B 2015, 985, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-W.; Gao, S.; Hu, C.-Y.; Wu, Y.-M. Modelling of migration from printing inks on paper packaging. Packag. Technol. Sci. 2015, 28, 357–366. [Google Scholar] [CrossRef]
- Bonini, M.; Errani, E.; Zerbinati, G.; Ferri, E.; Girotti, S. Extraction and gas chromatographic evaluation of plasticizers content in food packaging films. Microchem. J. 2008, 90, 31–36. [Google Scholar] [CrossRef]
- Mylchreest, E.; Sar, M.; Cattley, R.C.; Foster, P.M.D. Disruption of androgen-regulated male reproductive development by Di (n-butyl) phthalate during late gestation in rats is different from flutamide. Toxicol. Appl. Pharmacol. 1999, 156, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Naarala, J.; Korpi, A. Cell death and production of reactive oxygen species by murine macrophages after short term exposure to phthalates. Toxicol. Lett. 2009, 188, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, M.; Guo, M.; Chi, C.; Mo, F.; Shen, X. Pollution levels and characteristics of phthalate esters in indoor air in hospitals. J. Environ. Sci. 2015, 37, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Yuan, S.; Pan, X.; Winstead, C.; Wang, Q. Comparison, association, and risk assessment of phthalates in floor dust at different indoor environments in Delaware, USA. J. Environ. Sci. Health Part A Toxic/Hazard. Subst. Environ. Eng. 2015, 50, 1428–1439. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.; Ji, Y.; Liu, L.; Chen, L.; Zhao, X.; Wang, J.; Bai, Z.; Sun, Z. Spatial and temporal variation of phthalic acid esters (PAEs) in atmospheric PM10 and PM2.5 and the influence of ambient temperature in Tianjin, China. Atmos. Environ. 2013, 74, 199–208. [Google Scholar] [CrossRef]
- Kopelovich, L.; Perez, A.L.; Jacobs, N.; Mendelsohn, E.; Keenan, J.J. Screening-level human health risk assessment of toluene and dibutyl phthalate in nail lacquers. Food Chem. Toxicol. 2015, 81, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Fromme, H.; Lahrz, T.; Piloty, M.; Gebhart, H.; Oddoy, A.; Rüden, H. Occurrence of phthalates and musk fragrances in indoor air and dust from apartments and kindergartens in Berlin (Germany). Indoor Air 2004, 14, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Rudel, R.A.; Camann, D.E.; Spengler, J.D.; Korn, L.R.; Brody, J.G. Phthalates, alkylphenols, pesticides, polybrominated diphenyl ethers, and other endocrine-disrupting compounds in indoor air and dust. Environ. Sci. Technol. 2003, 37, 4543–4553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ding, P.; Hu, R.; Zhang, J.; Ma, X.; Luo, Z.; Li, G. A dibutyl phthalate sensor based on a nanofiber polyaniline coated quartz crystal monitor. Sensors 2013, 13, 3765–3775. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Fan, G.; Hu, R.; Li, G. Enhanced dibutyl phthalate sensing performance of a quartz crystal microbalance coated with au-decorated ZnO porous microspheres. Sensors 2015, 15, 21153–21168. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhang, K.; Fan, G.; Luo, Z.; Li, G. Development of a high-sensitivity plasticizer sensor based on a quartz crystal microbalance modified with a nanostructured nickel hydroxide film. Meas. Sci. Technol. 2015, 26, 055102. [Google Scholar] [CrossRef]
- Ogi, H.; Motoshisa, K.; Matsumoto, T.; Hatanaka, K.; Hirao, M. Isolated electrodeless high-frequency quartz crystal microbalance for immunosensors. Anal. Chem. 2006, 78, 6903–6909. [Google Scholar] [CrossRef] [PubMed]
- Erol, A.; Okur, S.; Yağmurcukardeş, N.; Arıkan, M.Ç. Humidity-sensing properties of a ZnO nanowire film as measured with a QCM. Sens. Actuators B Chem. 2011, 152, 115–120. [Google Scholar] [CrossRef]
- Rodahl, M.; Kasemo, B. A simple setup to simultaneously measure the resonant frequency and the absolute dissipation factor of a quartz crystal microbalance. Rev. Sci. Instrum. 1996, 67, 3238. [Google Scholar] [CrossRef]
- Ogi, H.; Motohisa, K.; Hatanaka, K.; Ohmori, T.; Hirao, M.; Nishiyama, M. Concentration dependence of IgG-protein A affinity studied by wireless-electrodeless QCM. Biosens. Bioelectron. 2007, 22, 3238–3242. [Google Scholar] [CrossRef] [PubMed]
- Ogi, H.; Fukunishi, Y.; Nagai, H.; Okamoto, K.; Hirao, M.; Nishiyama, M. Nonspecific-adsorption behavior of polyethylenglycol and bovine serum albumin studied by 55-MHz wireless-electrodeless quartz crystal microbalance. Biosens. Bioelectron. 2009, 24, 3148–3152. [Google Scholar] [CrossRef] [PubMed]
- Ogi, H.; Yanagida, T.; Hirao, M.; Nishiyama, M. Replacement-free mass-amplified sandwich assay with 180-MHz electrodeless quartz-crystal microbalance biosensor. Biosens. Bioelectron. 2011, 26, 4819–4822. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.C.; Lowe, C.R. Magnetic–acoustic–resonator sensors (MARS)—A new sensing methodology. Sens. Actuators A Phys. 1999, 72, 32–37. [Google Scholar] [CrossRef]
- Stevenson, A.C.; Araya-Kleinsteuber, B.; Sethi, R.S.; Mehta, H.M.; Lowe, C.R. Hypersonic evanescent waves generated with a planar spiral coil. Analyst 2003, 128, 1175. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, A.C.; Araya-Kleinsteuber, B.; Sethi, R.S.; Metha, H.M.; Lowe, C.R. Planar coil excitation of multifrequency shear wave transducers. Biosens. Bioelectron. 2005, 20, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Stevenson, A.C.; Lowe, C.R. An acoustic glucose sensor. Biosens. Bioelectron. 2012, 35, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Selyanchyn, R.; Wakamatsu, S.; Hayashi, K.; Lee, S.W. A nano-thin film-based prototype QCM sensor array for monitoring human breath and respiratory patterns. Sensors 2015, 15, 18834–18850. [Google Scholar] [CrossRef] [PubMed]
- Procek, M.; Stolarczyk, A.; Pustelny, T.; Maciak, E. A study of a QCM sensor based on TiO2 anostructures for the etection of NO2 and explosives vapours in air. Sensors 2015, 15, 9563–9581. [Google Scholar] [CrossRef] [PubMed]
- Sabri, Y.M.; Kandjani, A.E.; Ippolito, S.J.; Bhargava, S.K. Nanosphere monolayer on a transducer for enhanced detection of gaseous heavy metal. ACS Appl. Mater. Interfaces 2015, 7, 1491–1499. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Sun, X.; Zhang, K.; Fan, G.; Wang, Y.; Li, G.; Hu, R. A Noncontact Dibutyl Phthalate Sensor Based on a Wireless-Electrodeless QCM-D Modified with Nano-Structured Nickel Hydroxide. Sensors 2017, 17, 1681. https://doi.org/10.3390/s17071681
Chen D, Sun X, Zhang K, Fan G, Wang Y, Li G, Hu R. A Noncontact Dibutyl Phthalate Sensor Based on a Wireless-Electrodeless QCM-D Modified with Nano-Structured Nickel Hydroxide. Sensors. 2017; 17(7):1681. https://doi.org/10.3390/s17071681
Chicago/Turabian StyleChen, Daqi, Xiyang Sun, Kaihuan Zhang, Guokang Fan, You Wang, Guang Li, and Ruifen Hu. 2017. "A Noncontact Dibutyl Phthalate Sensor Based on a Wireless-Electrodeless QCM-D Modified with Nano-Structured Nickel Hydroxide" Sensors 17, no. 7: 1681. https://doi.org/10.3390/s17071681




