An Opto-Electronic Sensor for Detecting Soil Microarthropods and Estimating Their Size in Field Conditions
Abstract
:1. Introduction
2. Materials and Methods
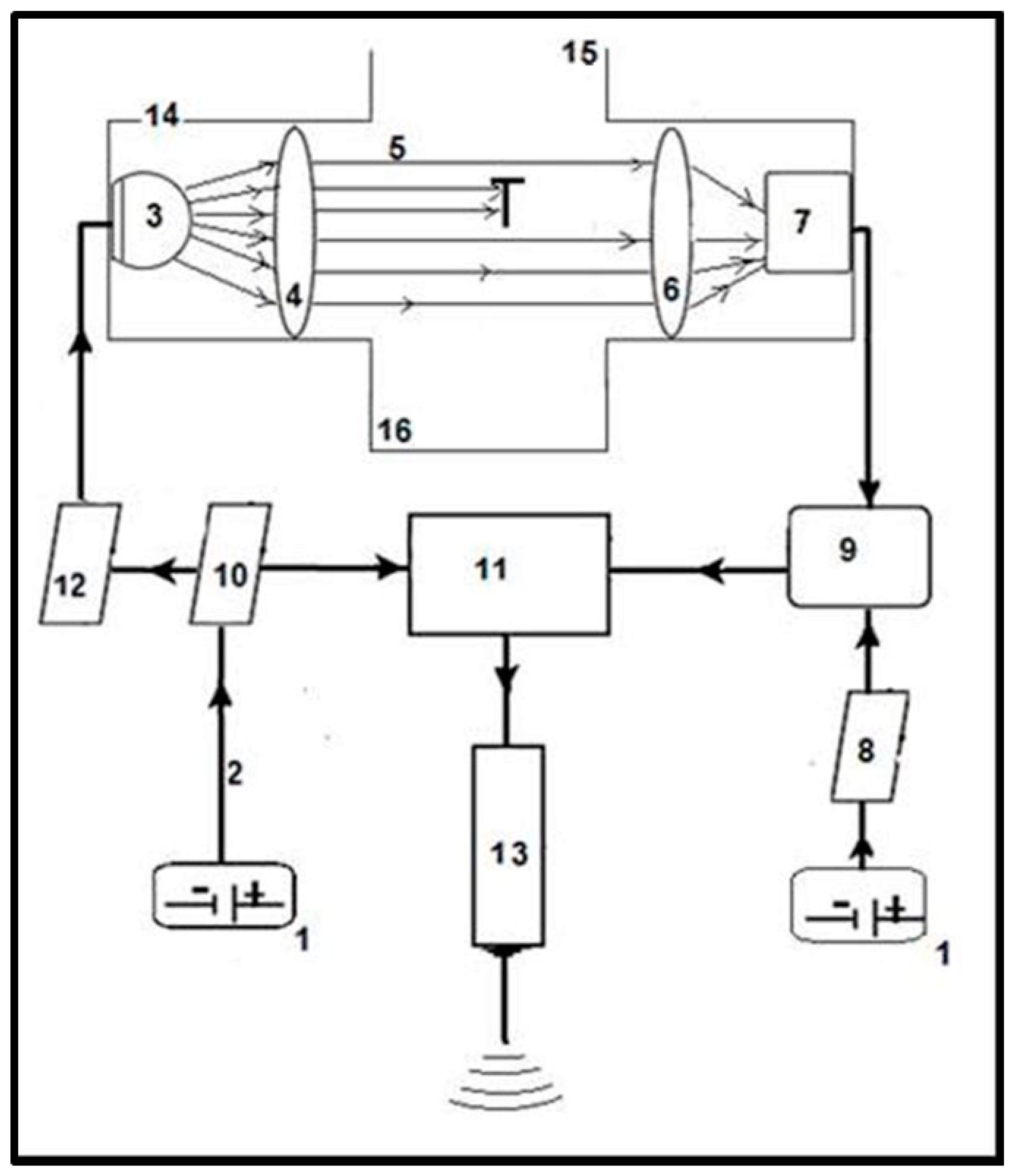
2.1. Operation of the Sensor
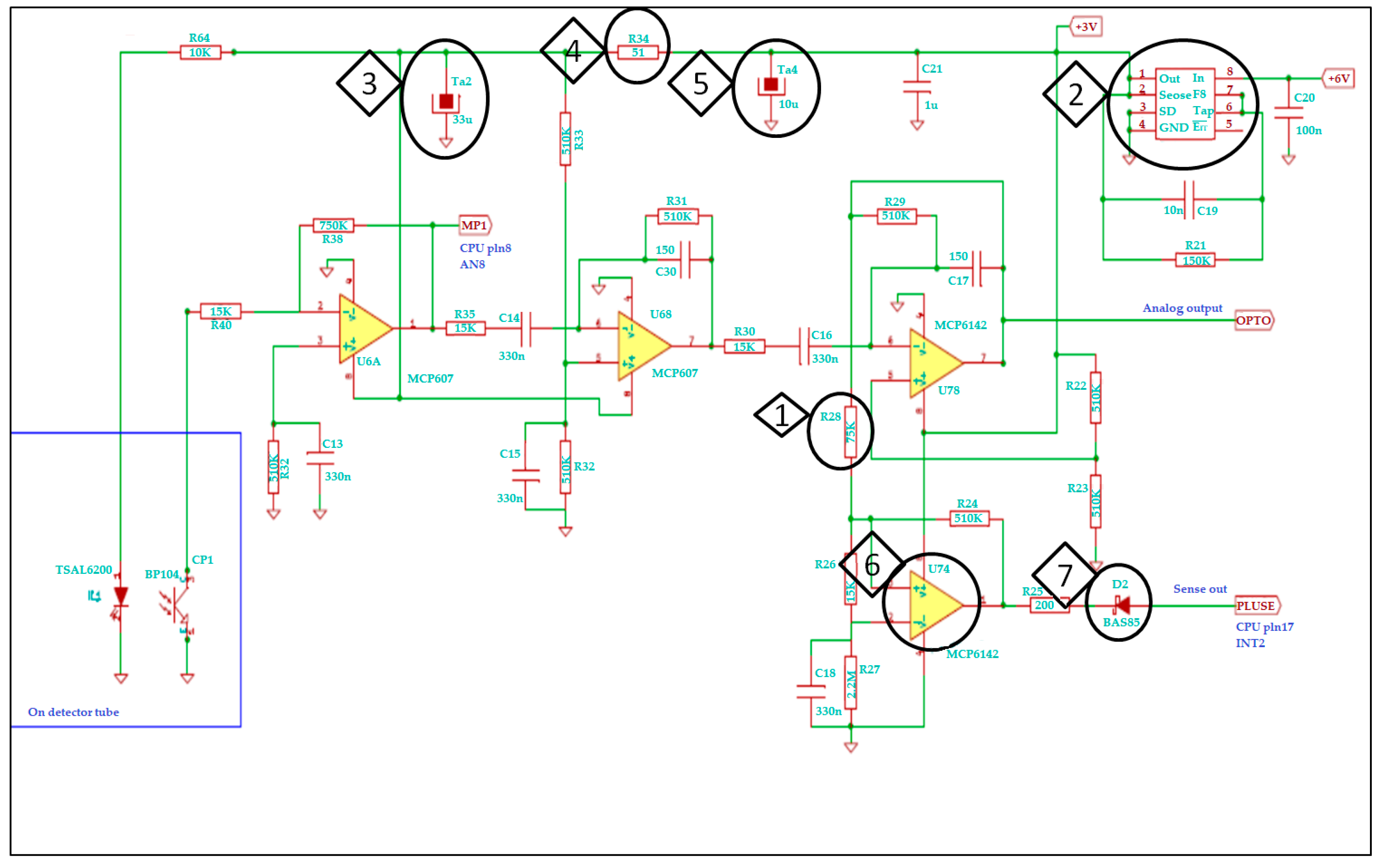
2.2. Signal Amplifier
2.3. CPU Unit
2.4. Laboratory Testing of Detection Efficiency
2.5. Signal Patterning during Detection and Size Estimation of Falling-In Objects
2.6. Statistical Analysis
3. Results
3.1. Baseline, Noise and Detection Threshold
3.2. Current Consumption
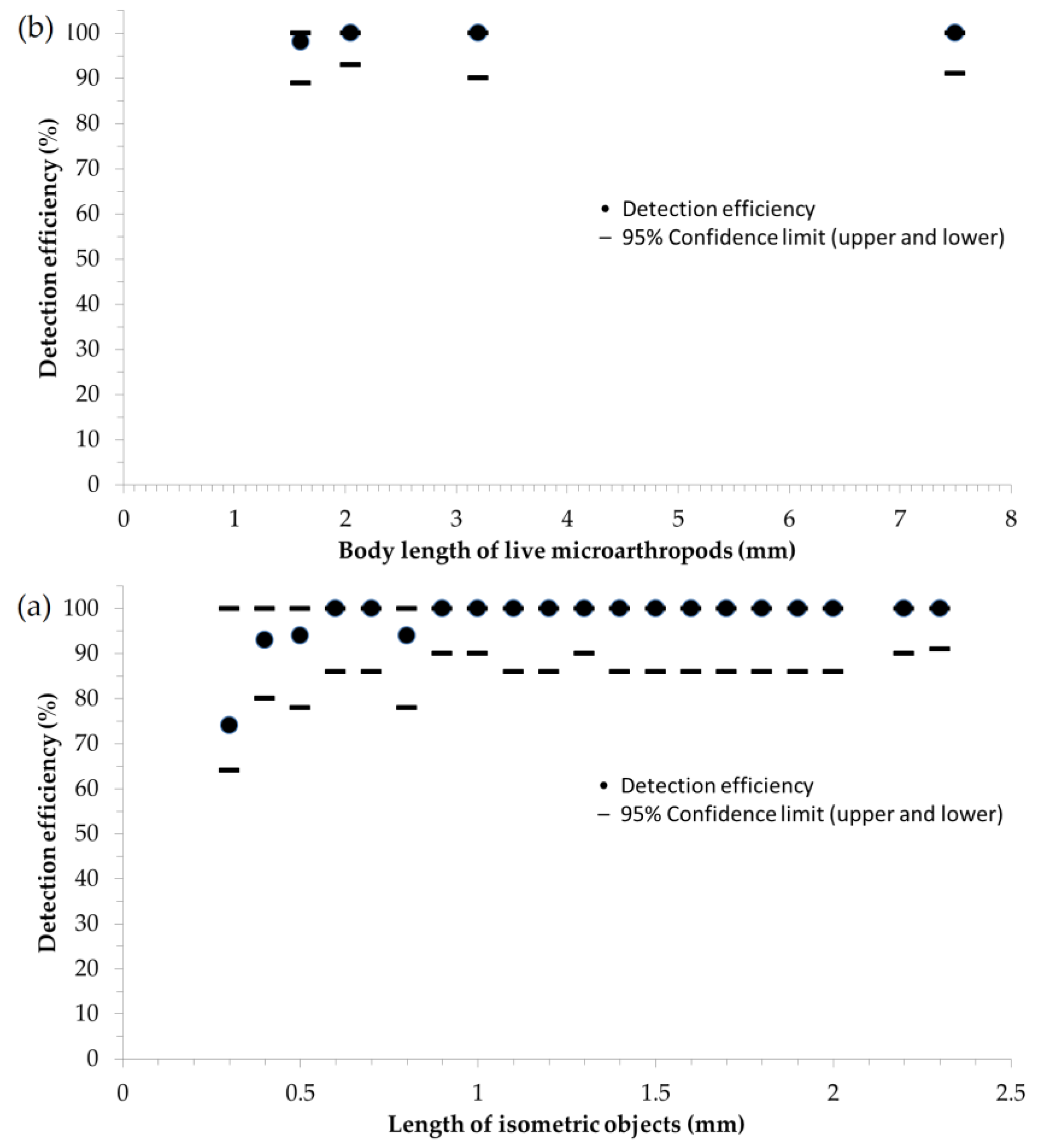
3.3. Efficiency and Minimum Threshold Level of Detection
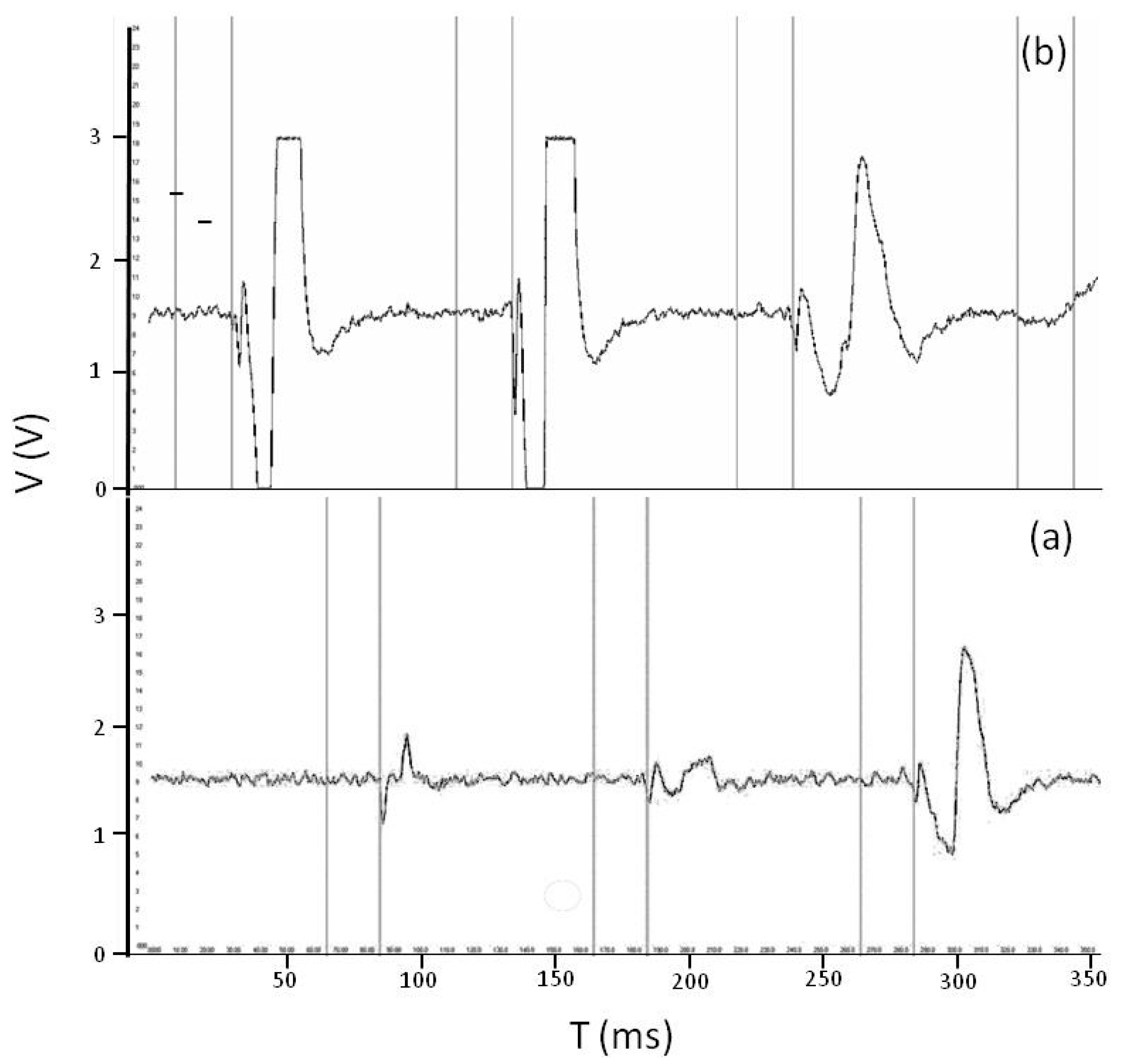
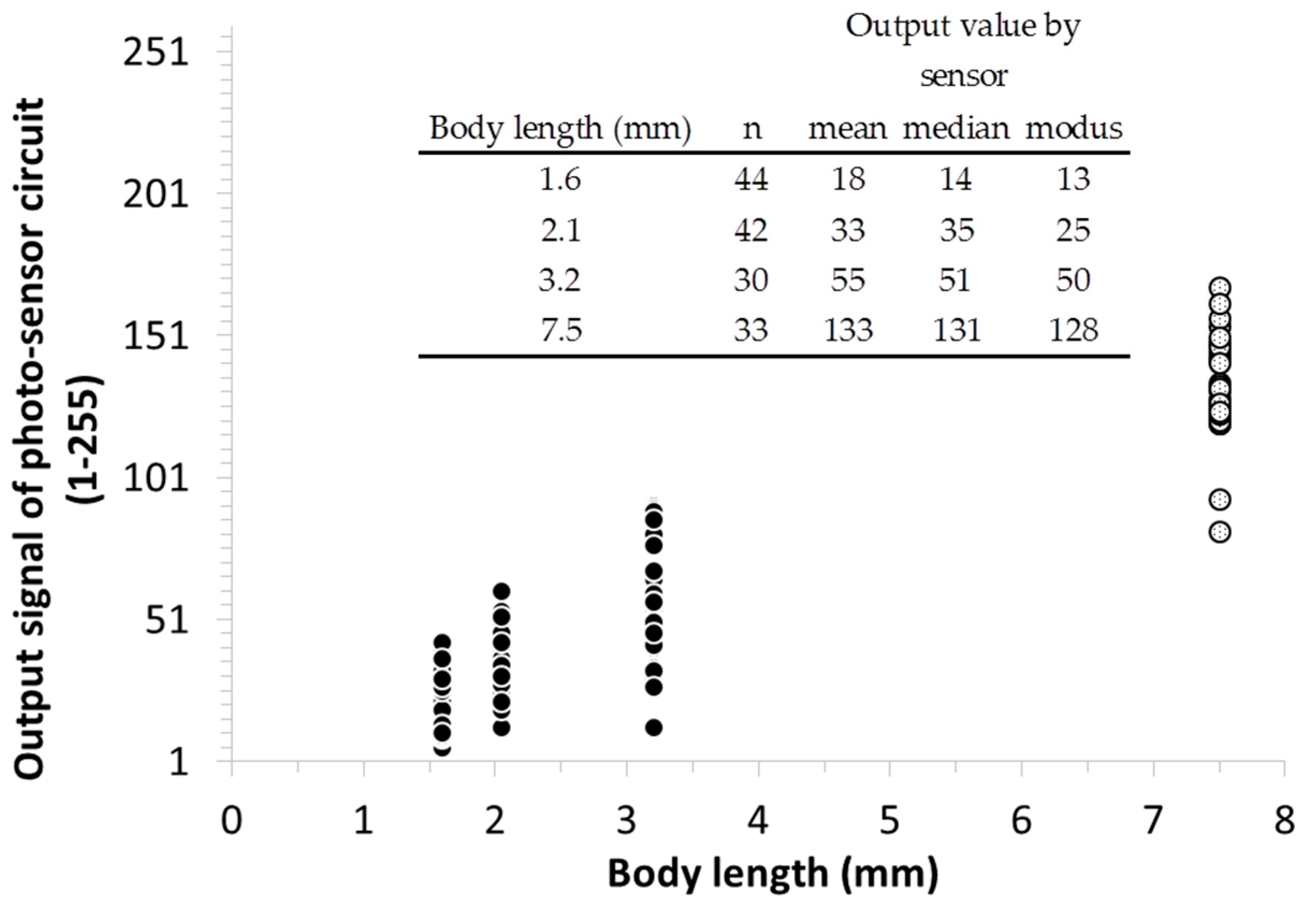
3.4. Signal Patterning during Detection and Size Estimation of Falling-In Objects
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bardgett, R.; Hopkins, D.; Usher, M. Biological Diversity and Function in Soils; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Wall, D.H.; Bardgett, R.D. Soil Ecology and Ecosystem Services; Oxford University Press: Cambridge, UK, 2012. [Google Scholar]
- Anderson, J.T.; Smith, L.M. Invertebrate response to moist-soil management of playa wetlands. Ecol. Appl. 2000, 10, 550–558. [Google Scholar] [CrossRef]
- Ettema, C.H.; Wardle, D.A. Spatial soil ecology. Trends Ecol. Evol. 2002, 17, 177–183. [Google Scholar] [CrossRef]
- Oliver, I.; Beattie, A.J. Designing a cost-effective invertebrate survey: A test of methods for rapid assessment of biodiversity. Ecol. Appl. 1996, 6, 594–607. [Google Scholar] [CrossRef]
- Eisenhauer, N.; Cesarz, S.; Koller, R.; Worm, K.; Reich, P.B. Global change belowground: Impacts of elevated CO2, nitrogen, and summer drought on soil food webs and biodiversity. Glob. Chang. Biol. 2012, 18, 435–447. [Google Scholar] [CrossRef]
- Giller, K.; Beare, M.; Lavelle, P.; Izac, A.-M.; Swift, M. Agricultural intensification, soil biodiversity and agroecosystem function. Appl. Soil Ecol. 1997, 6, 3–16. [Google Scholar] [CrossRef]
- Kardol, P.; Cregger, M.A.; Campany, C.E.; Classen, A.T. Soil ecosystem functioning under climate change: Plant species and community effects. Ecology 2010, 91, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Matson, P.A.; Parton, W.J.; Power, A.; Swift, M. Agricultural intensification and ecosystem properties. Science 1997, 277, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Gardi, C.; Montanarella, L.; Arrouays, D.; Bispo, A.; Lemanceau, P.; Jolivet, C.; Mulder, C.; Ranjard, L.; Römbke, J.; Rutgers, M. Soil biodiversity monitoring in Europe: Ongoing activities and challenges. Eur. J. Soil Sci. 2009, 60, 807–819. [Google Scholar] [CrossRef]
- Römbke, J.; Sousa, J.-P.; Schouten, T.; Riepert, F. Monitoring of soil organisms: A set of standardized field methods proposed by ISO. Eur. J. Soil Sci. 2006, 42, S61–S64. [Google Scholar] [CrossRef]
- Eisenbeis, G. Biology of soil invertebrates. In Intestinal Microorganisms of Termites and Other Invertebrates; Springer: Berlin/Heidelberg, Germany, 2006; Volume 6, pp. 3–53. [Google Scholar]
- Petersen, H.; Luxton, M. A comparative analysis of soil fauna populations and their role in decomposition processes. Oikos 1982, 39, 288–388. [Google Scholar] [CrossRef]
- Jones, J.P. Monitoring species abundance and distribution at the landscape scale. J. Appl. Ecol. 2011, 48, 9–13. [Google Scholar] [CrossRef]
- Ringold, P.L.; Alegria, J.; Czaplewski, R.L.; Mulder, B.S.; Tolle, T.; Burnett, K. Adaptive monitoring design for ecosystem management. Ecol. Appl. 1996, 6, 745–747. [Google Scholar] [CrossRef]
- Weidemann, G. Artenzahlen in den okosystemen des sollings. In Ökosystemforschung–Ergebnisse des Solling-Projekts; Verlag Eugen Ulmer: Stuttgart, Germany, 1966; Volume 198, pp. 219–225. [Google Scholar]
- Turbé, A.; de Toni, A.; Benito, P.; Lavelle, P.; Lavelle, P.; Camacho, N.R.; van der Putten, W.H.; Labouze, E.; Mudgal, S. Soil biodiversity: Functions, threats and tools for policy makers. Available online: https://hal-bioemco.ccsd.cnrs.fr/bioemco-00560420/document (accessed on 24 July 2017).
- Litzkow, C.A.; Shuman, D.; Kruss, S.; Coffelt, J.A. Electronic Grain Probe Insect Counter (EGPIC). U.S. Patent 5,646,404, 8 July 1997. [Google Scholar]
- Shuman, D.; Crompton, R.D. Sensor Output Analog Processing—A Microcontroller-Based Insect Monitoring System. U.S. Patent 6,882,279, 19 April 2005. [Google Scholar]
- Shuman, D.; Larson, R.G.; Epsky, N.D. A quantitative stored-product insect monitoring system using sensor output analog processing (SOAP). Trans. ASAE 2004, 47, 1857–1864. [Google Scholar] [CrossRef]
- Engel, J.E.; Wyttenbach, R.A. An opto-electronic sensor for monitoring small movements in insects. Fla. Entomol. 2001, 84, 336–343. [Google Scholar] [CrossRef]
- Hoffmann, W.C.; Jank, P.C.; Klun, J.A.; Fritz, B.K. Quantifying the movement of multiple insects using an optical insect counter. J. Am. Mosq. Control Assoc. 2010, 26, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Potamitis, I.; Rigakis, I.; Fysarakis, K. The electronic mcphail trap. Sensors 2014, 14, 22285–22299. [Google Scholar] [CrossRef] [PubMed]
- Potamitis, I.; Rigakis, I.; Fysarakis, K. Insect biometrics: Optoacoustic signal processing and its applications to remote monitoring of mcphail type traps. PLoS ONE 2015, 10, e0140474. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Lee, S.H.; Chahl, J.S. A review of recent sensing technologies to detect invertebrates on crops. Precis. Agric. 2016, 18, 1–32. [Google Scholar] [CrossRef]
- Dombos, M.; Kosztolányi, A.; Szlávecz, K.; Gedeon, C.; Flórián, N.; Groó, Z.; Dudás, P.; Bánszegi, O. Edapholog monitoring system: Automatic, real-time detection of soil microarthropods. Methods Ecol. Evol. 2017, 18, 635–666. [Google Scholar] [CrossRef]
- Crossley, D.; Blair, J.M. A high-efficiency, “low-technology” Tullgren-type extractor for soil microarthropods. Agric. Ecosyst. Environ. 1991, 34, 187–192. [Google Scholar] [CrossRef]
- Soil Quality—Sampling of Soil Invertebrates—Part 2: Sampling and Extraction of Microarthropods (Collembola and Acarina). Available online: https://www.iso.org/standard/37027.html (accessed on 24 July 2017).
- Querner, P.; Bruckner, A. Combining pitfall traps and soil samples to collect collembola for site scale biodiversity assessments. Appl. Soil Ecol. 2010, 45, 293–297. [Google Scholar] [CrossRef]
- Bánszegi, O.; Kosztolányi, A.; Bakonyi, G.; Szabó, B.; Dombos, M. New method for automatic body length measurement of the collembolan, Folsomia candida Willem 1902 (insecta: Collembola). PLoS ONE 2014, 9, e98230. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.; Bouchet-Valat, M.; Andronic, L.; Ash, M.; Boye, T.; Calza, S.; Chang, A.; Grosjean, P.; Heiberger, R.; Pour, K.K.; et al. R Commander. Available online: ftp://videolan.cs.pu.edu.tw/network/CRAN/web/packages/Rcmdr/Rcmdr.pdf (accessed on 24 July 2017).
- Drake, V.A.; Wang, H.K.; Harman, I.T. Insect monitoring radar: Remote and network operation. Comput. Electron. Agric. 2002, 35, 77–94. [Google Scholar] [CrossRef]
- Reynolds, D.R.; Riley, J.R. Remote-sensing, telemetric and computer-based technologies for investigating insect movement: A survey of existing and potential techniques. Comput. Electron. Agric. 2002, 35, 271–307. [Google Scholar] [CrossRef]
- Riley, J.R.; Drake, V.A. Technologies for insect movement and migration research. Comput. Electron. Agric. 2002, 35, 57–62. [Google Scholar] [CrossRef]
- Lensing, J.R.; Todd, S.; Wise, D.H. The impact of altered precipitation on spatial stratification and activity-densities of springtails (Collembola) and spiders (Araneae). Ecol. Entomol. 2005, 30, 194–200. [Google Scholar] [CrossRef]
- Schon, N.L.; Mackay, A.D.; Minor, M.A.; Yeates, G.W.; Hedley, M.J. Soil fauna in grazed New Zealand hill country pastures at two management intensities. Appl. Soil Ecol. 2008, 40, 218–228. [Google Scholar] [CrossRef]
- Mercer, R.D.; Gabriel, A.G.A.; Barendse, J.; Marshall, D.J.; Chown, S.L. Invertebrate body sizes from Marion Island. Antarct. Sci. 2001, 13, 135–143. [Google Scholar] [CrossRef]
- Tamura, H. A method for the measurement of curled body length of Collembola. In Revue D’écologie Et de Biologie Du Sol.; Gauthier-Villars: Paris, France, 1964. [Google Scholar]
- Salmon, S.; Ponge, J.F.; Gachet, S.; Deharveng, L.; Lefebvre, N.; Delabrosse, F. Linking species, traits and habitat characteristics of Collembola at European scale. Soil Biol. Biochem. 2014, 75, 73–85. [Google Scholar] [CrossRef]
- Ojala, R.; Huhta, V. Dispersal of microarthropods in forest soil. Pedobiologia 2001, 45, 443–450. [Google Scholar] [CrossRef]
- Dunger, W.; Schulz, H.J.; Zimdars, B. Colonization behaviour of Collembola under different conditions of dispersal, Proceedings of the Xth international Colloquium on Apterygota, České Budějovice 2000: Apterygota at the Beginning of the Third Millennium. Pedobiologia 2002, 46, 316–327. [Google Scholar]
- Ingimarsdóttir, M.; Caruso, T.; Ripa, J.; Magnúsdóttir, Ó.B.; Migliorini, M.; Hedlund, K. Primary assembly of soil communities: Disentangling the effect of dispersal and local environment. Oecologia 2012, 170, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sabu, T.K.; Nithya, S.; Vinod, K. Faunal survey, endemism and possible species loss of Scarabaeinae (Coleoptera: Scarabaeidae) in the western slopes of the moist south western ghats, south India. Zootaxa 2011, 2830, 29–38. [Google Scholar]


| Threshold Level (mV) | Average Number of False Detections per Two Hours during 24 h | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–2 h | 2–4 h | 4–6 h | 6–8 h | 8–10 h | 10–12 h | 12–14 h | 14–16 h | 16–18 h | 18–20 h | 20–22 h | 22–24 h | |
| 70 | 20 | 17 | 22 | 18 | 24 | 19 | 16 | 23 | 22 | 17 | 20 | 24 |
| 82 | 4 | 6 | 2 | 5 | 3 | 7 | 5 | 6 | 4 | 5 | 2 | 3 |
| 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gedeon, C.I.; Flórián, N.; Liszli, P.; Hambek-Oláh, B.; Bánszegi, O.; Schellenberger, J.; Dombos, M. An Opto-Electronic Sensor for Detecting Soil Microarthropods and Estimating Their Size in Field Conditions. Sensors 2017, 17, 1757. https://doi.org/10.3390/s17081757
Gedeon CI, Flórián N, Liszli P, Hambek-Oláh B, Bánszegi O, Schellenberger J, Dombos M. An Opto-Electronic Sensor for Detecting Soil Microarthropods and Estimating Their Size in Field Conditions. Sensors. 2017; 17(8):1757. https://doi.org/10.3390/s17081757
Chicago/Turabian StyleGedeon, Csongor I., Norbert Flórián, Péter Liszli, Beáta Hambek-Oláh, Oxána Bánszegi, Judit Schellenberger, and Miklós Dombos. 2017. "An Opto-Electronic Sensor for Detecting Soil Microarthropods and Estimating Their Size in Field Conditions" Sensors 17, no. 8: 1757. https://doi.org/10.3390/s17081757





