Detection of Pseudomonas aeruginosa Metabolite Pyocyanin in Water and Saliva by Employing the SERS Technique
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Silver Nanoparticles (Ag NPs) Synthesis
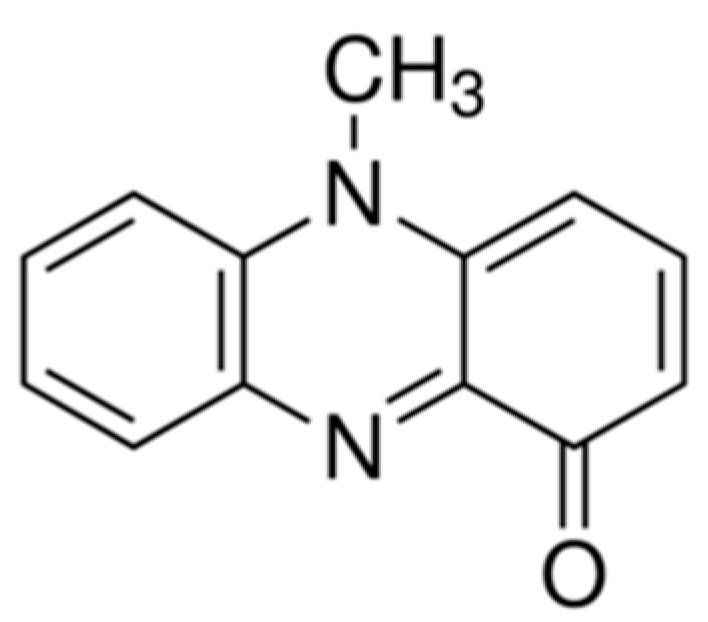
2.3. Pyocyanin Solution
2.4. Saliva Sample Preparation
2.5. Instrumentation
2.6. Data Processing
3. Results and Discussion
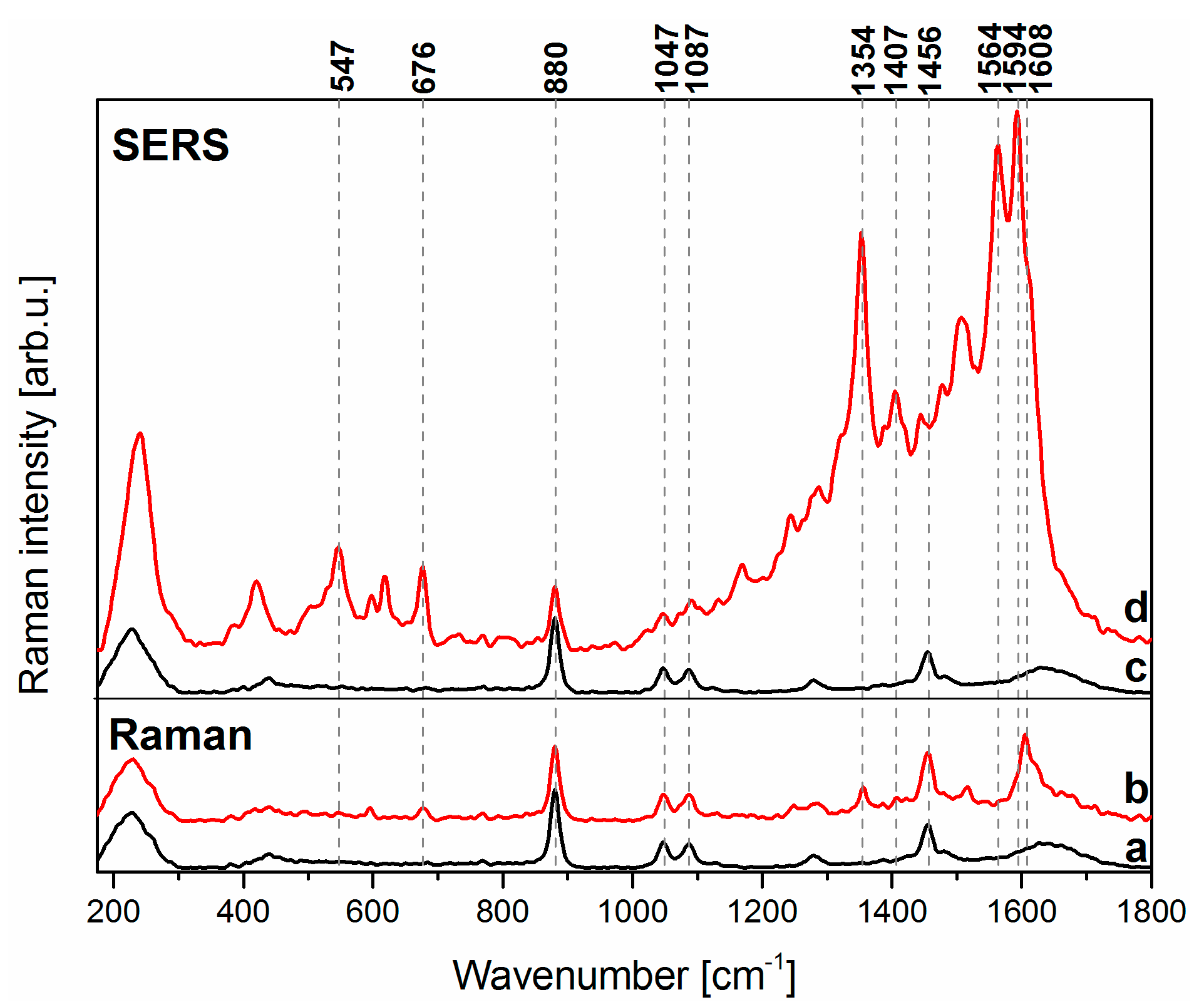
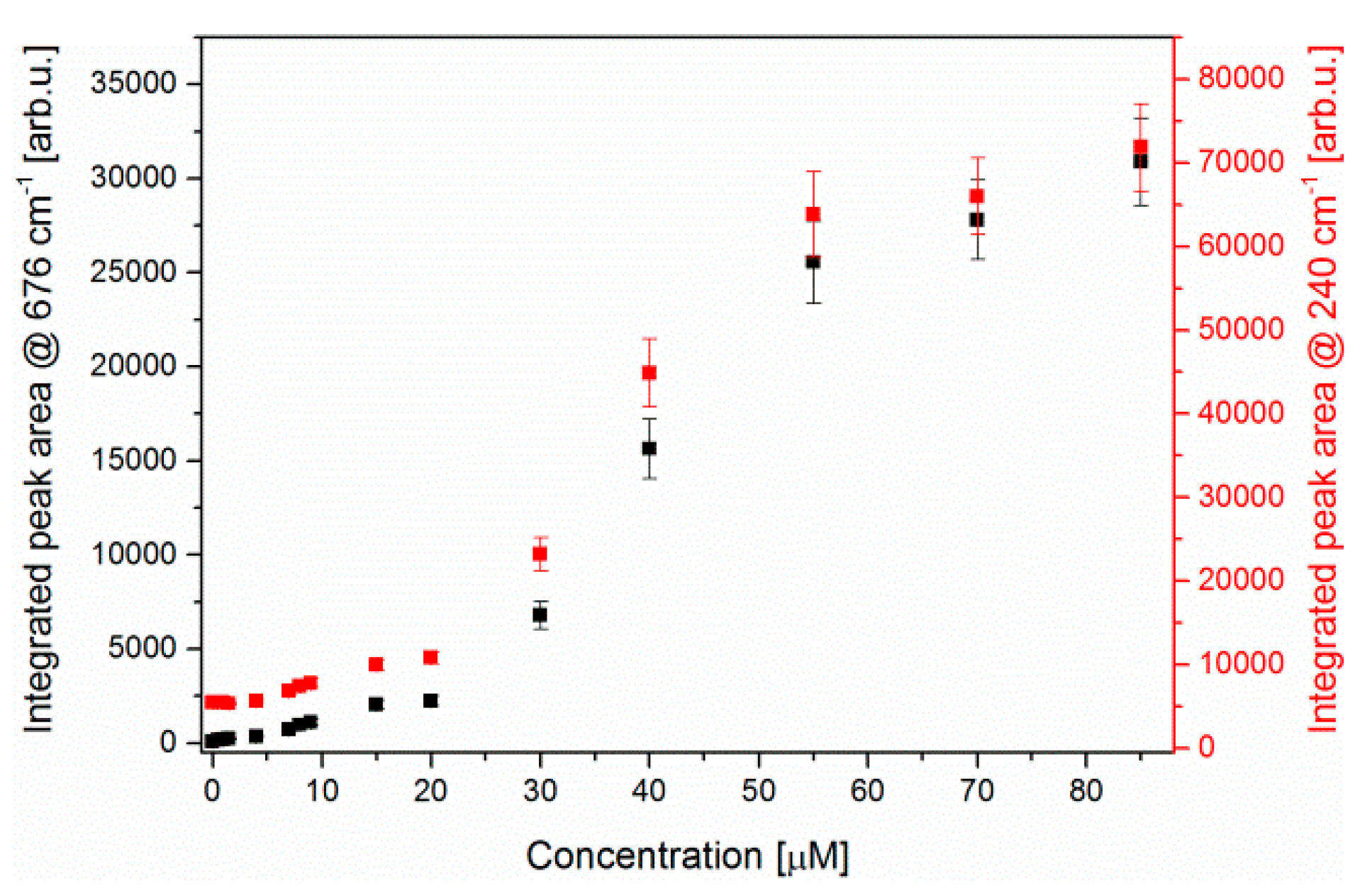
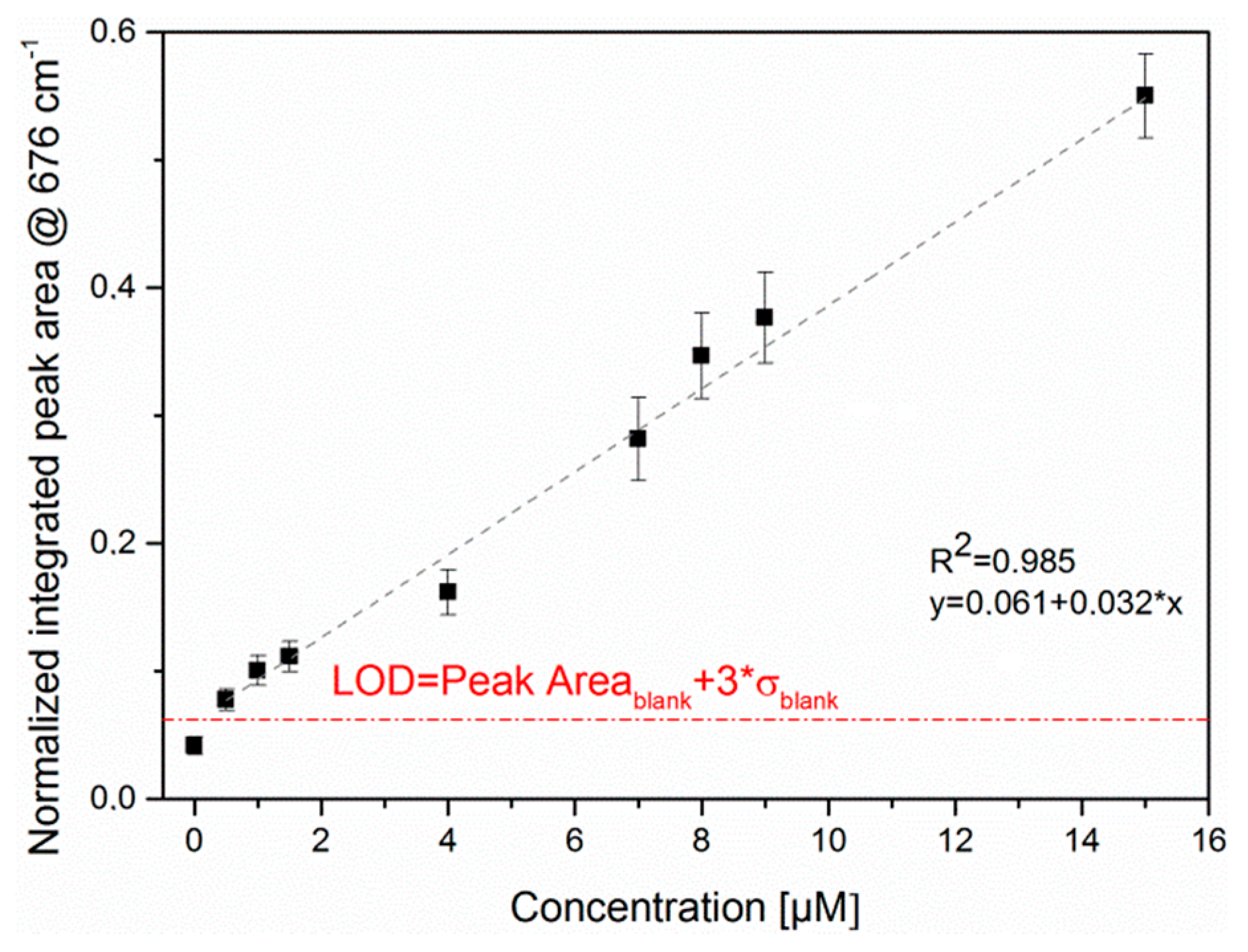
3.1. SERS Detection of Pyocyanin in Aqueous Solution
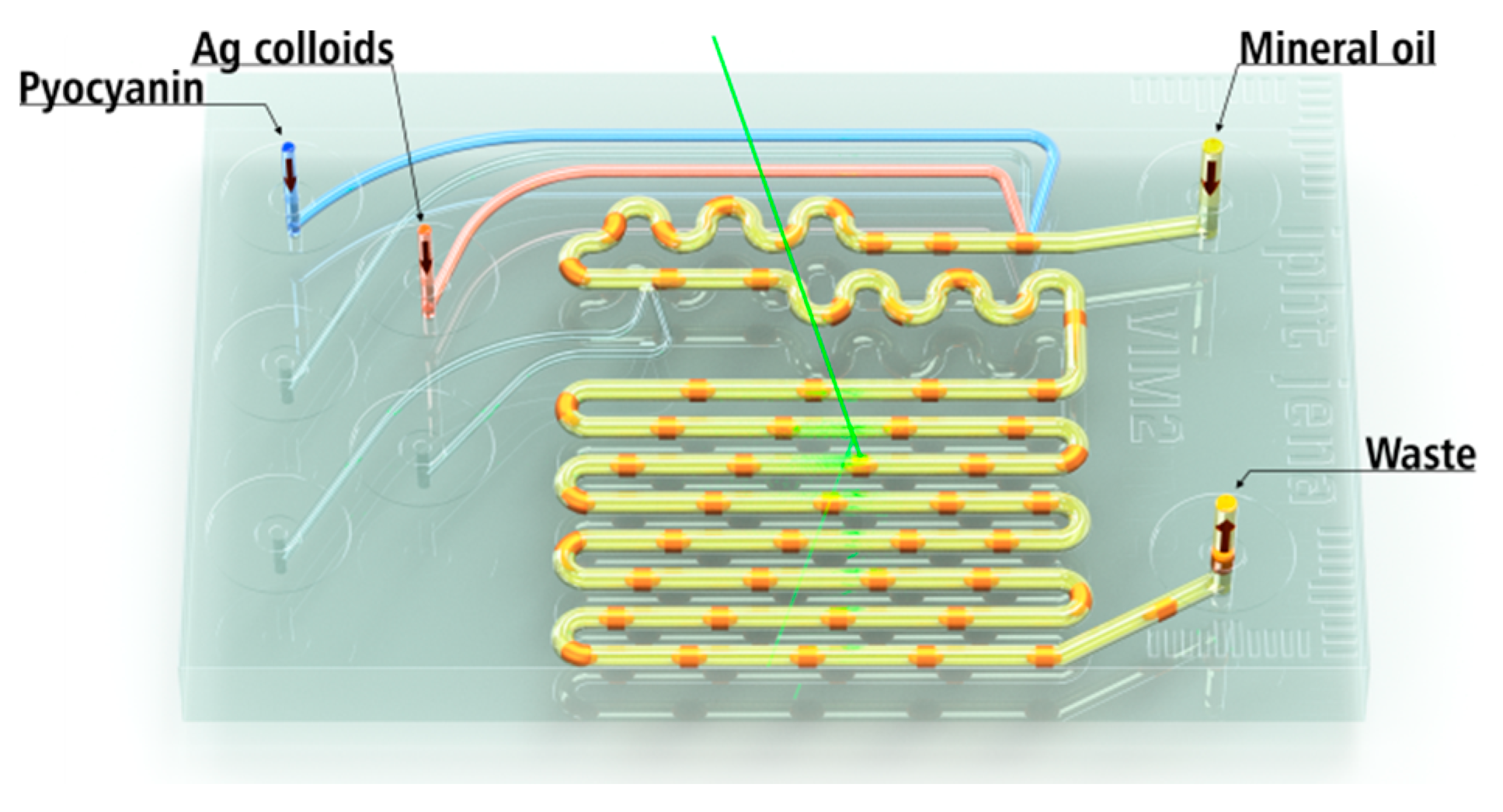
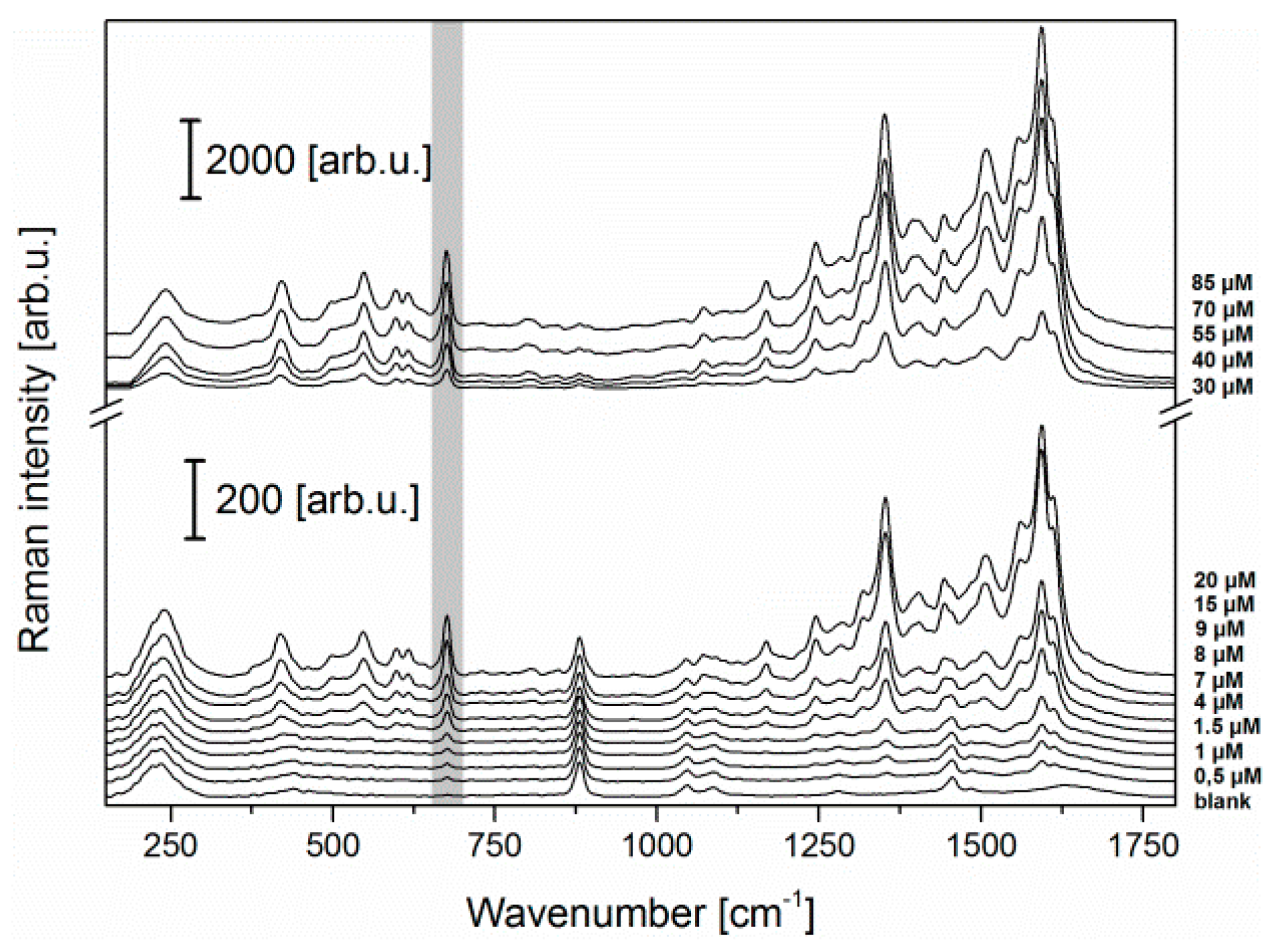
3.2. LoC-SERS Measurement of PYO in Aqueous Solution
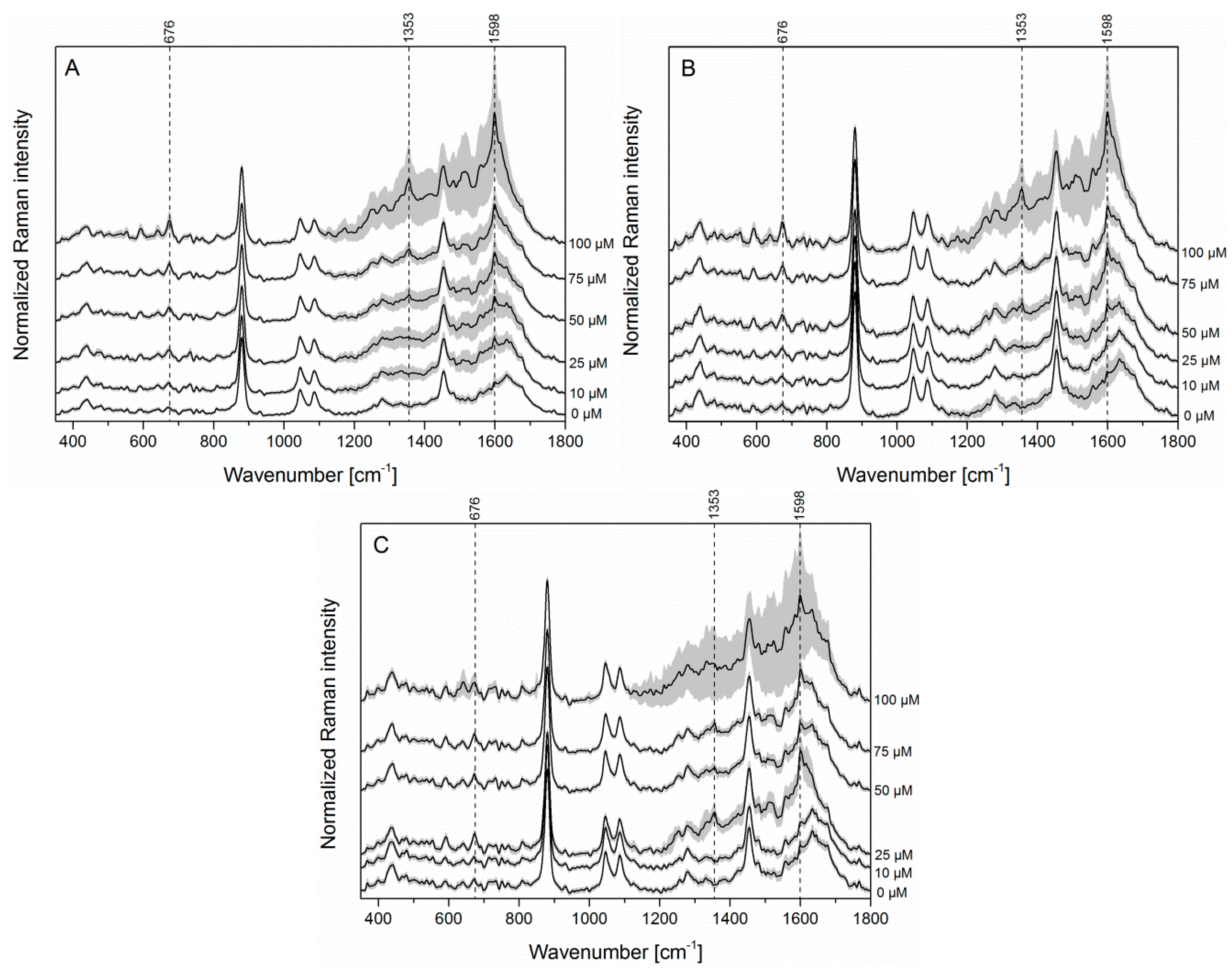
3.3. SERS Detection of PYO in Saliva
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Antibiotic Resistance. Available online: http://www.who.int/mediacentre/factsheets/antibiotic-resistance/en/ (accessed on 27 February 2017).
- Lambert, M.L.; Suetens, C.; Savey, A.; Palomar, M.; Hiesmayr, M.; Morales, I.; Agodi, A.; Frank, U.; Mertens, K.; Schumacher, M.; et al. Clinical outcomes of health-care-associated infections and antimicrobial resistance in patients admitted to European intensive-care units: A cohort study. Lancet Infect. Dis. 2011, 11, 30–38. [Google Scholar] [CrossRef]
- Lau, G.W.; Hassett, D.J.; Ran, H.M.; Kong, F.S. The role of pyocyanin in Pseudomonas aeruginosa infection. Trends Mol. Med. 2004, 10, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.C.; Whiteley, M. Metabolism and Pathogenicity of Pseudomonas aeruginosa Infections in the Lungs of Individuals with Cystic Fibrosis. Microbiol. Spectr. 2015, 3, 185–213. [Google Scholar] [CrossRef]
- Wilson, R.; Sykes, D.A.; Watson, D.; Rutman, A.; Taylor, G.W.; Cole, P.J. Measurement of Pseudomonas-aeruginosa phenazine pigments in sputum and assessment of their contribution to sputum sol toxicity for respiratory epithelium. Infect. Immun. 1988, 56, 2515–2517. [Google Scholar] [PubMed]
- Hunter, R.C.; Klepac-Ceraj, V.; Lorenzi, M.M.; Grotzinger, H.; Martin, T.R.; Newman, D.K. Phenazine Content in the Cystic Fibrosis Respiratory Tract Negatively Correlates with Lung Function and Microbial Complexity. Am. J. Respir. Cell Mol. Biol. 2012, 47, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.W.; Luo, J.; Zhong, C.J. SERS nanoprobes for bio-application. Front. Chem. Sci. Eng. 2015, 9, 428–441. [Google Scholar] [CrossRef]
- Cialla, D.; Pollok, S.; Steinbrucker, C.; Weber, K.; Popp, J. SERS-based detection of biomolecules. Nanophotonics 2014, 3, 383–411. [Google Scholar] [CrossRef]
- Pahlow, S.; Marz, A.; Seise, B.; Hartmann, K.; Freitag, I.; Kammer, E.; Bohme, R.; Deckert, V.; Weber, K.; Cialla, D.; et al. Bioanalytical application of surface- and tip-enhanced Raman spectroscopy. Eng. Life Sci. 2012, 12, 131–143. [Google Scholar] [CrossRef]
- Sharma, B.; Frontiera, R.R.; Henry, A.I.; Ringe, E.; Van Duyne, R.P. SERS: Materials, applications, and the future. Mater. Today 2012, 15, 16–25. [Google Scholar] [CrossRef]
- Polisetti, S.; Baig, N.F.; Morales-Soto, N.; Shrout, J.D.; Bohn, P.W. Spatial Mapping of Pyocyanin in Pseudomonas Aeruginosa Bacterial Communities Using Surface Enhanced Raman Scattering. Appl. Spectrosc. 2017, 71, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Bodelon, G.; Montes-Garcia, V.; Lopez-Puente, V.; Hill, E.H.; Hamon, C.; Sanz-Ortiz, M.N.; Rodal-Cedeira, S.; Costas, C.; Celiksoy, S.; Perez-Juste, I.; et al. Detection and imaging of quorum sensing in Pseudomonas aeruginosa biofilm communities by surface-enhanced resonance Raman scattering. Nat. Mater. 2016, 15, 1203–1211. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.M.; Chen, J.; Li, X.B.; Zhao, Y.P.; Zughaier, S.M. Culture-free diagnostics of Pseudomonas aeruginosa infection by silver nanorod array based SERS from clinical sputum samples. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.A.; Zhang, Y.L.; Ding, H.; Sun, H.B. SERS-Enabled Lab-on-a-Chip Systems. Adv. Opt. Mater. 2015, 3, 618–633. [Google Scholar] [CrossRef] [Green Version]
- Jahn, I.J.; Zukovskaja, O.; Zheng, X.S.; Weber, K.; Bocklitz, T.W.; Cialla-May, D.; Popp, J. Surface-enhanced Raman spectroscopy and microfluidic platforms: Challenges, solutions and potential applications. Analyst 2017, 142, 1022–1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yu, C.X. Analytical characterization using surface-enhanced Raman scattering (SERS) and microfluidic sampling. Nanotechnology 2015, 26, 092001. [Google Scholar] [CrossRef] [PubMed]
- Andreou, C.; Hoonejani, M.R.; Barmi, M.R.; Moskovits, M.; Meinhart, C.D. Rapid Detection of Drugs of Abuse in Saliva Using Surface Enhanced Raman Spectroscopy and Microfluidics. ACS Nano 2013, 7, 7157–7164. [Google Scholar] [CrossRef] [PubMed]
- Israelsen, N.D.; Hanson, C. Nanoparticle properties and synthesis effects on surface-enhanced Raman scattering enhancement factor: An introduction. Sci. World J. 2015, 2015, 124582. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.M.; Righini, M.; Gonzalez, M.U.; Quidant, R.; Kall, M. Optical aggregation of metal nanoparticles in a microfluidic channel for surface-enhanced Raman scattering analysis. Lab Chip 2009, 9, 193–195. [Google Scholar] [CrossRef] [PubMed]
- Leopold, N.; Lendl, B. A new method for fast preparation of highly surface-enhanced Raman scattering (SERS) active silver colloids at room temperature by reduction of silver nitrate with hydroxylamine hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Marz, A.; Ackermann, K.R.; Malsch, D.; Bocklitz, T.; Henkel, T.; Popp, J. Towards a quantitative SERS approach—Online monitoring of analytes in a microfluidic system with isotope-edited internal standards. J. Biophotonics 2009, 2, 232–242. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. Development Core R: A Language and Environment for Statistical Computing; The R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Ryan, C.G.; Clayton, E.; Griffin, W.L.; Sie, S.H.; Cousens, D.R. SNIP, a statistics-sensitive background treatment for the quantitative-analysis of pixe spectra in geoscience applications. Nucl. Instrum. Methods Phys. Res. B 1988, 34, 396–402. [Google Scholar] [CrossRef]
- Atkinson, K. An Introduction to Numerical Analysis, 2nd ed.; Wiley: Hoboken, NJ, USA, 1989; pp. 251–263. ISBN 978-0-471-62489-9. [Google Scholar]
- Hassan, H.M.; Fridovich, I. Mechanism of the antibiotic action of pyocyanine. J. Bacteriol. 1980, 141, 156–163. [Google Scholar] [PubMed]
- Zhang, Y.Y.; Walkenfort, B.; Yoon, J.H.; Schlucker, S.; Xie, W. Gold and silver nanoparticle monomers are non-SERS-active: A negative experimental study with silica-encapsulated Raman-reporter-coated metal colloids. Phys. Chem. Chem. Phys. 2015, 17, 21120–21126. [Google Scholar] [CrossRef] [PubMed]
- Baia, M.; Baia, L.; Kiefer, W.; Popp, J. Surface-enhanced Raman scattering and density functional theoretical study of anthranil adsorbed on colloidal silver particles. J. Phys. Chem. B 2004, 108, 17491–17496. [Google Scholar] [CrossRef]
- Castro, J.L.; Lopez-Ramirez, M.R.; Arenas, J.F.; Soto, J.; Otero, J.C. Evidence of Deprotonation of Aromatic Acids and Amides Adsorbed on Silver Colloids by Surface-Enhanced Raman Scattering. Langmuir 2012, 28, 8926–8932. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, J.; Mukherjee, K.M.; Misra, T.N. A pH dependent surface-enhanced Raman scattering study of hypoxanthine. J. Raman Spectrosc. 2000, 31, 427–431. [Google Scholar] [CrossRef]
- Hidi, I.J.; Jahn, M.; Weber, K.; Cialla-May, D.; Popp, J. Droplet based microfluidics: Spectroscopic characterization of levofloxacin and its SERS detection. Phys. Chem. Chem. Phys. 2015, 17, 21236–21242. [Google Scholar] [CrossRef] [PubMed]
- Greabu, M.; Battino, M.; Mohora, M.; Totan, A.; Didilescu, A.; Spinu, T.; Totan, C.; Miricescu, D.; Radulescu, R. Saliva—A diagnostic window to the body, both in health and in disease. J. Med. Life 2009, 2, 124–132. [Google Scholar] [PubMed]
- Li, X.Z.; Yang, T.Y.; Lin, J.X. Spectral analysis of human saliva for detection of lung cancer using surface-enhanced Raman spectroscopy. J. Biomed. Opt. 2012, 17, 0370031–0370035. [Google Scholar] [CrossRef] [PubMed]
- Yuen, C.; Zheng, W.; Huang, Z.W. Optimization of extinction efficiency of gold-coated polystyrene bead substrates improves surface-enhanced Raman scattering effects by post-growth microwave heating treatment. J. Raman Spectrosc. 2010, 41, 374–380. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žukovskaja, O.; Jahn, I.J.; Weber, K.; Cialla-May, D.; Popp, J. Detection of Pseudomonas aeruginosa Metabolite Pyocyanin in Water and Saliva by Employing the SERS Technique. Sensors 2017, 17, 1704. https://doi.org/10.3390/s17081704
Žukovskaja O, Jahn IJ, Weber K, Cialla-May D, Popp J. Detection of Pseudomonas aeruginosa Metabolite Pyocyanin in Water and Saliva by Employing the SERS Technique. Sensors. 2017; 17(8):1704. https://doi.org/10.3390/s17081704
Chicago/Turabian StyleŽukovskaja, Olga, Izabella Jolan Jahn, Karina Weber, Dana Cialla-May, and Jürgen Popp. 2017. "Detection of Pseudomonas aeruginosa Metabolite Pyocyanin in Water and Saliva by Employing the SERS Technique" Sensors 17, no. 8: 1704. https://doi.org/10.3390/s17081704



