A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-μm CMOS Technology for Cancer Biomarker Detection
Abstract
:1. Introduction
2. Materials and Capacitive Sensing Element
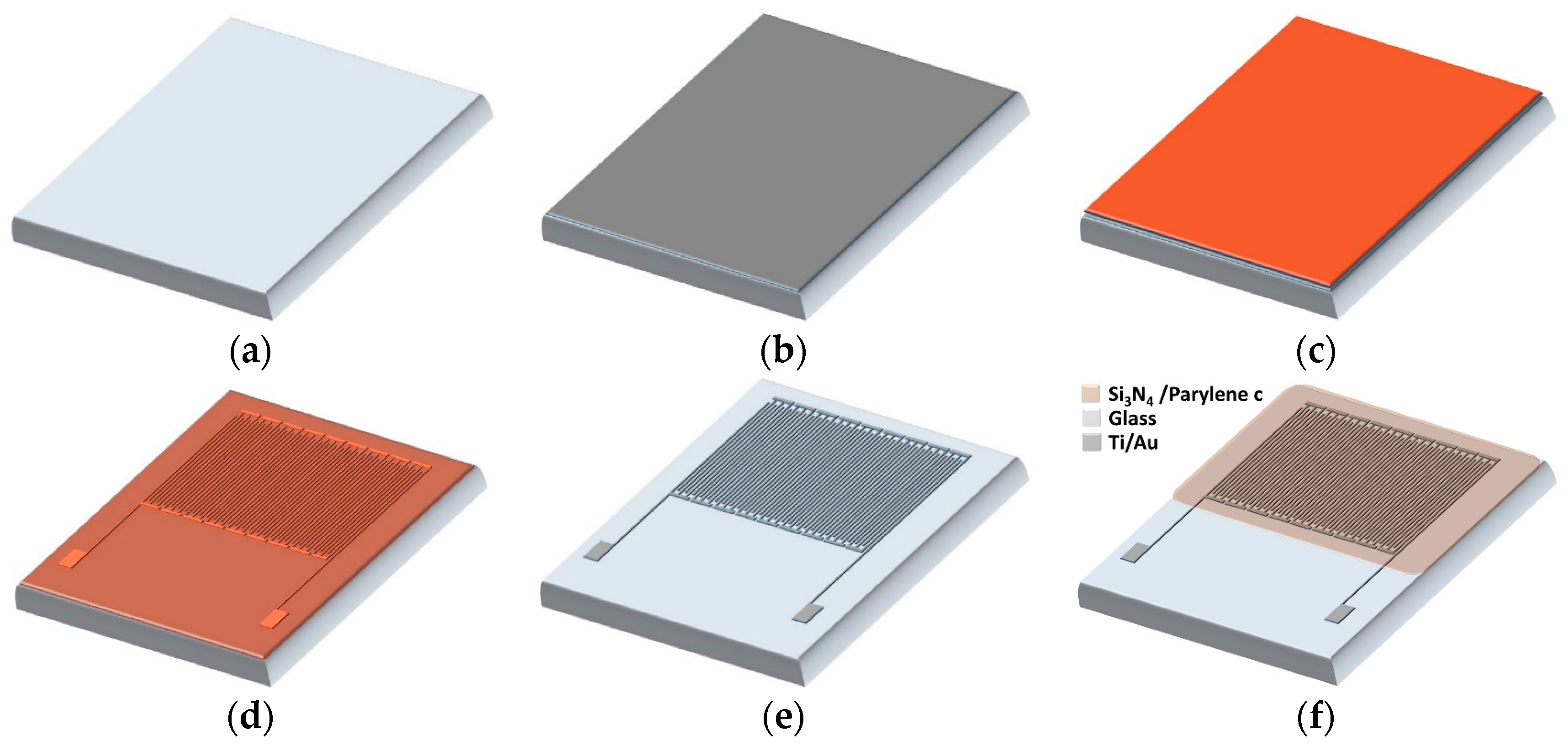
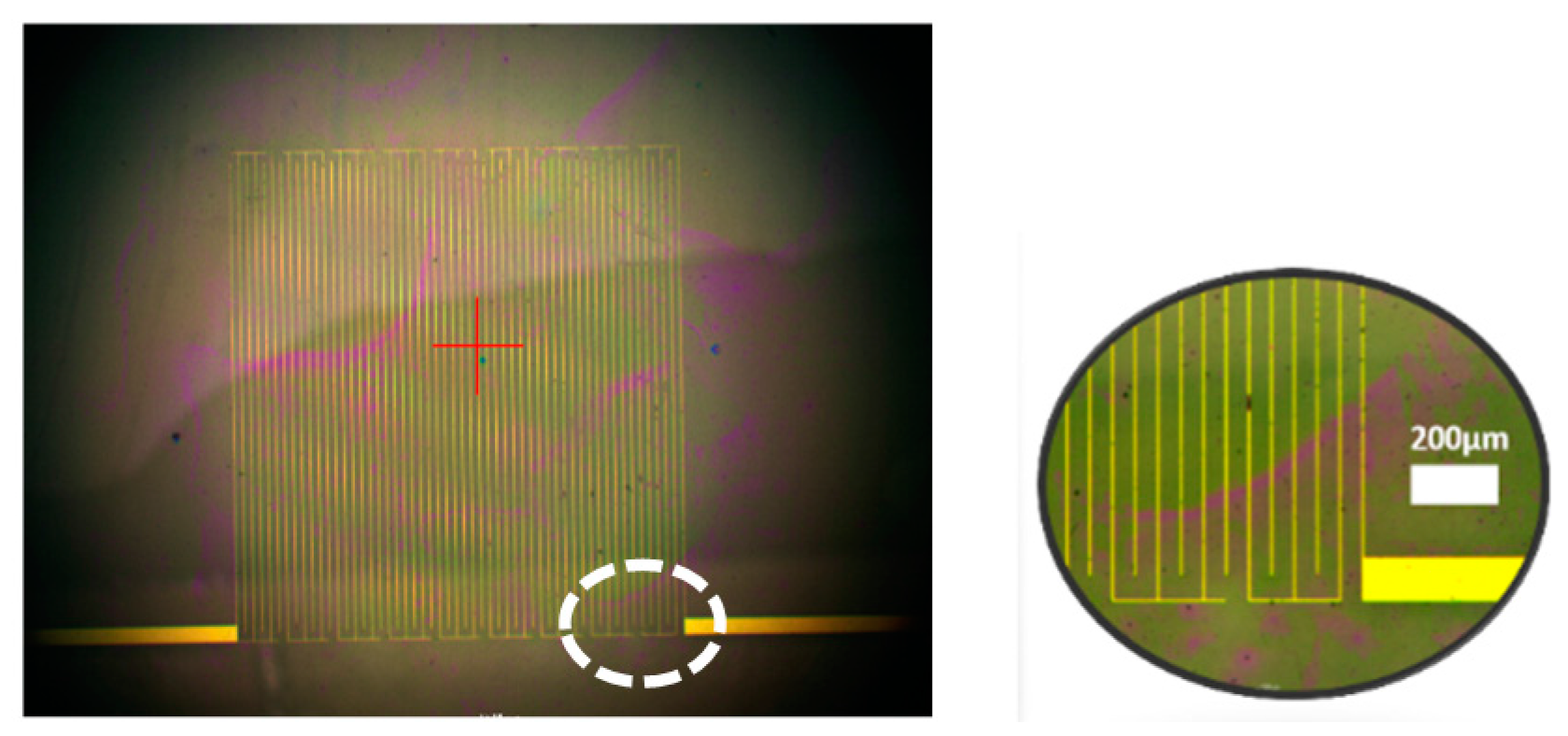
2.1. Capacitive Biosensor
2.2. Sample Sites
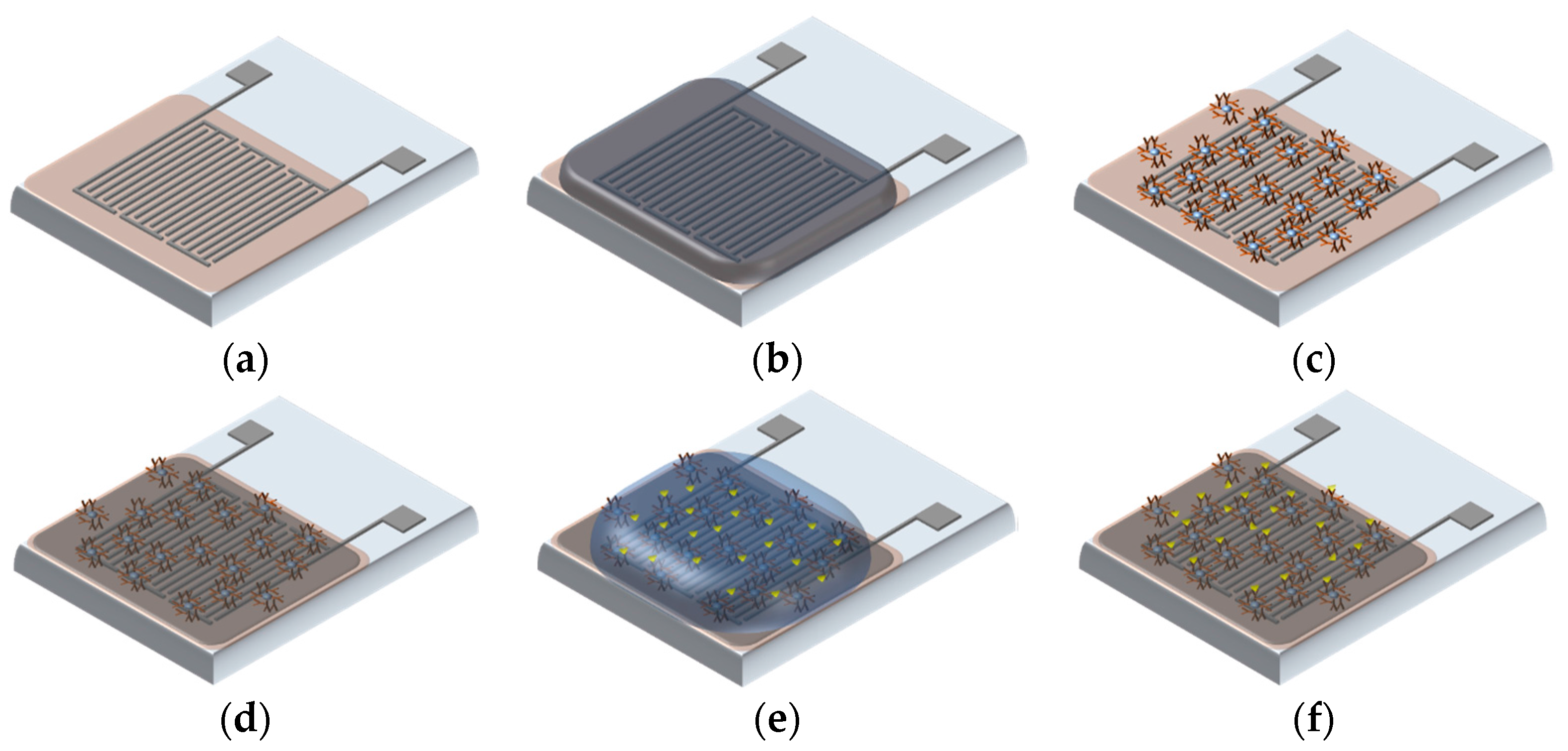
2.3. Surface Modification and Antibody immobilization
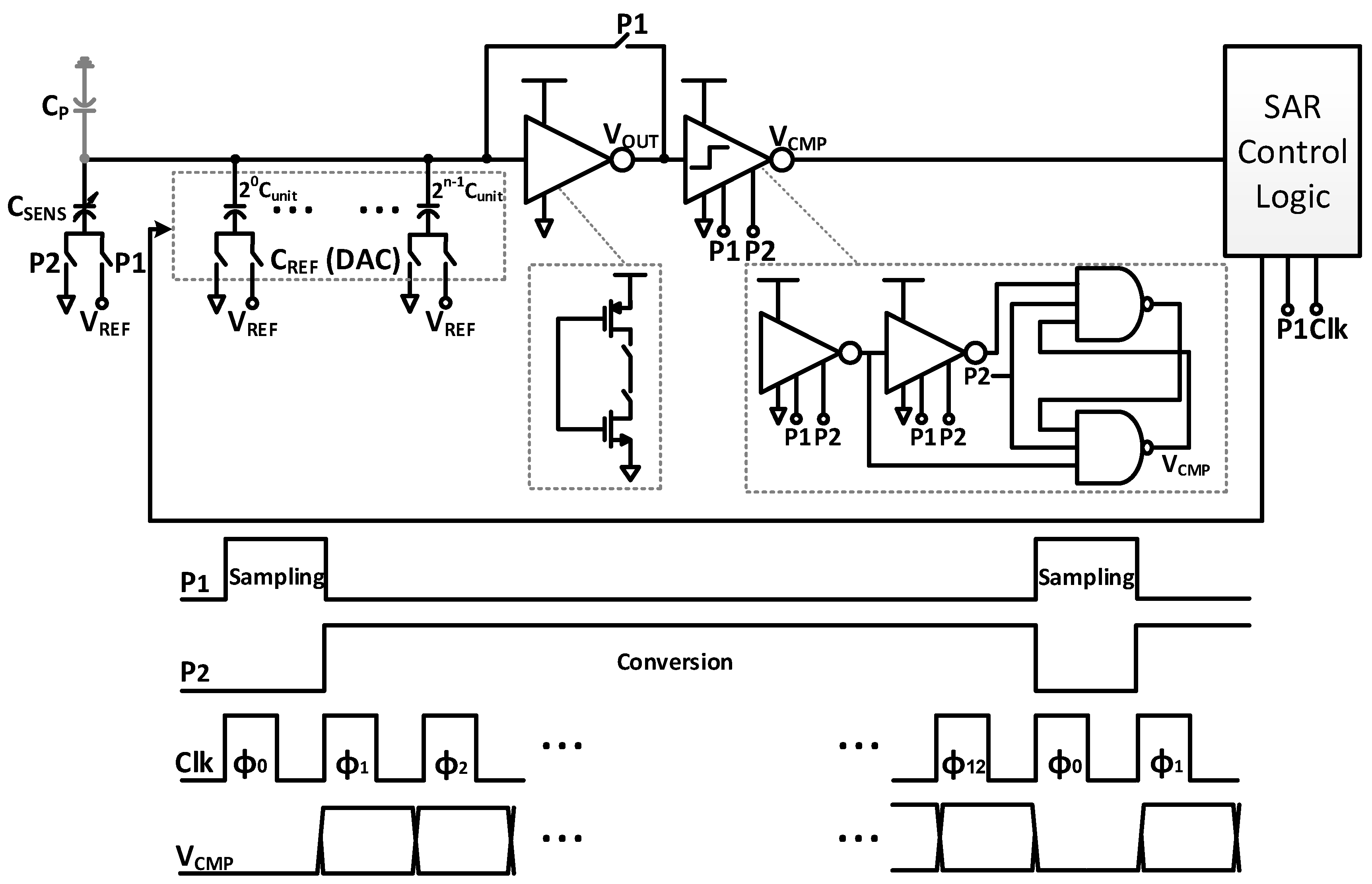
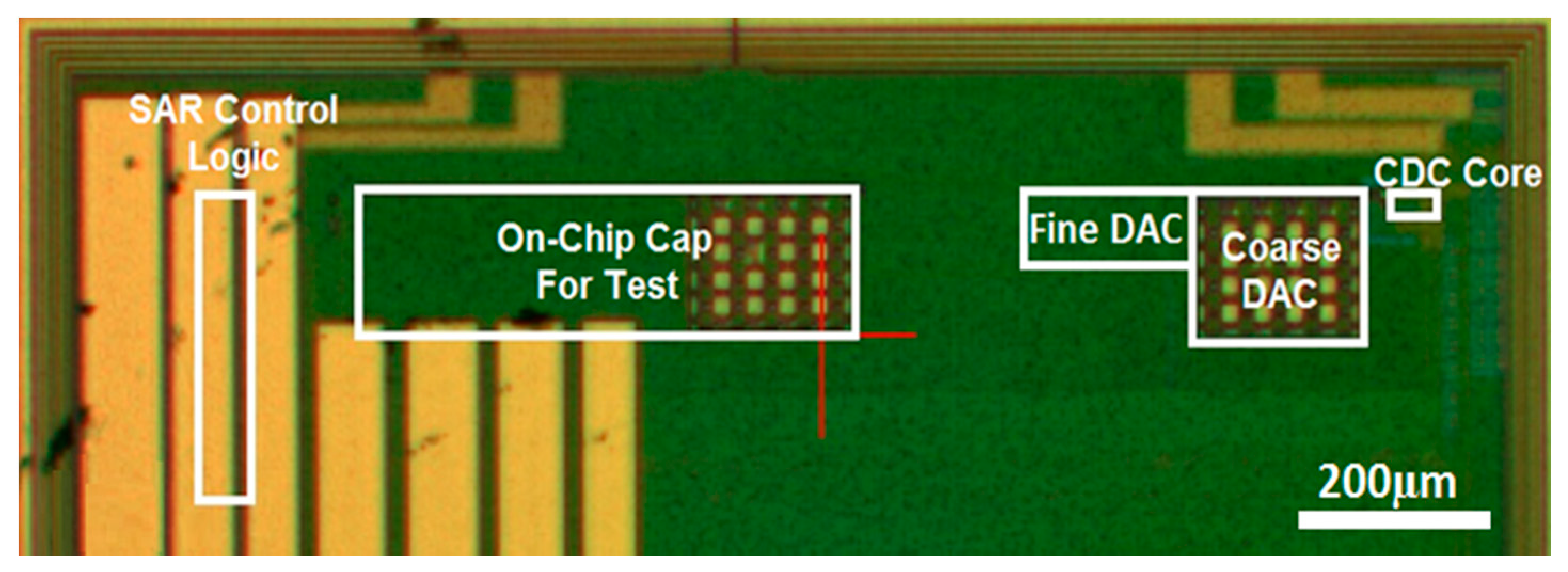
3. Successive Approximation Readout Circuit
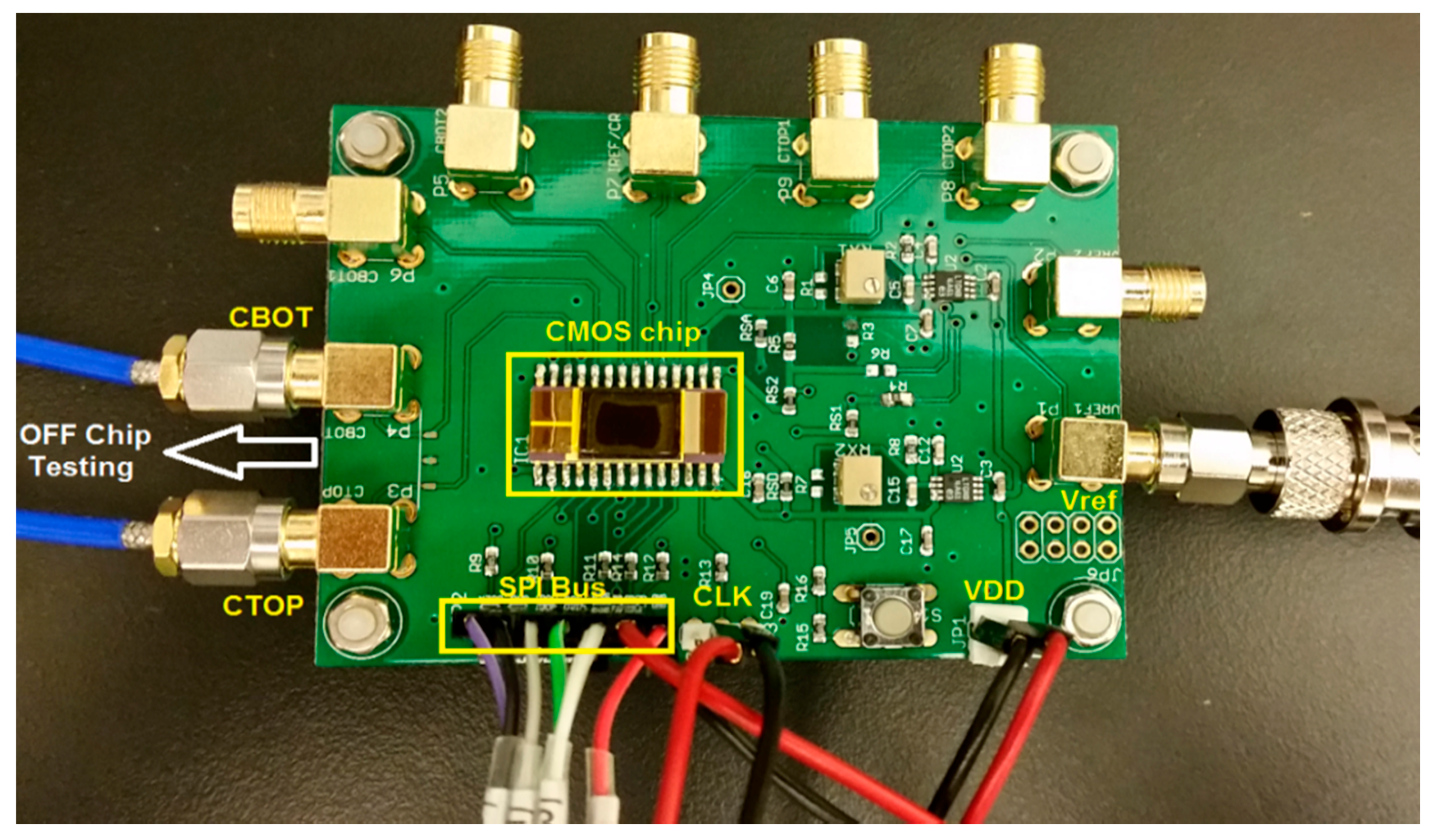
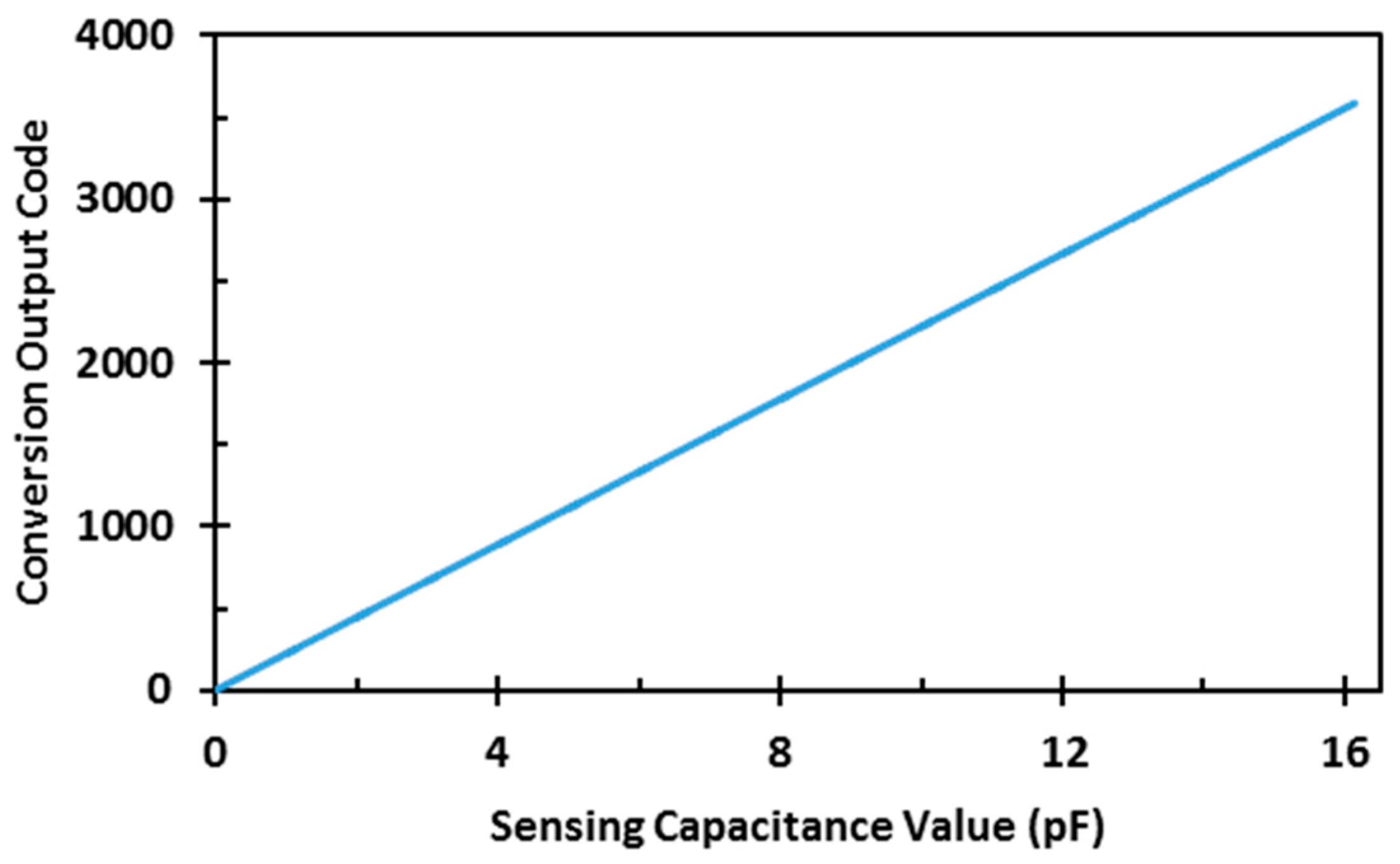
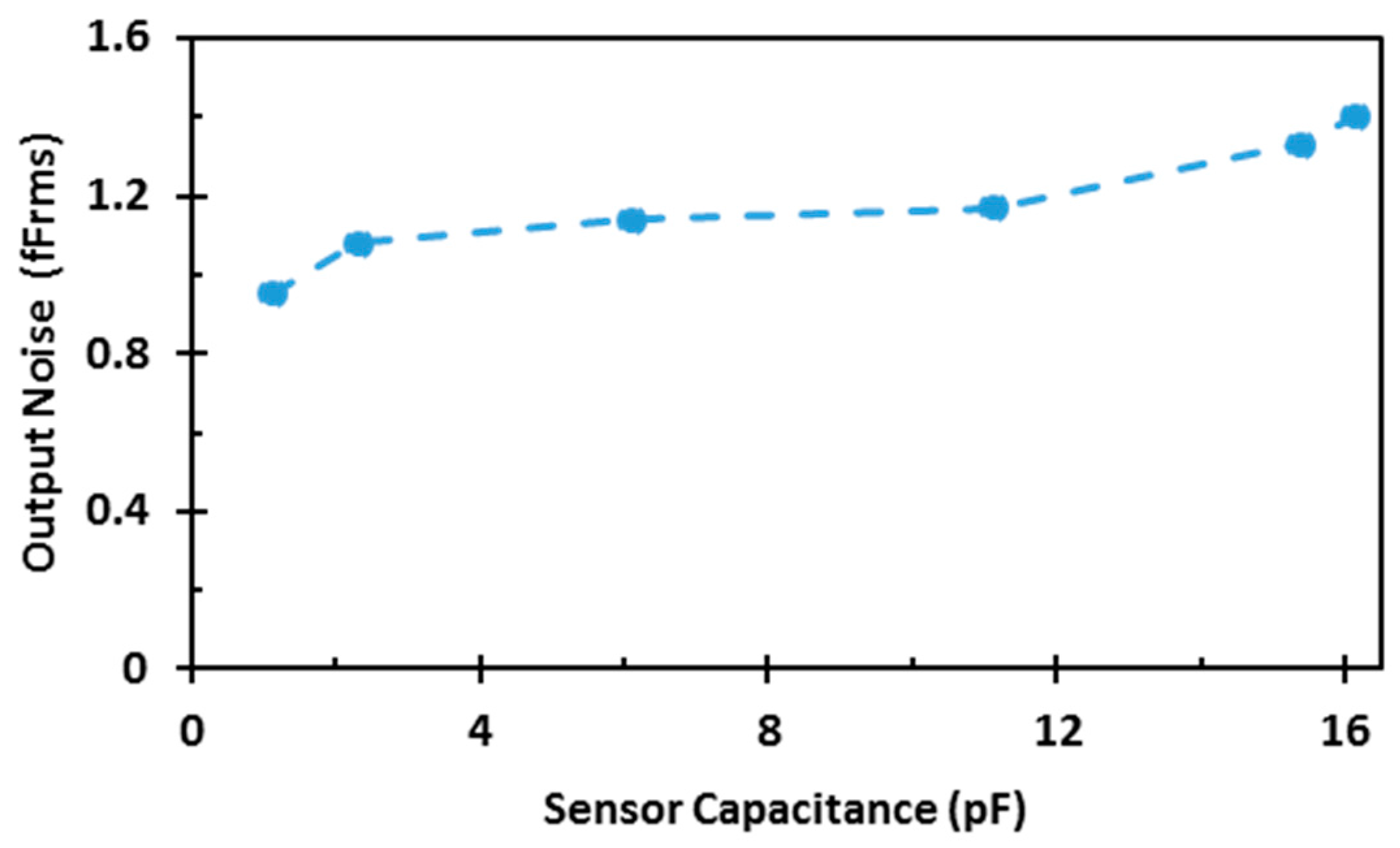
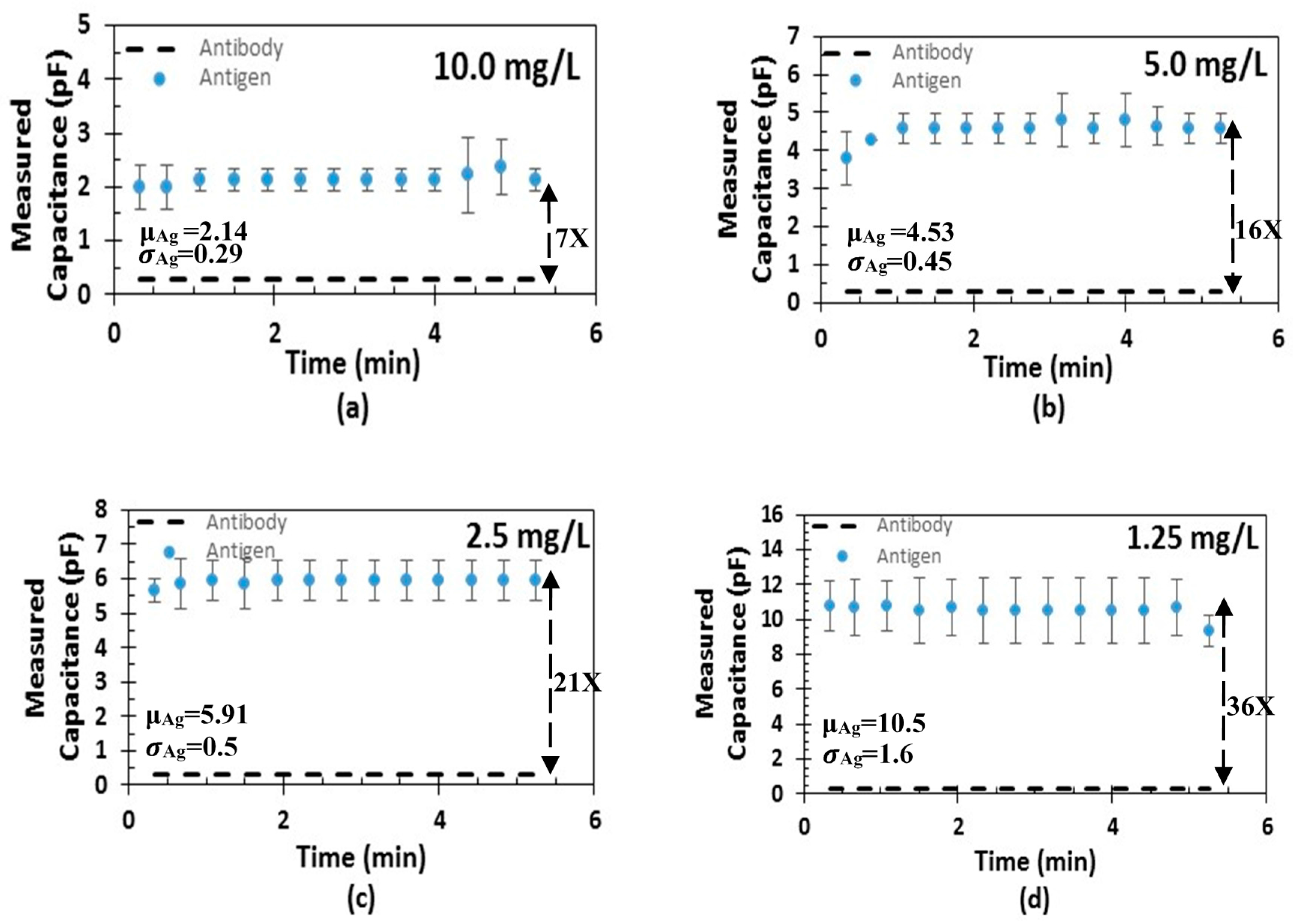
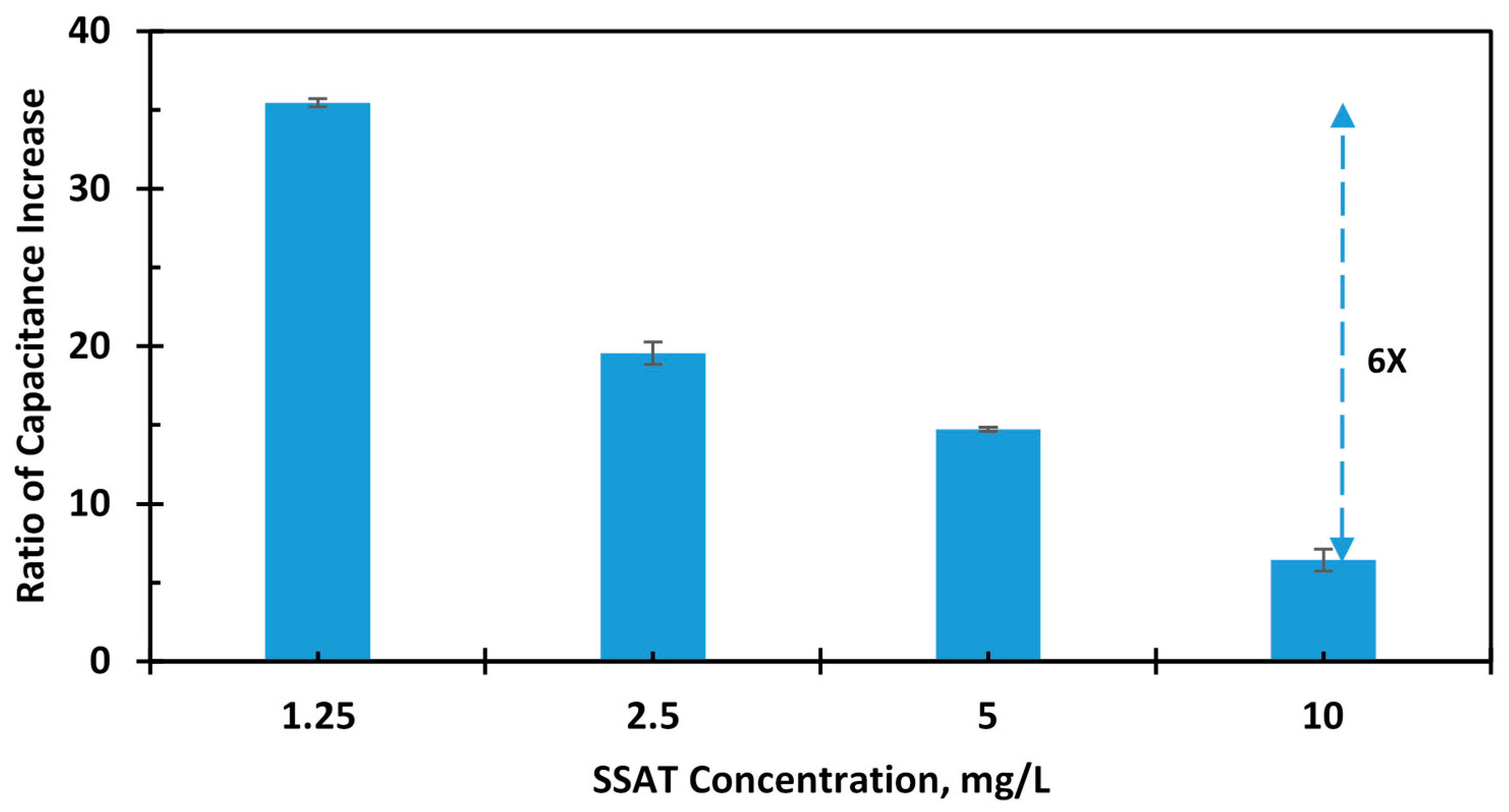
4. Measurement Results
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Okeke, I.N. Diagnostic insufficiency in Africa. Clin. Infect. Dis. 2006, 42, 1501–1503. [Google Scholar] [CrossRef] [PubMed]
- Serkova, N.J.; Glunde, K. Metabolomics of cancer. In Tumor Biomarker Discovery: Methods and Protocols; Tainsky, M.A., Ed.; Humana Press: Totowa, NJ, USA, 2009; pp. 273–295. [Google Scholar]
- Sitar, D.S.; Bras, A.P.M. Method for Assaying Non-Spermine/Spermidine Activity of Spermidine/Spermine N1-acetyltransferase (SSAT). U.S. Patent 6811967 B2, 2 November 2004. [Google Scholar]
- Vo-Dinh, T.; Allain, L.R.; Stokes, D.L. Cancer gene detection using surface-enhanced Raman scattering (SERS). J. Raman Spectrosc. 2002, 33, 511–516. [Google Scholar] [CrossRef]
- Yu, W.W.; White, I.M. A simple filter-based approach to surface enhanced Raman spectroscopy for trace chemical detection. Analyst 2012, 137, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Hajisalem, G.; Li, W.; Hof, F.; Gordon, R. Quantification of an exogenous cancer biomarker in urinalysis by Raman Spectroscopy. Analyst 2014, 139, 5375–5378. [Google Scholar] [CrossRef] [PubMed]
- Soda, K. The mechanisms by which polyamines accelerate tumor spread. J. Exp. Clin. Cancer Res. 2011, 30, 95. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Choi, S.; Lee, J.O.; Yoon, J.B.; Cho, G.H. CMOS capacitive biosensor with enhanced sensitivity for label-free DNA detection. In Proceedings of the 2012 IEEE International Solid-State Circuits Conference, San Francisco, CA, USA, 19–23 February 2012. [Google Scholar]
- Pai, A.; Khachaturian, A.; Chapman, S.; Hu, A.; Wang, H.; Hajimiri, A. A handheld magnetic sensing platform for antigen and nucleic acid detection. Analyst 2014, 139, 1403–1411. [Google Scholar] [CrossRef] [PubMed]
- Norian, H.; Field, R.M.; Kymissis, I.; Shepard, K.L. An integrated CMOS quantitative-polymerase-chain-reaction lab-on-chip for point-of-care diagnostics. Lab Chip 2014, 14, 4076–4084. [Google Scholar] [CrossRef] [PubMed]
- Manickam, A.; Chevalier, A.; McDermott, M.; Ellington, A.D.; Hassibi, A. A CMOS Electrochemical Impedance Spectroscopy (EIS) biosensor array. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Gambini, S.; Skucha, K.; Liu, P.P.; Kim, J.; Krigel, R. A 10 kPixel CMOS hall sensor array with baseline suppression and parallel readout for immunoassays. IEEE J. Solid State Circuits 2013, 48, 302–317. [Google Scholar] [CrossRef]
- Hong, L.; McManus, S.; Yang, H.; Sengupta, K. A fully integrated CMOS fluorescence biosensor with on-chip nanophotonic filter. In Proceedings of the 2015 Symposium on VLSI Circuits (VLSI Circuits), Kyoto, Japan, 16–19 June 2015. [Google Scholar]
- Berggren, C.; Bjarnason, B.; Johansson, G. An immunological Interleukine-6 capacitive biosensor using perturbation with a potentiostatic step. Biosens. Bioelectron. 1998, 13, 1061–1068. [Google Scholar] [CrossRef]
- Carrara, S.; Bhalla, V.; Stagni, C.; Benini, L.; Ferretti, A.; Valle, F.; Samorì, B. Label-free cancer markers detection by capacitance biochip. Sens. Actuators B Chem. 2009, 136, 163–172. [Google Scholar] [CrossRef]
- Prakash, S.B.; Abshire, P. Tracking cancer cell proliferation on a CMOS capacitance sensor chip. Biosens. Bioelectron. 2008, 23, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mahdavi, A.; Tirrell, D.A.; Hajimiri, A. A magnetic cell-based sensor. Lab Chip 2012, 12, 4465–4471. [Google Scholar] [CrossRef] [PubMed]
- Dupont, E.P.; Labonne, E.; Maruyama, Y.; Vandevyver, C.; Lehmann, U.; Gijs, M.A.; Charbon, E. Fluorescent magnetic bead and cell differentiation/counting using a CMOS SPAD matrix. Sens. Actuators B Chem. 2012, 174, 609–615. [Google Scholar] [CrossRef]
- Baker, K.L.; Bolger, F.B.; Lowry, J.P. Development of a microelectrochemical biosensor for the real-time detection of choline. Sens. Actuators B Chem. 2017, 243, 412–420. [Google Scholar] [CrossRef]
- Oseev, A.; Schmidt, M.P.; Hirsch, S.; Brose, A.; Schmidt, B. Two-component dielectric dispersion impedance biosensor for in-line protein monitoring. Sens. Actuators B Chem. 2017, 239, 1213–1220. [Google Scholar] [CrossRef]
- Hall, D.A.; Gaster, R.S.; Makinwa, K.A.A.; Wang, S.X.; Murmann, B. A 256 pixel magnetoresistive biosensor microarray in 0.18 μm CMOS. IEEE J. Solid State Circuits 2013, 48, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Sivashankar, S.; Sapsanis, C.; Buttner, U.; Salama, K.N. Flexible low-cost cardiovascular risk marker biosensor for point-of-care applications. Electron. Lett. 2015, 51, 1746–1748. [Google Scholar] [CrossRef]
- Mashraei, Y.; Sivashankar, S.; Buttner, U.; Salama, K.N. Integration of fractal biosensor in a digital microfluidic platform. IEEE Sens. J. 2016, 16, 8775–8783. [Google Scholar] [CrossRef]
- Jung, W.; Jeong, S.; Oh, S.; Sylvester, D.; Blaauw, D. 27.6 A 0.7pF-to-10nF fully digital capacitance-to-digital converter using iterative delay-chain discharge. In Proceedings of the 2015 IEEE International Solid-State Circuits Conference—(ISSCC), San Francisco, CA, USA, 22–26 February 2015. [Google Scholar]
- Oh, S.; Jung, W.; Yang, K.; Blaauw, D.; Sylvester, D. 15.4b Incremental Sigma-Delta Capacitance-to-Digital Converter with Zoom-in 9b Asynchronous SAR; VLSI Circuits Digest of Technical Papers; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2014; pp. 1–2. [Google Scholar]
- Yuming, H.; Zu-yao, C.; Pakula, L.; Shalmany, S.H.; Pertijs, M. 27.7 A 0.05 mm2 1V capacitance-to-digital converter based on period modulation. In Proceedings of the 2015 IEEE International Solid-State Circuits Conference—(ISSCC), San Francisco, CA, USA, 22–26 February 2015. [Google Scholar]
- Alhoshany, A.; Omran, H.; Salama, K.N. A 45.8 fJ/Step, energy-efficient, differential SAR capacitance-to-digital converter for capacitive pressure sensing. Sens. Actuators A Phys. 2016, 245, 10–18. [Google Scholar] [CrossRef]
- Omran, H.; Alhoshany, A.; Alahmadi, H.; Salama, K.N. A 33fJ/Step SAR capacitance-to-digital converter using a chain of inverter-based amplifiers. IEEE Trans. Circuits Syst. I Regul. Papers 2017, 64, 310–321. [Google Scholar] [CrossRef]
- Sanjurjo, J.P.; Prefasi, E.; Buffa, C.; Gaggl, R. An energy-efficient 17-bit noise-shaping Dual-Slope Capacitance-to-Digital Converter for MEMS sensors. In Proceedings of the ESSCIRC Conference 2016: 42nd European Solid-State Circuits Conference, Lausanne, Switzerland, 12–15 September 2016. [Google Scholar]
- Santa Cruz Biotechnology, Inc. Available online: https://www.scbt.com (accessed on 23 August 2017).
- Terlingen, J.G.A.; Jan, F.; Hoffman, A.S. Immobilization of surface active compounds on polymer supports using a gas discharge process. J. Biomater. Sci. Polym. Ed. 1993, 4, 31–33. [Google Scholar] [CrossRef]
- Kim, D.; Herr, A.E. Protein immobilization techniques for microfluidic assays. Biomicrofluidics 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Sapsanis, C.; Sivashankar, S.; Omran, H.; Buttner, U.; Salama, K.N. Capacitive immunosensor for C-reactive protein quantification. In Proceedings of the 2015 IEEE 58th International Midwest Symposium on Circuits and Systems (MWSCAS), Fort Collins, CO, USA, 2–5 August 2015. [Google Scholar]
- Sapsanis, C.; Buttner, U.; Omran, H.; Belmabkhout, Y.; Shekhah, O.; Eddaoudi, M.; Salama, K.N. A nafion coated capacitive humidity sensor on a flexible PET substrate. In Proceedings of the 2016 IEEE 59th International Midwest Symposium on Circuits and Systems (MWSCAS), Abu Dhabi, UAE, 16–19 October 2016. [Google Scholar]
- Yabuki, S. How to lengthen the long-term stability of enzyme membranes: Trends and strategies. Catalysts 2017, 7, 36. [Google Scholar] [CrossRef]
- Mank-Seymour, A.R.; Murray, T.R.; Berkey, K.A.; Xiao, L.; Kern, S.; Casero, R.A. Two active copies of the X-linked gene spermidine/spermine N-1 acetyltransferase (SSAT) in a female lung cancer cell line are associated with an increase in sensitivity to an antitumor polyamine analogue. Clin. Cancer Res. 1998, 4, 2003–2008. [Google Scholar] [PubMed]
- Pledgie-Tracy, A.; Billam, M.; Hacker, A.; Sobolewski, M.D.; Woster, P.M.; Zhang, Z.; Casero, R.A.; Davidson, N.E. The role of the polyamine catabolic enzymes SSAT and SMO in the synergistic effects of standard chemotherapeutic agents with a polyamine analogue in human breast cancer cell lines. Cancer Chemother. Pharmacol. 2010, 65, 1067–1081. [Google Scholar] [CrossRef] [PubMed]
- Babbar, N.; Gerner, E.W.; Casero, R.A., Jr. Induction of spermidine/spermine N1-acetyltransferase (SSAT) by aspirin in Caco-2 colon cancer cells. Biochem. J. 2006, 394, 317–324. [Google Scholar] [CrossRef] [PubMed]


| Technology | CMOS 180 nm |
|---|---|
| Power Supply (Analog/Digital) | 0.9 V/1 V |
| Absolute Resolution | 4.5 fF |
| Capacitance Range | 16.137 pF |
| Noise (rms) | <1.4 fF |
| Power (Analog) | 1.8 μW |
| Power (Digital) | 0.3 μW |
| Resolution | 12 Bits |
| Measurement Time | 42.5 μs |
| Reference | BIOCAS 2010 [11] | JSSC 2013 [12] | VLSI 2015 [13] | JSSC 2013 [21] | This Work |
|---|---|---|---|---|---|
| Target | Protein | Protein | Protein | Protein | Protein |
| Technology (nm) | 350 | 180 | 65 | 180 | 180 |
| Sensing Parameter | Impedance | Magnetism | Fluorescence | Magnetism | Capacitive |
| Target Protein | Protein G | Human serum albumin | Streptavidin | SLPI cancer marker | SSAT enzyme |
| Transducer | Au electrodes | Hall sensor | Photodetector | GMR sensor | Au electrodes |
| Labeling | Label-free | Magnetic particle | Qdot 800 fluorophore | Magnetic particle | Label-free |
| Immobilization | Yes | Yes | Yes | Yes | Yes |
| Power | 84.8 mW | 300 mW | 66 mW | 50.4 mW | 2.1 μW |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhoshany, A.; Sivashankar, S.; Mashraei, Y.; Omran, H.; Salama, K.N. A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-μm CMOS Technology for Cancer Biomarker Detection. Sensors 2017, 17, 1942. https://doi.org/10.3390/s17091942
Alhoshany A, Sivashankar S, Mashraei Y, Omran H, Salama KN. A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-μm CMOS Technology for Cancer Biomarker Detection. Sensors. 2017; 17(9):1942. https://doi.org/10.3390/s17091942
Chicago/Turabian StyleAlhoshany, Abdulaziz, Shilpa Sivashankar, Yousof Mashraei, Hesham Omran, and Khaled N. Salama. 2017. "A Biosensor-CMOS Platform and Integrated Readout Circuit in 0.18-μm CMOS Technology for Cancer Biomarker Detection" Sensors 17, no. 9: 1942. https://doi.org/10.3390/s17091942





