A Novel Organic Electrochemical Transistor-Based Platform for Monitoring the Senescent Green Vegetative Phase of Haematococcus pluvialis Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
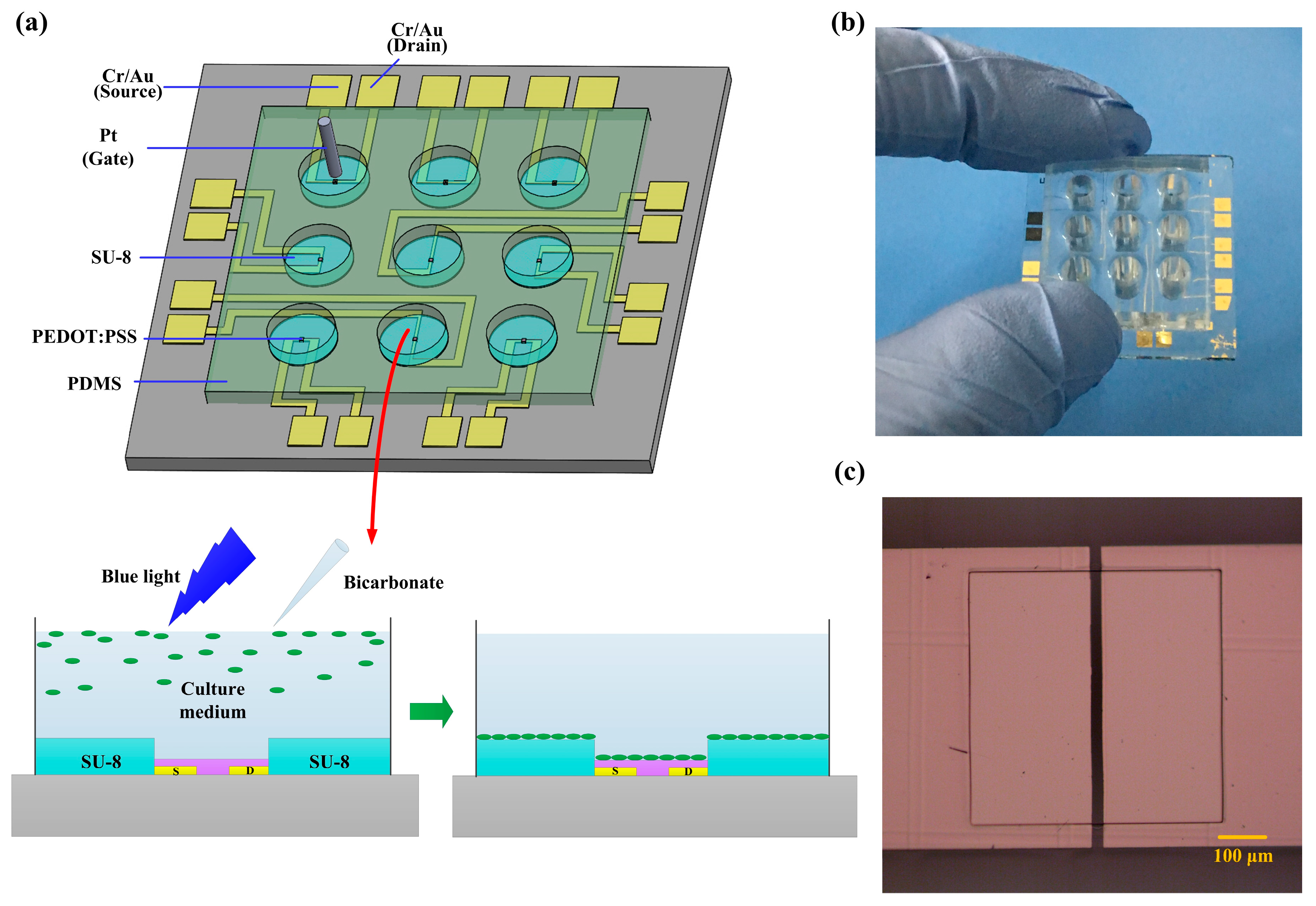
2.2. Device Fabrication
2.3. Device Characterization and Image Acquisition
2.4. H. pluvialis Preparation
3. Results and Discussion
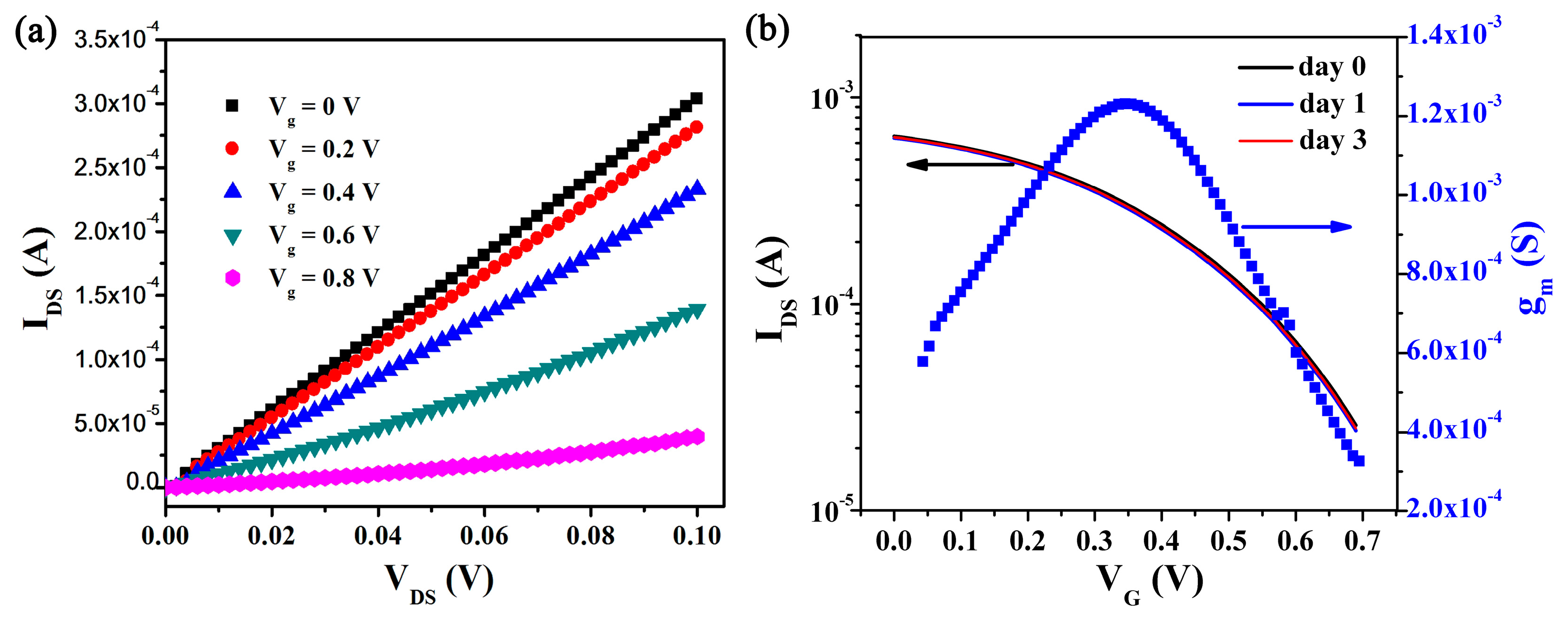
3.1. Preparation and Characterization of OECT Array
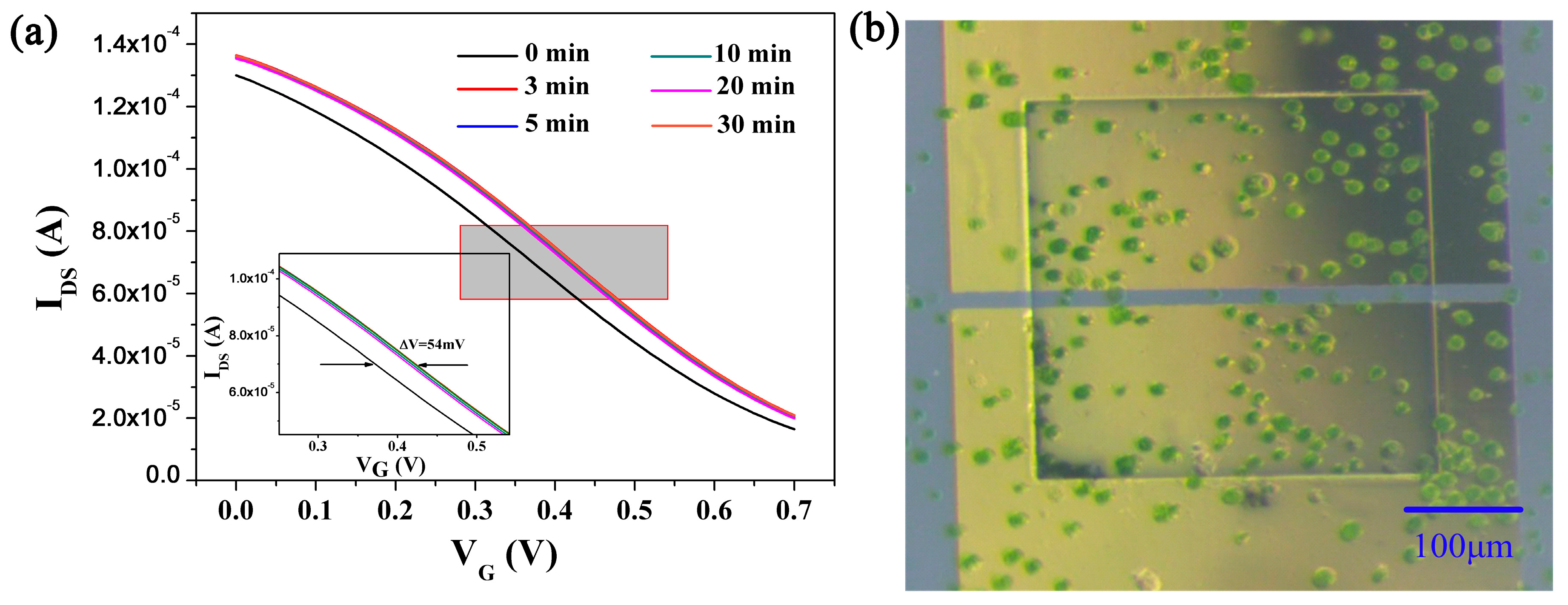
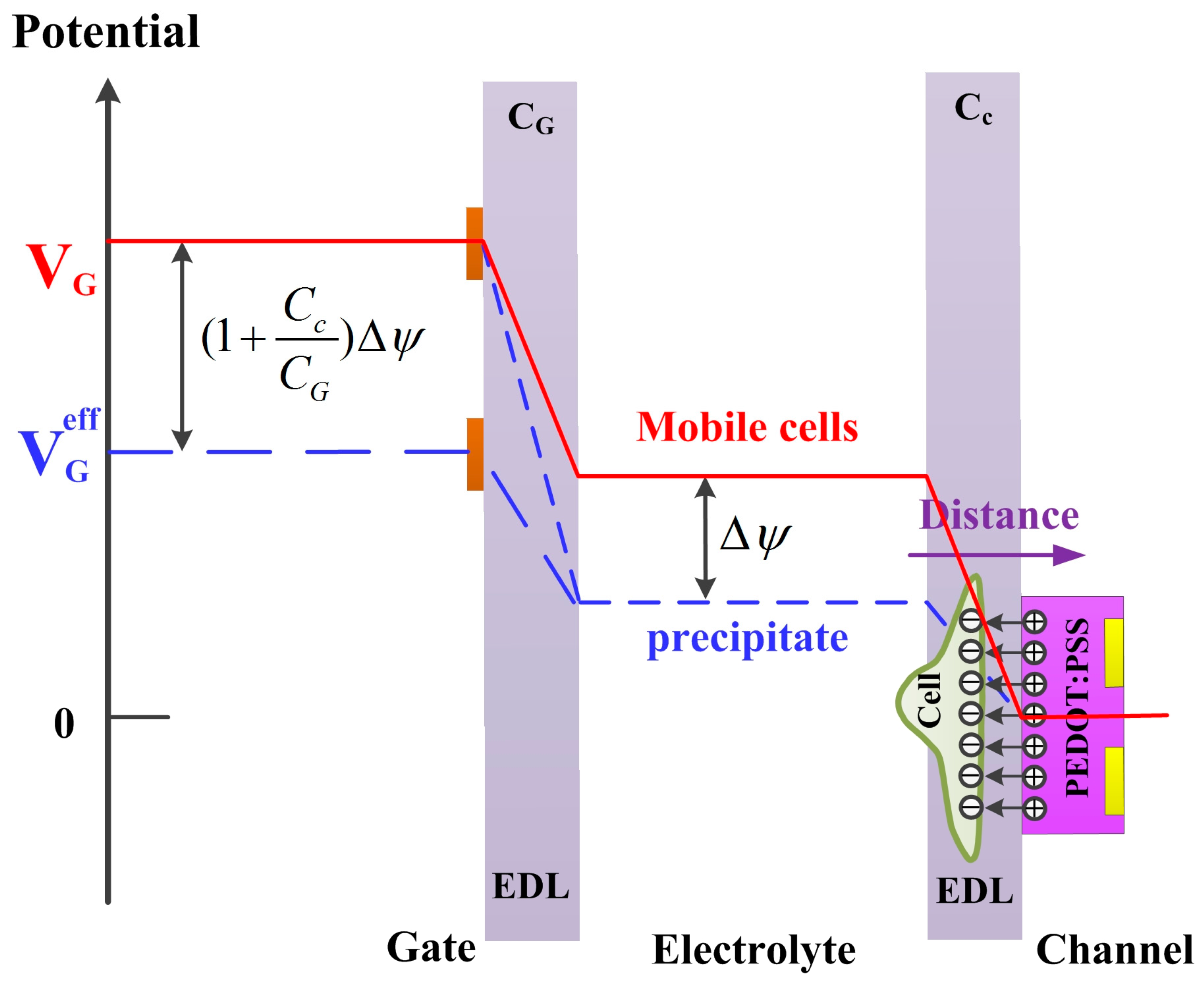
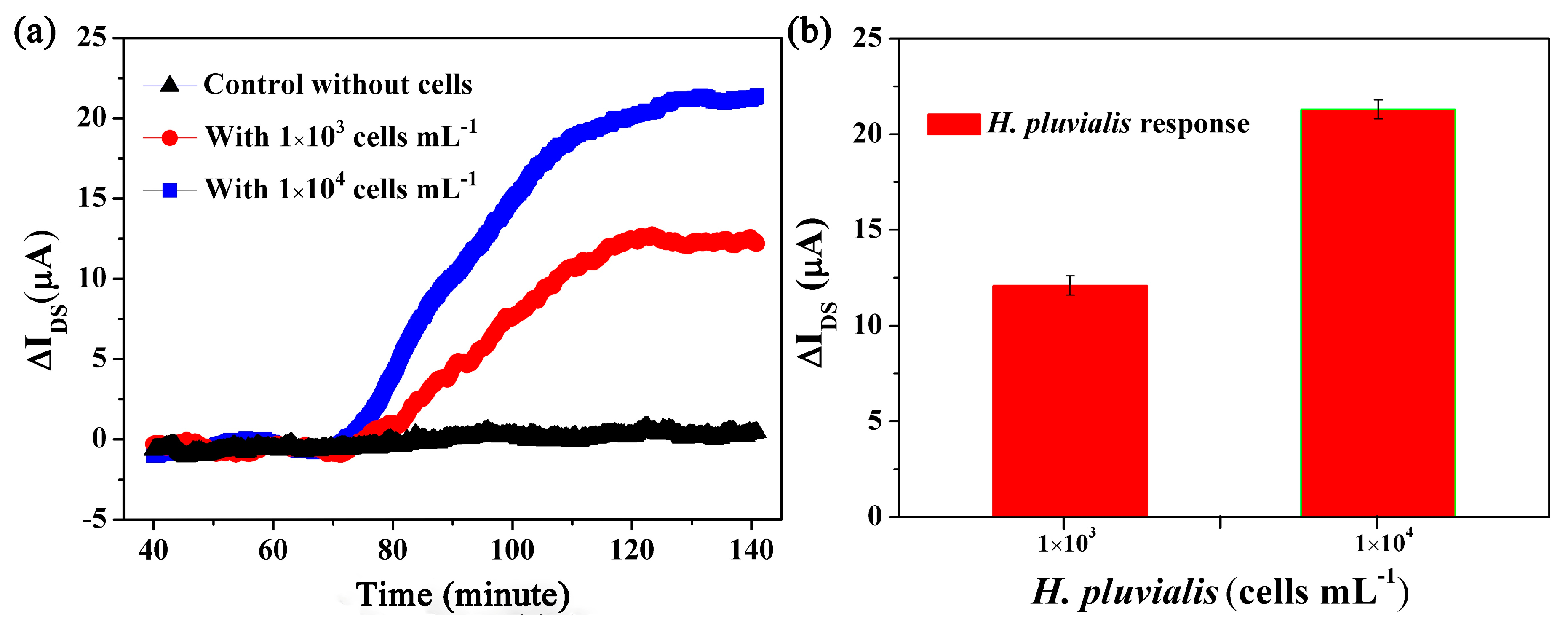
3.2. Real-Time Algal Cells Detection
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vanmaekelbergh, D.; Houtepen, A.J.; Kelly, J.J. Electrochemical gating: A method to tune and monitor the (opto) electronic properties of functional materials. Electrochem. Acta 2007, 53, 1140–1149. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G. Steady-state and transient behavior of organic electrochemical transistors. Adv. Funct. Mater. 2007, 17, 3538–3544. [Google Scholar] [CrossRef]
- Dabke, R.B.; Singh, G.D.; Dhanabalan, A.; Lal, R.; Contractor, A.Q. An ion-activated molecular electronic device. Anal. Chem. 1997, 69, 724–727. [Google Scholar] [CrossRef]
- Bernards, D.A.; Malliaras, G.G.; Toombes, G.E.; Gruner, S.M. Gating of an organic transistor through a bilayer lipid membrane with ion channels. Appl. Phys. Lett. 2006, 89, 053505. [Google Scholar] [CrossRef]
- Kim, D.J.; Lee, N.E.; Park, J.S.; Park, I.J.; Kim, J.G.; Cho, H.J. Organic electrochemical transistor based immunosensor for prostate specific antigen (PSA) detection using gold nanoparticles for signal amplification. Biosens. Bioelectron. 2010, 25, 2477–2482. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Gokhale, R.S.; Contractor, A.Q.; Kumar, A. Novel label-free DNA sensors based on poly (3, 4-ethylenedioxythiophene). Chem. Commun. 2004, 7, 820–821. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Lin, P.; Chan, H.L.; Yan, F. Highly sensitive dopamine biosensors based on organic electrochemical transistors. Biosens. Bioelectron. 2011, 26, 4559–4563. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Curto, V.F.; Fraser, K.J.; Gurfinkel, M.; Byrne, R.; Diamond, D.; Owens, R.M. Organic electrochemical transistor incorporating an ionogel as a solid state electrolyte for lactate sensing. J. Mater. Chem. 2012, 22, 4440–4443. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.T.; Mabeck, J.T.; Zhu, C.; Cady, N.C.; Batt, C.A.; Malliaras, G.G. A simple poly (3, 4-ethylene dioxythiophene)/poly (styrene sulfonic acid) transistor for glucose sensing at neutral pH. Chem. Commun. 2004, 13, 1556–1557. [Google Scholar] [CrossRef] [PubMed]
- Macaya, D.J.; Nikolou, M.; Takamatsu, S.; Mabeck, J.T.; Owens, R.M.; Malliaras, G.G. Simple glucose sensors with micromolar sensitivity based on organic electrochemical transistors. Sens. Actuators B Chem. 2007, 123, 374–378. [Google Scholar] [CrossRef]
- Bernards, D.A.; Macaya, D.J.; Nikolou, M.; DeFranco, J.A.; Takamatsu, S.; Malliaras, G.G. Enzymatic sensing with organic electrochemical transistors. J. Mater. Chem. 2008, 18, 116–120. [Google Scholar] [CrossRef]
- Bolin, M.H.; Svennersten, K.; Nilsson, D.; Sawatdee, A.; Jager, E.W.; Richter-Dahlfors, A.; Berggren, M. Active Control of Epithelial Cell-Density Gradients Grown Along the Channel of an Organic Electrochemical Transistor. Adv. Mater. 2009, 21, 4379–4382. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, F.; Yu, J.; Chan, H.L.; Yang, M. The Application of Organic Electrochemical Transistors in Cell-Based Biosensors. Adv. Mater. 2010, 22, 3655–3660. [Google Scholar] [CrossRef] [PubMed]
- Khodagholy, D.; Doublet, T.; Quilichini, P.; Gurfinkel, M.; Leleux, P.; Ghestem, A.; Malliaras, G.G. In vivo recordings of brain activity using organic transistors. Nat. Commun. 2013, 4, 1575. [Google Scholar] [CrossRef] [PubMed]
- Glombitza, K.W.; Koch, M. Secondary Metabolites of Pharmaceutical Potential; Cresswell, R.C., Rees, T.A.V., Shah, N., Eds.; Algal and Cyanobacterial Biotechnology, Longman Scientific & Technical: Essex, UK, 1989; pp. 161–238. [Google Scholar]
- Jin, E.; Polle, J.E.; Lee, H.K.; Hyun, S.M.; Chang, M. Xanthophylls in microalgae: From biosynthesis to biotechnological mass production and application. J. Microbiol. Biotechnol. 2003, 13, 165–174. [Google Scholar]
- Aziz, M.A.; Ng, W.J. Feasibility of wastewater treatment using the activated-algae process. Bioresour. Technol. 1992, 40, 205–208. [Google Scholar] [CrossRef]
- Lorenz, R.T.; Cysewski, G.R. Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends biotechnol. 2000, 18, 160–167. [Google Scholar] [CrossRef]
- Shah, M.M.; Liang, Y.; Cheng, J.J.; Daroch, M. Astaxanthin-Producing Green Microalga Haematococcus pluvialis: From Single Cell to High Value Commercial Products. Front. Plant. Sci. 2016, 7, 531. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.E.; Yun, Y.S.; Park, J.M.; Yang, J.W. Determination of the time transferring cells for astaxanthin production considering two-stage process of Haematococcus pluvialis cultivation. Bioresour. Technol. 2011, 102, 11249–11253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lin, P.; Yang, M.; Yan, F. Fabrication of organic electrochemical transistor arrays for biosensing. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 4402–4406. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.S.; Yoon, H.C.; Yang, H.; Pyo, H.B.; Chung, K.H.; Kim, S.J.; Kim, Y.T. A polymer-based microfluidic device for immunosensing biochips. Lab Chip 2003, 3, 106–113. [Google Scholar]
- Zheng, Y.H.; Tao, M.; Li, Z.; Hu, Z.L. Cloning and characterization of selenoprotein thioredoxin reductase gene in Haematococcus pluvialis. Algal Res. 2016, 17, 97–104. [Google Scholar] [CrossRef]
- Berggren, M.; Richter-Dahlfors, A. Organic bioelectronics. Adv. Mater. 2007, 19, 3201–3213. [Google Scholar] [CrossRef]
- Ghosh, S.; Inganäs, O. Conducting polymer hydrogels as 3D electrodes: Applications for supercapacitors. Adv. Mater. 1999, 11, 1214–1218. [Google Scholar] [CrossRef]
- Cicoira, F.; Sessolo, M.; Yaghmazadeh, O.; DeFranco, J.A.; Yang, S.Y.; Malliaras, G.G. Influence of device geometry on sensor characteristics of planar organic electrochemical transistors. Adv. Mater. 2010, 22, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Yan, F.; Chan, H.L. Ion-sensitive properties of organic electrochemical transistors. ACS Appl. Mater. Interfaces 2010, 2, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Q.; Zhong, L.N.; Yao, S.J. Zeta potential as a diagnostic tool to evaluate the biomass electrostatic adhesion during ion-exchange expanded bed application. Biotechnol. Bioeng. 2006, 95, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, B.; de Haar, C.; Lee, L.; Oortgiesen, M. The surface charge of visible particulate matter predicts biological activation in human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2002, 178, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, M.; Park, J.H.; Singelyn, J.; Ma, H.; Sailor, M.J.; Ozkan, C. A Surface-Charge Study on Cellular-Uptake Behavior of F3-Peptide-Conjugated Iron Oxide Nanoparticles. Small 2009, 5, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: London, UK, 2013. [Google Scholar]
- Rosenhahn, A.; Finlay, J.A.; Pettit, M.E.; Ward, A.; Wirges, W.; Gerhard, R.; Callow, J.A. Zeta potential of motile spores of the green alga Ulva linza and the influence of electrostatic interactions on spore settlement and adhesion strength. Biointerphases 2009, 4, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Henderson, R.K.; Parsons, S.A.; Jefferson, B. Successful removal of algae through the control of zeta potential. Sep. Sci. Technol. 2008, 43, 1653–1666. [Google Scholar] [CrossRef]
- Sukenik, A.; Shelef, G. Algal autoflocculation—Verification and proposed mechanism. Biotechnol. Bioeng. 1984, 26, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Hancock, C.; Flores, M. Zeta Potential: Key to Harvesting Algae for Biofuels and Bioproducts; Utah State University: Logan, UT, USA, 2016. [Google Scholar]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, W.; Xiao, K.; Tao, M.; Nie, L.; Liu, D.; Ke, S.; Zeng, X.; Hu, Z.; Lin, P.; Zhang, Y. A Novel Organic Electrochemical Transistor-Based Platform for Monitoring the Senescent Green Vegetative Phase of Haematococcus pluvialis Cells. Sensors 2017, 17, 1997. https://doi.org/10.3390/s17091997
Wei W, Xiao K, Tao M, Nie L, Liu D, Ke S, Zeng X, Hu Z, Lin P, Zhang Y. A Novel Organic Electrochemical Transistor-Based Platform for Monitoring the Senescent Green Vegetative Phase of Haematococcus pluvialis Cells. Sensors. 2017; 17(9):1997. https://doi.org/10.3390/s17091997
Chicago/Turabian StyleWei, Weiwei, Kang Xiao, Ming Tao, Lifu Nie, Dan Liu, Shanming Ke, Xierong Zeng, Zhangli Hu, Peng Lin, and Yu Zhang. 2017. "A Novel Organic Electrochemical Transistor-Based Platform for Monitoring the Senescent Green Vegetative Phase of Haematococcus pluvialis Cells" Sensors 17, no. 9: 1997. https://doi.org/10.3390/s17091997



