Aptamer-Based Carboxyl-Terminated Nanocrystalline Diamond Sensing Arrays for Adenosine Triphosphate Detection
Abstract
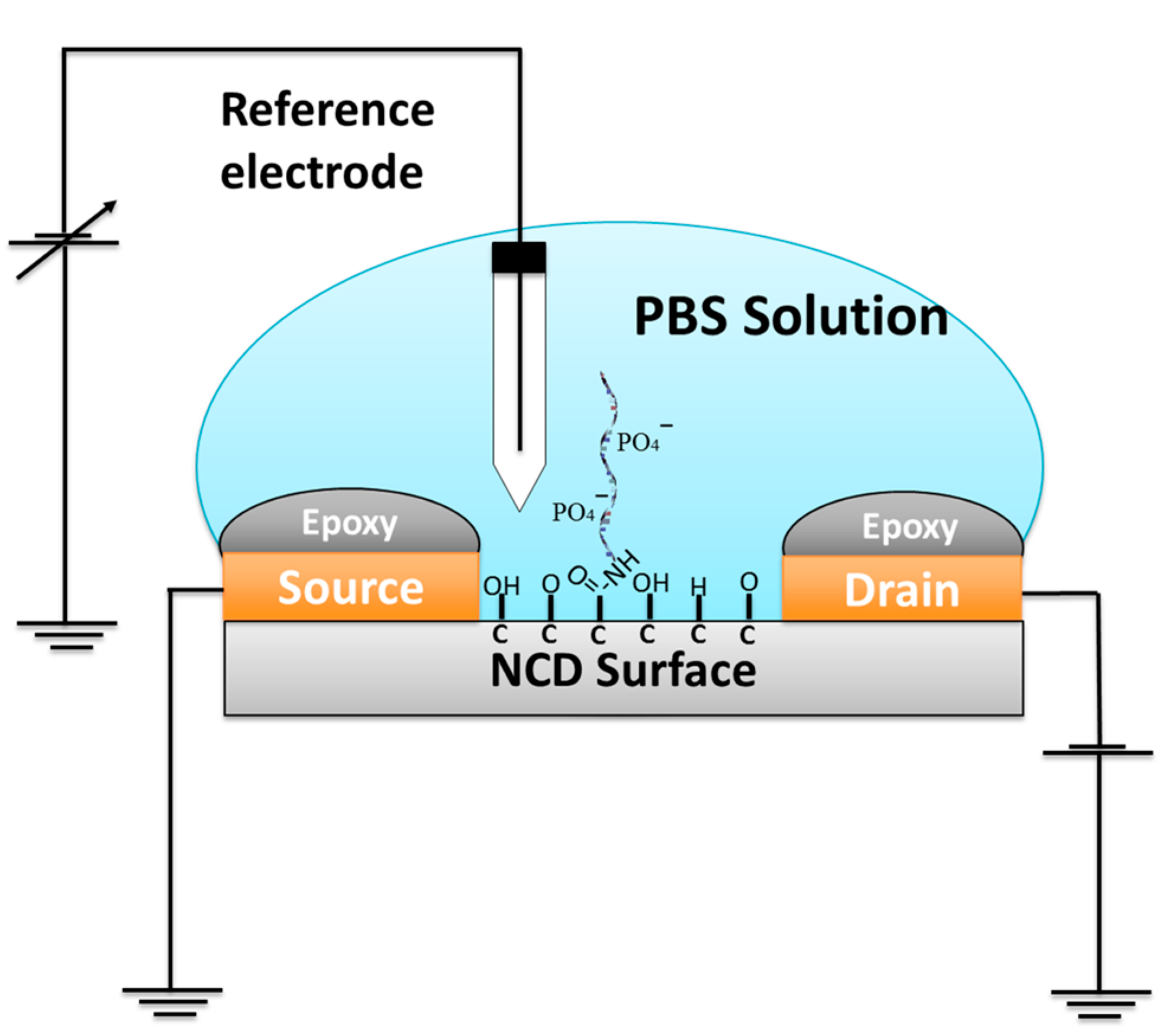
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. X-ray Photoelectron Spectroscopy Analysis
3.2. Fluorescence Signal Detection
3.3. Threshold Voltage Shift for Carboxyl Termination
3.4. Reusability of the Aptasensor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stoltenburg, R.; Reinemann, C.; Strehlitz, B. FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal. Bioanal. Chem. 2005, 383, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.E.; Zhang, Y.; Cai, J.; Cai, W.; Gao, T. Aptamer based Fluorescent Biosensor. Curr. Med. Chem. 2011, 18, 4175–4184. [Google Scholar] [CrossRef] [PubMed]
- Šmuc, T.; Ahn, I.-Y.; Ulrich, H. Nucleic acid aptamers as high affinity ligands in biotechnology and biosensorics. J. Pharm. Biomed. Anal. 2013, 81–82, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Famulok, M.; Mayer, G. Aptamer modules as sensors and detectors. Acc. Chem. Res. 2011, 44, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Huang, P.J.; Ding, J.; Liu, J. Aptamer-based biosensors for biomedical diagnostics. Analyst 2014, 139, 2627–2640. [Google Scholar] [CrossRef] [PubMed]
- Base Pair Biotechnologies. Aptamer vs. Antibody. Available online: https://www.basepairbio.com/aptamers-vs-antibodies/ (accessed on 28 April 2017).
- Wang, Y.; Liu, B. ATP detection using a label-free DNA aptamer and a cationic tetrahedralfluorene. Analyst 2008, 133, 1593–1598. [Google Scholar] [CrossRef] [PubMed]
- Kawarada, H.; Ruslinda, A.R. Diamond electrolyte solution gate FETs for DNA and protein sensors using DNA/RNA aptamers. Phys. Status Solidi 2011, 208, 2005–2016. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, W. Diamond nanostructures for drug delivery, bioimaging, and biosensing. Chem. Soc. Rev. 2017, 46, 734–760. [Google Scholar] [CrossRef] [PubMed]
- Nebel, C.E.; Rezek, B.; Shin, D. Diamond for bio-sensor applications. J. Phys. D Appl. Phys. 2007, 40, 6443–6466. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, S.; Liu, B.; Wu, J.; Zhou, Y.; He, L.; Ding, J.; Liu, J. Intracellular Detection of ATP Using an Aptamer Beacon Covalently Linked to Graphene Oxide Resisting Nonspecific Probe Displacement. Anal. Chem. 2014, 86, 12229–12235. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Liu, B. Fluorescent detection of ATP based on signaling DNA aptamer attached silica nanoparticles. Nanotechnology 2008, 19, 415605. [Google Scholar] [CrossRef] [PubMed]
- Paci, J.T.; Man, H.B.; Saha, B.; Ho, D.; Schatz, G.C. Understanding the Surfaces of Nanodiamonds. J. Phys. Chem. C 2013, 117, 17256–17267. [Google Scholar] [CrossRef]
- Wang, X.; Kurihara, S.; Hasegawa, M.; Ruslinda, A.R.; Kawarada, H. High Priority of Nanocrystalline Diamond as a Biosensing Platform. Jpn. J. Appl. Phys. 2012, 51, 1–7. [Google Scholar] [CrossRef]
- Dincer, C.; Laubender, E.; Hees, J.; Nebel, C.E.; Urban, G.; Heinze, J. SECM detection of single boron doped diamond nanodes and nanoelectrode arrays using phase-operated shear force technique. Electrochem. Commun. 2012, 24, 123–127. [Google Scholar] [CrossRef]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2011, 7, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Härtl, A.; Schmich, E.; Garrido, J.A.; Hernando, J.; Catharino, S.C.R.; Walter, S.; Feulner, P.; Kromka, A.; Steinmüller, D.; Stutzmann, M.; et al. Protein-modified nanocrystallyne diamond thin films for biosensor applications. Nat. Mater. 2004, 3, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Dincer, C.; Ktaich, R.; Laubender, E.; Hees, J.J.; Kieninger, J.; Nebel, C.E.; Heinze, J.; Urban, G. Nanocrystalline boron-doped diamond nanoelectrode arrays for ultrasensitive dopamine detection. Electrochim. Acta 2015, 185, 101–106. [Google Scholar] [CrossRef]
- Wang, W.X.; Ishii, Y.; Ruslinda, A.R.; Hasegawa, M.; Kawarada, H. Effective surface functionalization of nanocrystalline diamond films by direct carboxylation for PDGF detection via aptasensor. ACS Appl. Mater. Interfaces 2012, 4, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Hees, J.; Hoffmann, R.; Kriele, A.; Smirnov, W.; Obloh, H.; Glorer, K.; Raynor, B.; Driad, R.; Yang, N.; Williams, O.A.; et al. Nanocrystalline Diamond Nanoelectrode Arrays and Ensembles. ACS Nano 2011, 5, 3339–3346. [Google Scholar] [CrossRef] [PubMed]
- Martínez-lozano, T.P.C.; Tomás, V.; Martín, J. Determination of ATP via the photochemical generation of hydrogen peroxide using flow injection luminol chemiluminescence detection. Anal. Bioanal. Chem. 2003, 377, 189–194. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Database; CID=60961. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/60961 (accessed on 28 April 2017).
- Huizenga, D.E.; Szostak, J.W. A DNA Aptamer That Binds Adenosine and ATP. Biochemistry 1995, 34, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, T.; Suzuki, E.; Nakamuara, G.K. Solution structure of an ATP-binding RNA aptamer reveals a novel fold. RNA Soc. 1996, 2, 628–640. [Google Scholar]
- Suaebah, E.; Seshimo, Y.; Shibata, M.; Kono, S.; Hasegawa, M.; Kawarada, H. Aptamer strategy for ATP detection on nanocrystalline diamond functionalized by a nitrogen and hydrogen radical beam system. J. Appl. Phys. 2017, 44506. [Google Scholar] [CrossRef]
- Sun, W.; Gu, Z. ATP-Responsive Drug Delivery Systems. Expert Opin. Drug Deliv. 2016, 13, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Nutiu, R.; Li, Y. Structure-Switching Signaling Aptamers: Transducing Molecular Recognition into Fluorescence Signaling. Chemistry 2004, 10, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. Selection in vitro of single-stranded DNA molecules that fold into specific ligand-binding structures. Nature 1992, 355, 850–852. [Google Scholar] [CrossRef] [PubMed]
- Sazani, P.L.; Larralde, R.; Szostak, J.W. A small aptamer with strong and specific recognition of the triphosphate of ATP. J. Am. Chem. Soc. 2004, 126, 8370–8371. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Song, K.S.; Zhang, G.J.; Degawa, M.; Sasaki, Y.; Ohdomari, I.; Kawarada, H. Characterization of DNA Hybridization on Partially Aminated Diamond by Aromatic Compounds. Langmuir 2006, 22, 11245–11250. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Boukherroub, R. Different strategies for functionalization of diamond surfaces. J. Solid State Electrochem. 2007, 12, 1205–1218. [Google Scholar] [CrossRef]
- Zhang, G.J.; Song, K.S.; Nakamura, Y.; Ueno, T.; Funatsu, T.; Ohdomari, I.; Kawarada, H. DNA micropatterning on polycrystalline diamond via one-step direct amination. Langmuir 2006, 22, 3728–3734. [Google Scholar] [CrossRef] [PubMed]
- Tsugawa, K.; Ishihara, M.; Kim, J.; Hasegawa, M.; Koga, Y. Growth and Applications of Nanocrystalline-Diamond Films. Hyomen Kagaku 2009, 30, 267–272. [Google Scholar] [CrossRef]
- Penmatsa, V.; Rahim, R.A.; Kawarada, H.; Wang, C. Functionalized carbon microarrays platform for high sensitive detection of HIV-Tat peptide. RSC Adv. 2015, 5, 65042–65047. [Google Scholar] [CrossRef]
- Wang, X.; Ruslinda, A.R.; Ishiyama, Y.; Ishii, Y.; Kawarada, H. Higher coverage of carboxylic acid groups on oxidized single crystal diamond (001). Diam. Relat. Mater. 2011, 20, 1319–1324. [Google Scholar] [CrossRef]
- Zheng, H.Y.; Alsager, O.A.; Wood, C.S.; Hodgkiss, J.M.; Plank, N.O.V. Carbon nanotube field effect transistor aptasensors for estrogen detection in liquids. J. Vac. Sci. Technol. B Nanotechnol. Microelectron. 2015, 33, 06F904. [Google Scholar] [CrossRef]
- Murray, R.K.; Rodwell, V.W.; Bender, D.A.; Botham, K.M.; Kennelly, P.J.; Weil, P.A. Harper’s Illustrated Biochemistry, 30th ed.; McGraw-Hill: New York, NY, USA, 2015. [Google Scholar]
- Kim, S.G.; Lee, J.S.; Jun, J.; Shin, D.H.; Jang, J. Ultrasensitive Bisphenol A Field-Effect Transistor Sensor Using an Aptamer-Modified Multichannel Carbon Nanofiber Transducer. ACS Appl. Mater. Interfaces 2016, 8, 6602–6610. [Google Scholar] [CrossRef] [PubMed]
- Kageura, T.; Kato, K.; Suaebah, E.; Kajiya, M.; Kawai, S.; Inaba, M.; Tanii, T.; Haruyama, M.; Yamada, K.; Onoda, S.; et al. Effect of a radical exposure nitridation surface on the charge stability of shallow nitrogen vacancy centers in diamond. Appl. Phys. Express 2017, 10, 055503. [Google Scholar] [CrossRef]








| Chem. State | Position (eV) | Height (C in S) | Area (Gaussian %) | % Area |
|---|---|---|---|---|
| Sp2 | 283.93 | 1154 | 1928 | 10.3 |
| C-C | 284.85 | 14607 | 14730 | 78.7 |
| C-O | 285.86 | 1411 | 1108 | 5.9 |
| C=O | 286.57 | 529 | 738 | 3.9 |
| COOH | 288.12 | 233 | 214 | 1.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suaebah, E.; Naramura, T.; Myodo, M.; Hasegawa, M.; Shoji, S.; Buendia, J.J.; Kawarada, H. Aptamer-Based Carboxyl-Terminated Nanocrystalline Diamond Sensing Arrays for Adenosine Triphosphate Detection. Sensors 2017, 17, 1686. https://doi.org/10.3390/s17071686
Suaebah E, Naramura T, Myodo M, Hasegawa M, Shoji S, Buendia JJ, Kawarada H. Aptamer-Based Carboxyl-Terminated Nanocrystalline Diamond Sensing Arrays for Adenosine Triphosphate Detection. Sensors. 2017; 17(7):1686. https://doi.org/10.3390/s17071686
Chicago/Turabian StyleSuaebah, Evi, Takuro Naramura, Miho Myodo, Masataka Hasegawa, Shuichi Shoji, Jorge J. Buendia, and Hiroshi Kawarada. 2017. "Aptamer-Based Carboxyl-Terminated Nanocrystalline Diamond Sensing Arrays for Adenosine Triphosphate Detection" Sensors 17, no. 7: 1686. https://doi.org/10.3390/s17071686




