Size Controlled Copper (I) Oxide Nanoparticles Influence Sensitivity of Glucose Biosensor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
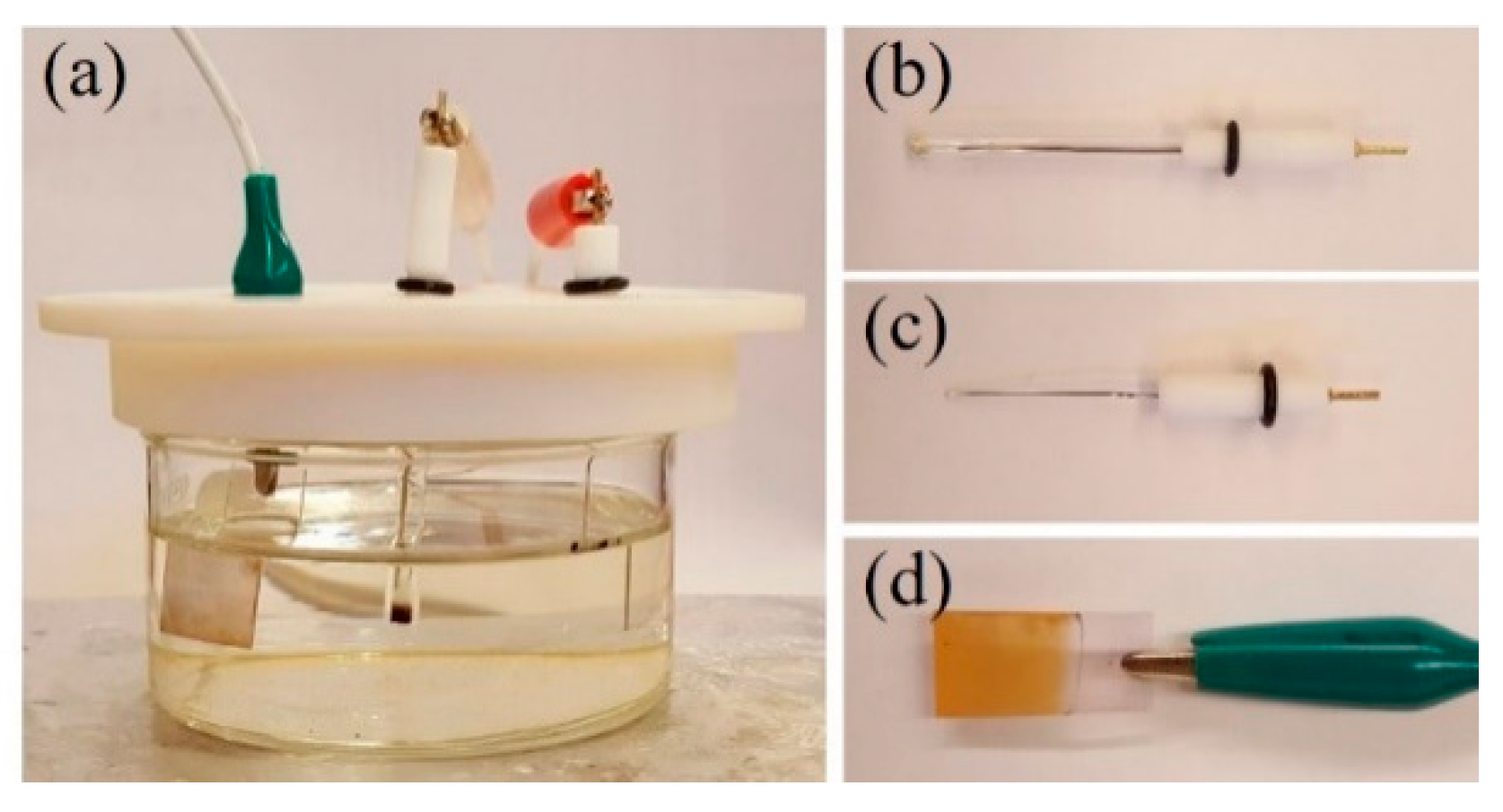
2.2. Fabrication of Cu2O Electrode via Electrodeposition
2.3. Fabrication of Enzymatic Biosensor
2.4. Characterization
3. Results and Discussion
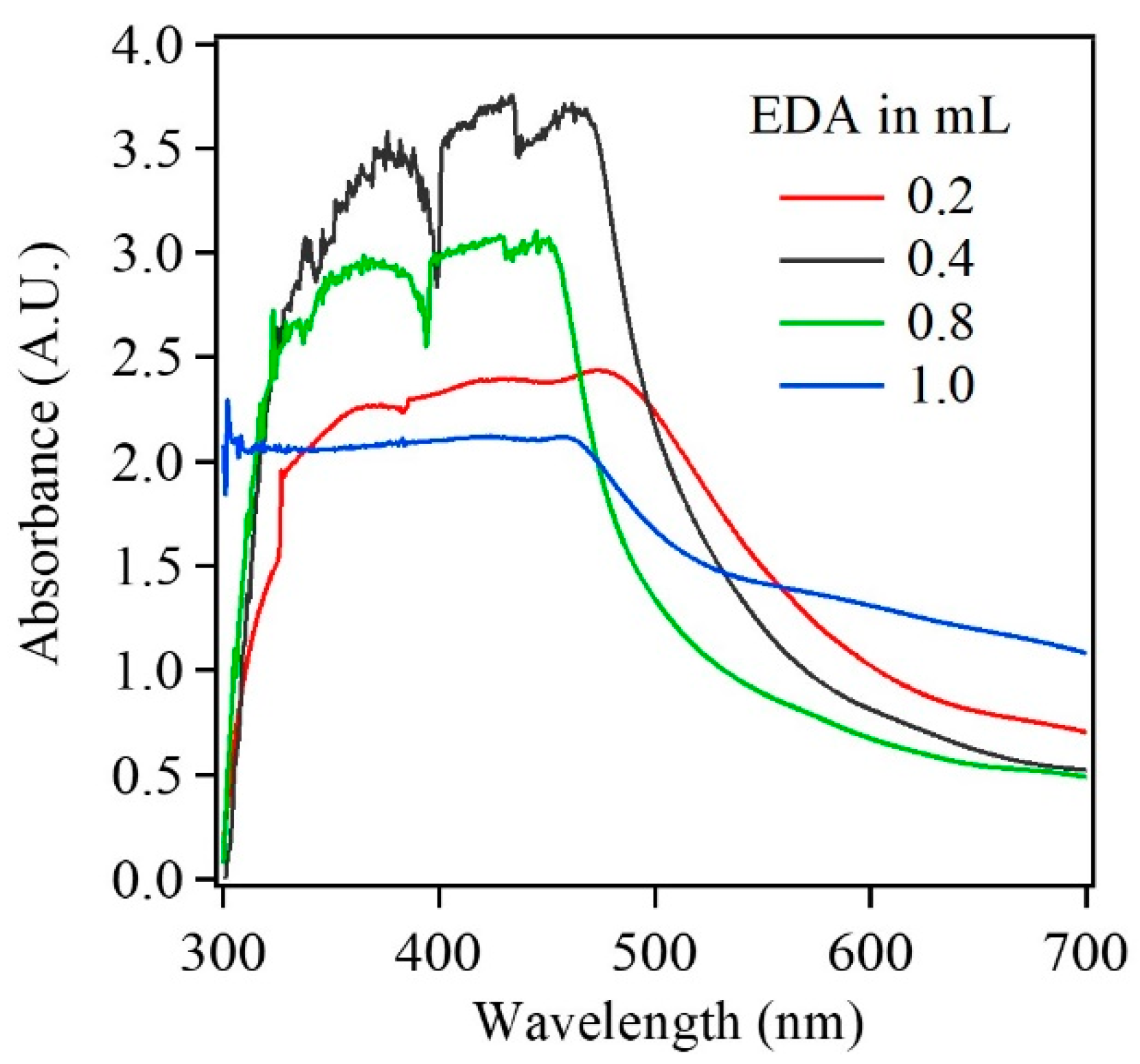
3.1. Characterization of Electrodeposited Cu2O Nanoparticles
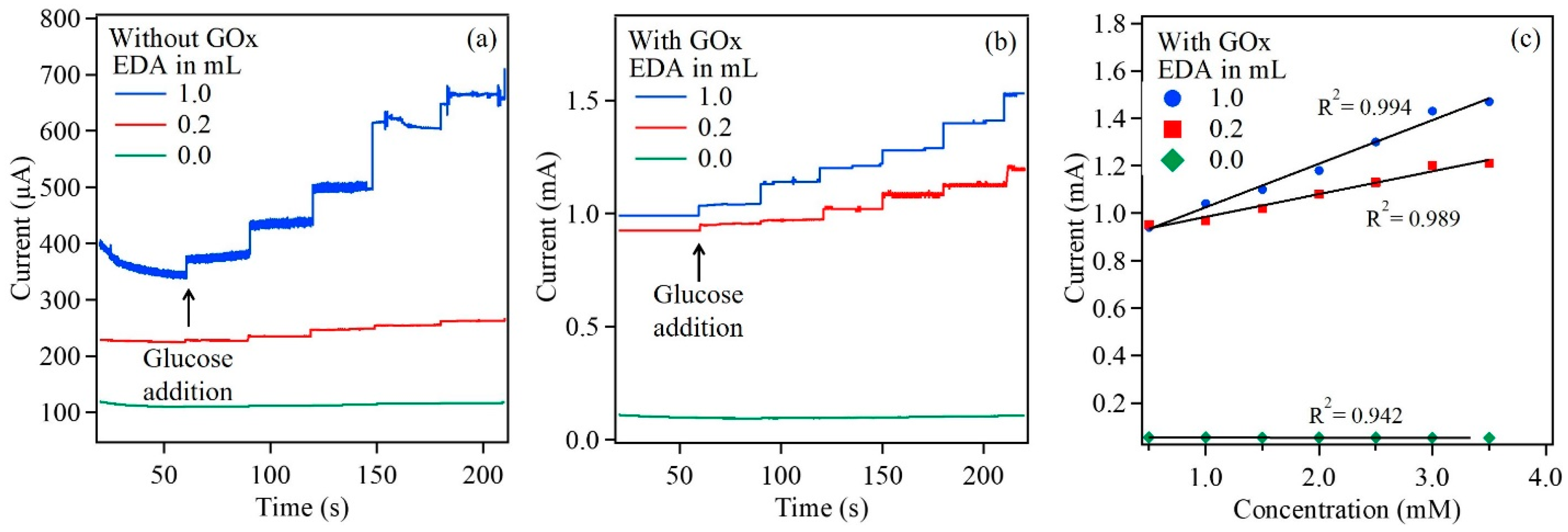
3.2. Cu2O Nanoparticles as Biosensing Platform
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Siripala, W.; Ivanovskaya, A.; Jaramillo, T.F.; Baeck, S.-H.; McFarland, E.W. A Cu2O/TiO2 heterojunction thin film cathode for photoelectrocatalysis. Sol. Energy Mater. Sol. Cells 2003, 77, 229–237. [Google Scholar] [CrossRef]
- Xiang, J.Y.; Tu, J.P.; Huang, X.H.; Yang, Y.Z. A comparison of anodically grown CuO nanotube film and Cu2O film as anodes for lithium ion batteries. J. Solid State Electrochem. 2008, 12, 941–945. [Google Scholar] [CrossRef]
- Won, Y.-H.; Stanciu, L.A. Cu2O and Au/Cu2O particles: Surface properties and applications in glucose sensing. Sensors 2012, 12, 13019–13033. [Google Scholar] [CrossRef] [PubMed]
- Rai, B.P. Cu2O solar cells: A review. Sol. Cells 1988, 25, 265–272. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Eom, K.; Kim, S.; Lee, D.; Seo, H. Physicochemical interface effect in Cu2O-ZnO heterojunction on photocurrent spectrum. RSC Adv. 2015, 5, 103803–103810. [Google Scholar] [CrossRef]
- Luo, J.; Steier, L.; Son, M.-K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Kondo, T.; Komoda, M.; Ikeda, S.; Kondo, J.; Domen, K.; Hara, M.; Shinohara, K.; Tanaka, A. Cu2O as a photocatalyst for overall water splitting under visible light irradiation. Chem. Commun. 1998, 3, 357–358. [Google Scholar] [CrossRef]
- Morales-Guio, C.G.; Liardet, L.; Mayer, M.T.; Tilley, S.D.; Grätzel, M.; Hu, X. Photoelectrochemical hydrogen production in alkaline solutions using Cu2O coated with earth-abundant hydrogen evolution catalysts. Angew. Chem. Int. Ed. 2015, 54, 664–667. [Google Scholar] [CrossRef]
- Paracchino, A.; Laporte, V.; Sivula, K.; Grätzel, M.; Thimsen, E. Highly active oxide photocathode for photoelectrochemical water reduction. Nat. Mater. 2011, 10, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wang, J.; Xu, G. Fast synthesis of Cu2O hollow microspheres and their application in DNA biosensor of hepatitis B virus. Cryst. Growth Des. 2009, 9, 633–638. [Google Scholar] [CrossRef]
- Elahi, M.Y.; Khodadasi, A.A.; Mortazavi, Y. A glucose biosensor based on glucose oxidase immobilized on ZnO/Cu2O graphene oxide nanocomposite electrode. J. Electrochem. Soc. 2014, 161, B81–B87. [Google Scholar] [CrossRef]
- Radi, A.; Pradhan, D.; Sohn, Y.; Leung, K.T. Nanoscale shape and size control of cubic, cuboctahedral, and octahedral Cu−Cu2O core−shell nanoparticles on Si(100) by one-step, templateless, capping-agent-free electrodeposition. ACS Nano 2010, 4, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Peng, Q.; Wang, X.; Li, Y. Nearly monodisperse Cu2O and CuO nanospheres: Preparation and applications for sensitive gas sensors. Chem. Mater. 2006, 18, 867–871. [Google Scholar] [CrossRef]
- Xu, H.; Wang, W.; Zhu, W. Shape evolution and size-controllable synthesis of Cu2O octahedra and their morphology-dependent photocatalytic properties. J. Phys. Chem. B 2006, 110, 13829–13834. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Zhang, C.; Gao, G.; Cui, D. Facile synthesis of hollow Cu2O octahedral and spherical nanocrystals and their morphology-dependent photocatalytic properties. Nanoscale Res. Lett. 2012, 7, 276. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, P.E.; Vanmaekelbergh, D.; Kelly, J.J.D. Photoelectrochemistry of electrodeposited Cu2O. J. Electrochem. Soc. 2000, 147, 486–489. [Google Scholar] [CrossRef]
- Hu, C.-C.; Nian, J.-N.; Teng, H. Electrodeposited p-type Cu2O as photocatalyst for H2 evolution from water reduction in the presence of WO3. Sol. Energy Mater. Sol. Cells 2008, 92, 1071–1076. [Google Scholar] [CrossRef]
- Nian, J.-N.; Hu, C.-C.; Teng, H. Electrodeposited p-type Cu2O for H2 evolution from photoelectrolysis of water under visible light illumination. Int. J. Hydrogen Energy 2008, 33, 2897–2903. [Google Scholar] [CrossRef]
- Zerbino, J.O.; Sanchez, R.M.T.; Sustersic, M.G. Effect of oxalate on the growth of cuprous oxide layers on copper electrodes. Ellipsometric and isoelectric point study. Acta Chim. Slov. 2009, 56, 124–130. [Google Scholar]
- Wang, G.; van den Berg, R.; de Mello Donega, C.; de Jong, K.P.; de Jongh, P.E. Silica-supported Cu2O nanoparticles with tunable size for sustainable hydrogen generation. Appl. Catal. B Environ. 2016, 192, 199–207. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Z.; Han, Z.; Wang, K.; Zhou, Y.; Huang, J.; Ye, Z. Synthesis of mesoporous multiwall ZnO nanotubes by replicating silk and application for enzymatic biosensor. Biosens. Bioelectron. 2013, 49, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, R.; Chen, W. Graphene wrapped Cu2O nanocubes: Non-enzymatic electrochemical sensors for the detection of glucose and hydrogen peroxide with enhanced stability. Biosens. Bioelectron. 2013, 45, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Su, Y.; Zhang, S.; Lv, X.; Xia, H.; Wang, Y. An improved sensitivity nonenzymatic glucose biosensor based on a CuxO modified electrode. Biosens. Bioelectron. 2010, 26, 903–907. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Xu, J.; Le, Z.G. Synthesis of cuprous oxide (Cu2O) nanoparticles/graphene composite with an excellent electrocatalytic activity towards glucose. Int. J. Electrochem. Sci. 2012, 7, 10063–10073. [Google Scholar]
- Wang, L.; Fu, J.; Hou, H.; Song, Y. A facile strategy to prepare Cu2O/Cu electrode as a sensitive enzyme-free glucose sensor. Int. J. Electrochem. Sci. 2012, 7, 12587–12600. [Google Scholar]
- El Khatib, K.M.; Hameed, R.A. Development of Cu2O/Carbon vulcan XC-72 as non-enzymatic sensor for glucose determination. Biosens. Bioelectron. 2011, 26, 3542–3548. [Google Scholar] [CrossRef] [PubMed]
- Felix, S.; Kollu, P.; Raghupathy, B.P.; Jeon, S.K.; Grace, A.N. Electrocatalytic activity of Cu2O nanocubes-based electrode for glucose oxidation. J. Chem. Sci. 2014, 126, 25–32. [Google Scholar] [CrossRef]
- Yang, Y.; Han, J.; Ning, X.; Cao, W.; Xu, W.; Guo, L. Controllable morphology and conductivity of electrodeposited Cu2O thin film: Effect of surfactants. ACS Appl. Mater. Interfaces 2014, 6, 22534–22543. [Google Scholar] [CrossRef] [PubMed]
- Siegfried, M.J.; Choi, K.S. Electrochemical crystallization of cuprous oxide with systematic shape evolution. Adv. Mater. 2004, 16, 1743–1746. [Google Scholar] [CrossRef]
- Chen, M.; Xia, Z.; Liu, Q. Ethylenediamine-assisted hydrothermal synthesis of NaCaSiO3OH: Controlled morphology, mechanism, and luminescence properties by doping Eu3+/Tb3+. Inorg. Chem. 2016, 55, 11316–11322. [Google Scholar] [CrossRef] [PubMed]
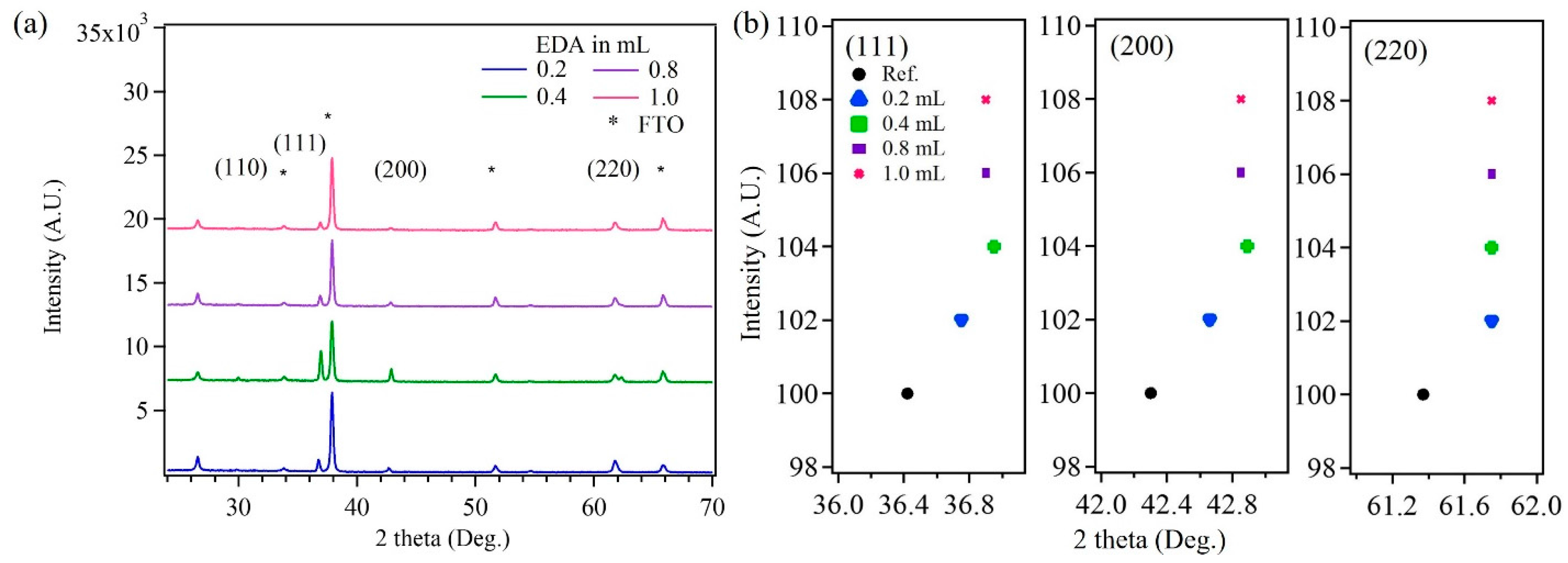
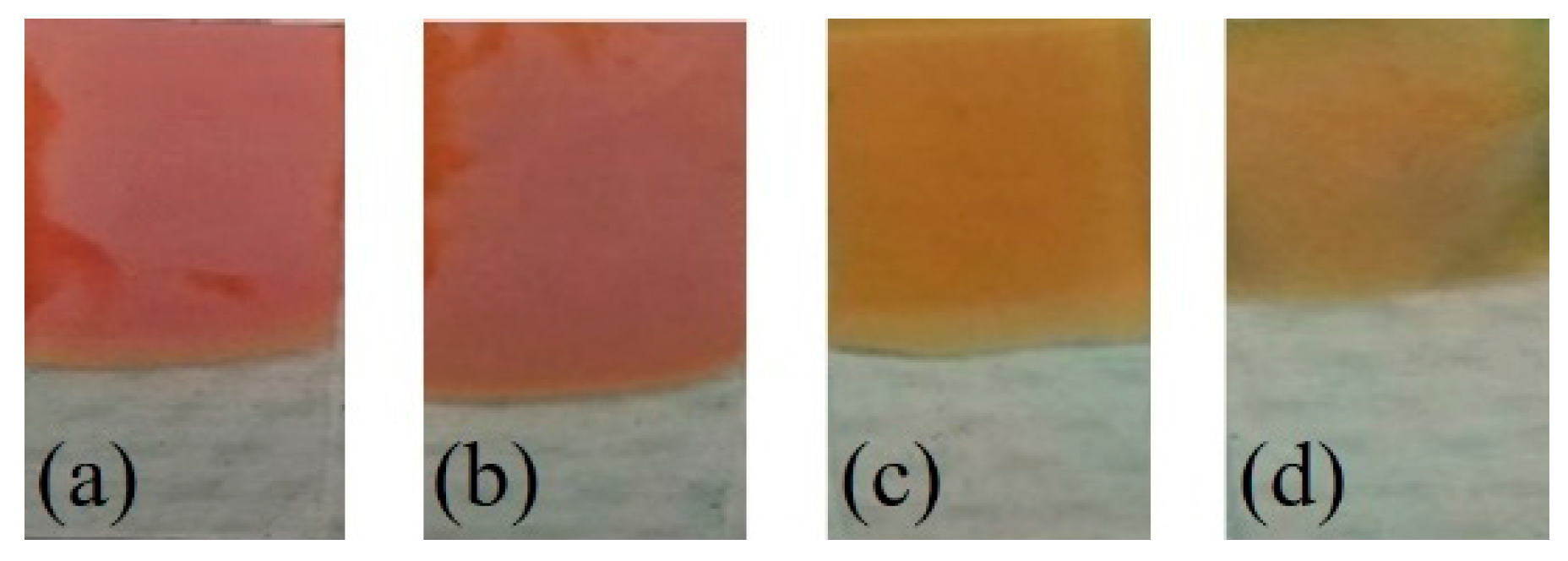
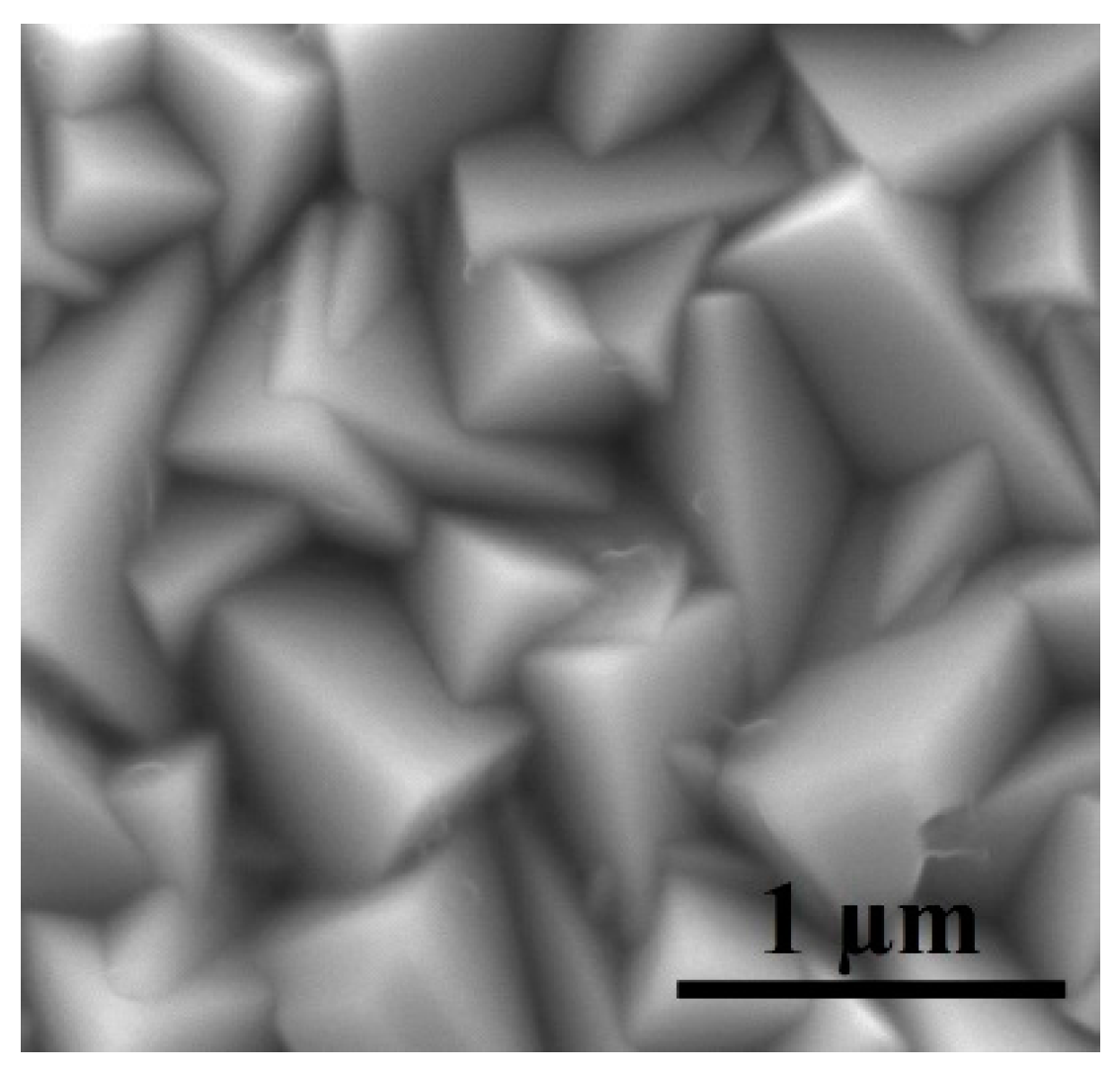
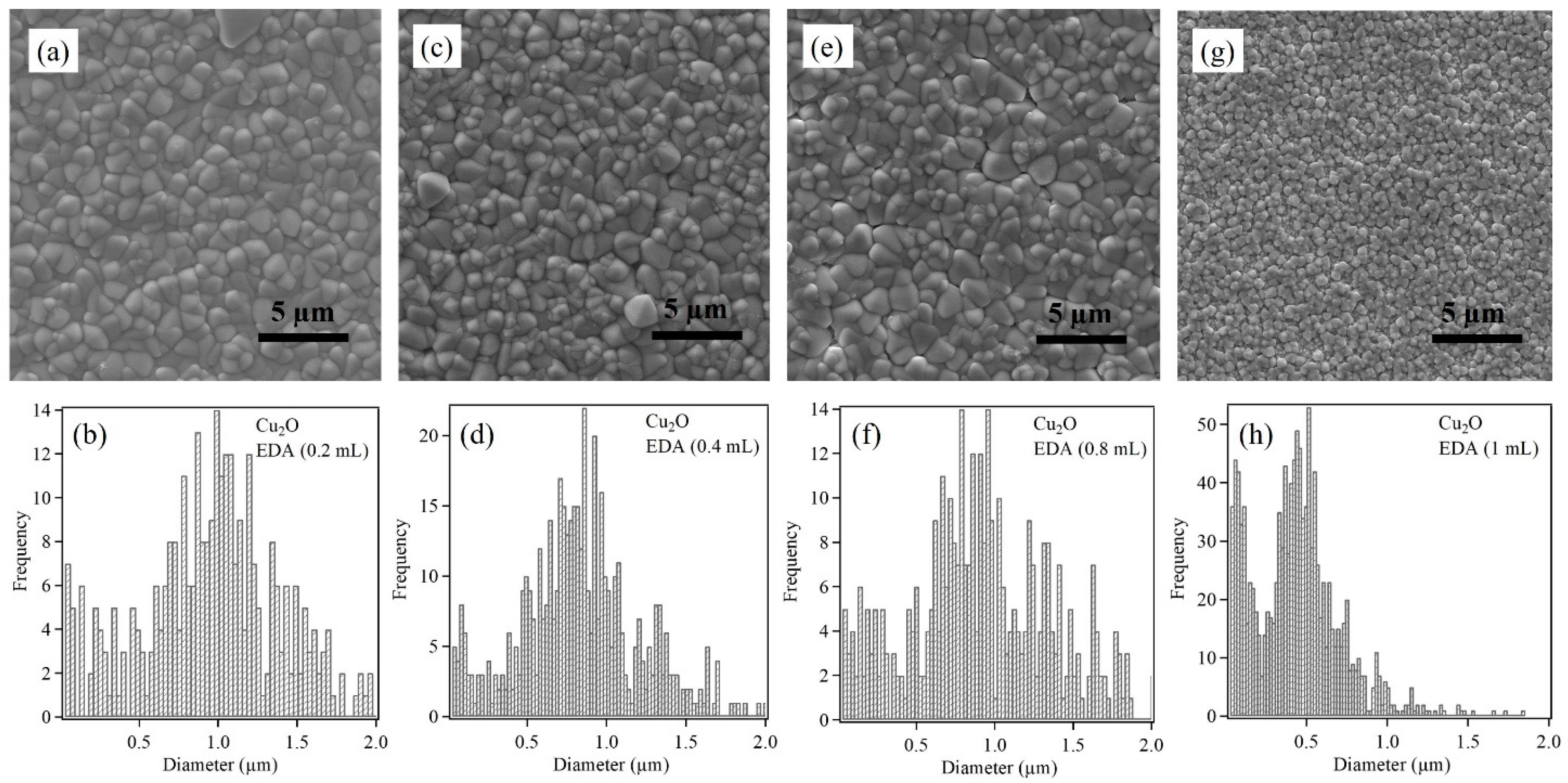
| Cu2O Sample with Varying EDA Content in mL | Particle Size (nm) | |
|---|---|---|
| 0.2 | 966 ± 23 | |
| 0.4 | 792 ± 16 | |
| 0.8 | 802 ± 34 | |
| 1.0 | 54 ± 20 | 427 ± 20 |
| Working Electrode | Sensitivity (μA/cm2·mM) | (mM) | LOD (μM) | Linear Range (mM) | Reference |
|---|---|---|---|---|---|
| GOx/Cu2O | 55.32 | 0.79 | 0.2 | 0.2–3.5 | The work |
| GOx/Cu2O/EDA (0.2) | 1243.2 | 1.00 | 0.1 | 0.1–3.5 | The work |
| GOx/Cu2O/EDA (1) | 1538 | 1.25 | 0.1 | 0.1–3.5 | The work |
| Cu2O/GNs | 285 | ------- | 3.3 | 0.3–3.3 | [23] |
| CuxO/Cu | 1620 | ------- | 49 | 0–4 | [24] |
| Cu2O/CRG | ------- | ------- | 1.2 | 0.1–1.1 | [25] |
| Cu2O/Cu | 62.29 | ------- | 37 | 0.05–6.75 | [26] |
| Cu2O/Carbon Vulcan XC-72 | 629 | ------- | 2.4 | 0–6 | [27] |
| Cu2O/Nafion/Glassy Carbon | 121.7 | ------- | 38 | 0–0.5 | [28] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, T.; Fallatah, A.; Suiter, E.; Padalkar, S. Size Controlled Copper (I) Oxide Nanoparticles Influence Sensitivity of Glucose Biosensor. Sensors 2017, 17, 1944. https://doi.org/10.3390/s17091944
Lan T, Fallatah A, Suiter E, Padalkar S. Size Controlled Copper (I) Oxide Nanoparticles Influence Sensitivity of Glucose Biosensor. Sensors. 2017; 17(9):1944. https://doi.org/10.3390/s17091944
Chicago/Turabian StyleLan, Tian, Ahmad Fallatah, Elliot Suiter, and Sonal Padalkar. 2017. "Size Controlled Copper (I) Oxide Nanoparticles Influence Sensitivity of Glucose Biosensor" Sensors 17, no. 9: 1944. https://doi.org/10.3390/s17091944





