Fast and Sensitive Ellipsometry-Based Biosensing
Abstract
:1. Introduction
2. Methods
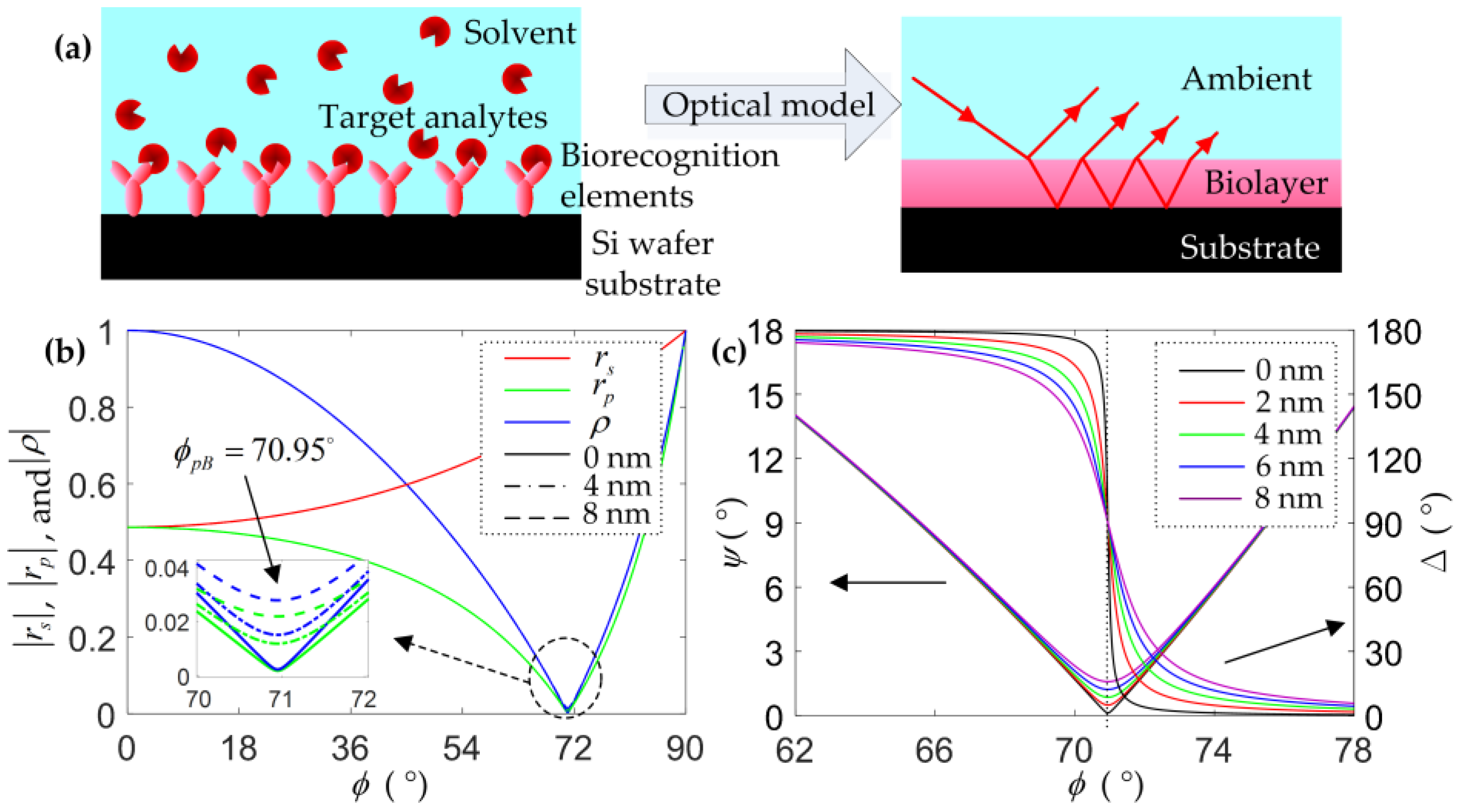
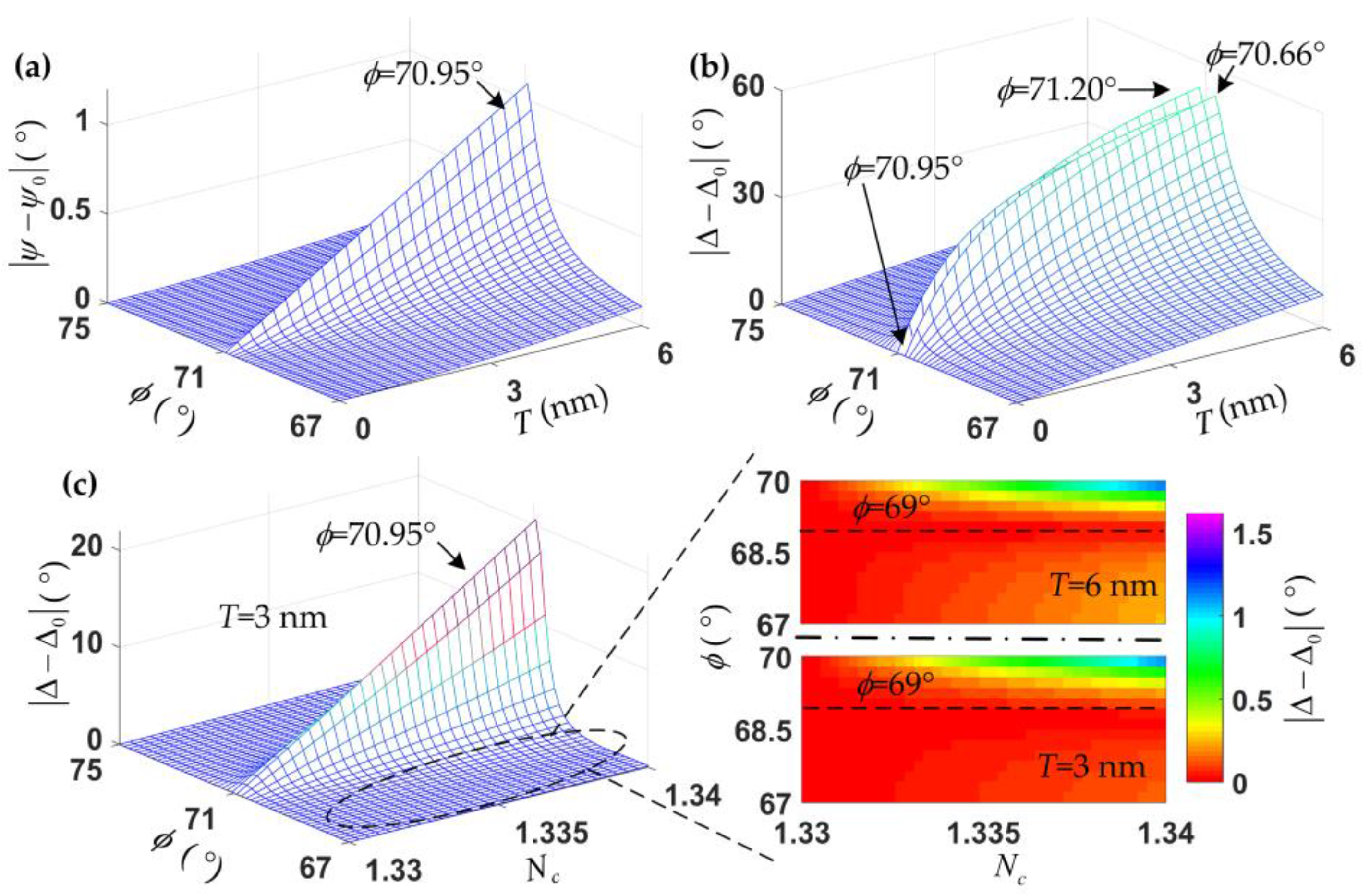
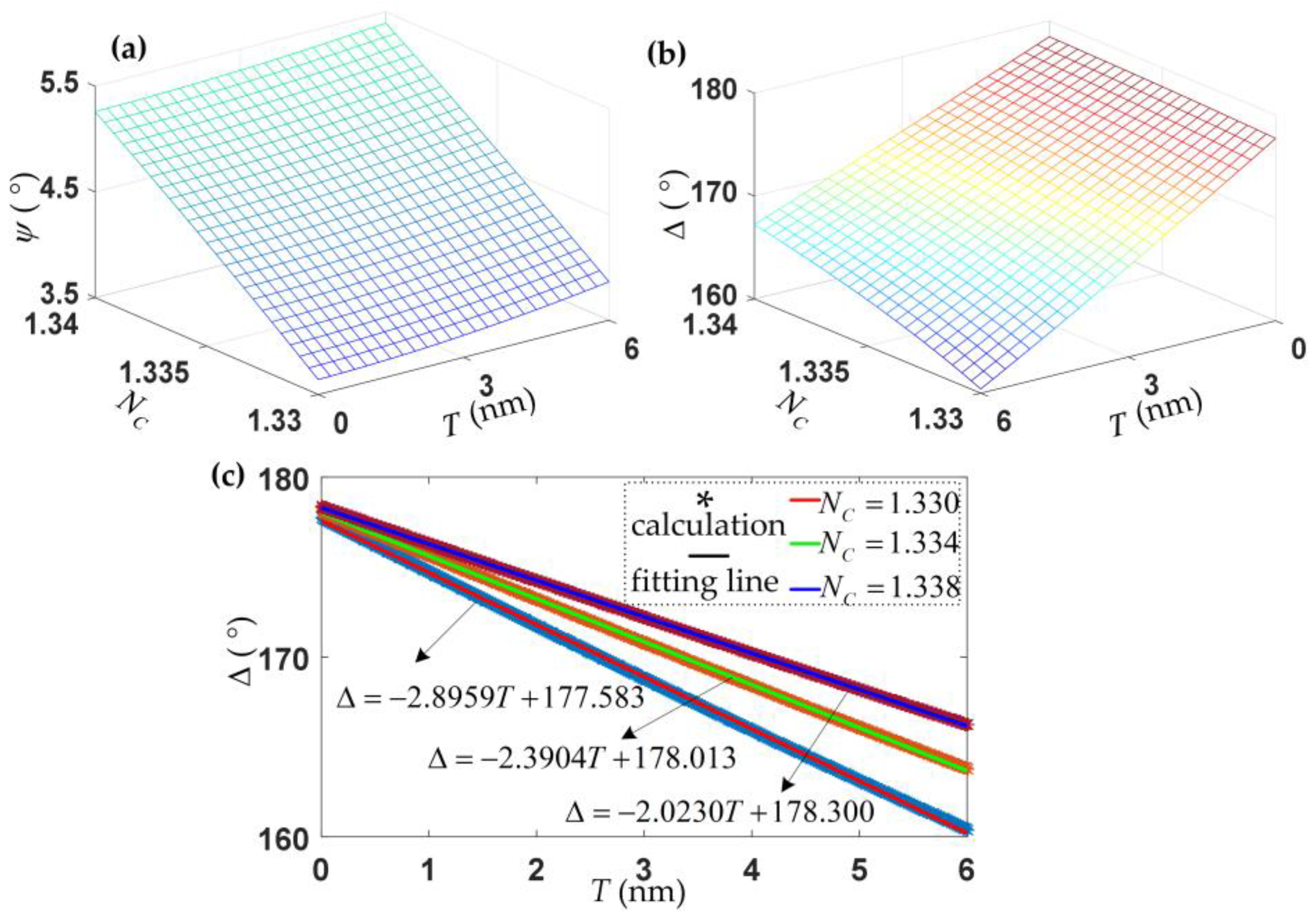
2.1. Optical Model and Numerical Calculation
2.2. Biosensing Scheme
3. Experiments
3.1. Materials
3.2. Bare Si Substrate Functionalized and Sensor Cell Fabricated
3.3. Ellipsometry Apparatus
4. Results and Discussion
4.1. Baseline Determination
4.2. Biointeraction Observation
4.3. Specificity Evaluation
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cooper, M.A. Optical biosensors in drug discovery. Nat. Rev. Drug Discov. 2002, 1, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.S.; Toh, C.S. Novel biosensing methodologies for ultrasensitive detection of viruses. Analyst 2013, 138, 6219–6229. [Google Scholar] [CrossRef] [PubMed]
- Zeimpekis, I.; Papadimitriou, K.I.; Sun, K.; Hu, C.; Ashburn, P.; Morgan, H.; Prodromakis, T. A Sub-30 mpH Resolution Thin Film Transistor-Based Nanoribbon Biosensing Platform. Sensors 2017, 17, 2000. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; White, I.M.; Shopova, S.I.; Zhu, H.; Suter, J.D.; Sun, Y. Sensitive optical biosensors for unlabeled targets: A review. Anal. Chim. Acta 2008, 620, 8–26. [Google Scholar] [CrossRef] [PubMed]
- Hoa, X.D.; Kirk, A.G.; Tabrizian, M. Towards integrated and sensitive surface plasmon resonance biosensors: A review of recent progress. Biosens. Bioelectron. 2007, 23, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.D. Optical halide sensing using fluorescence quenching: Theory, simulations and applications—A review. Meas. Sci. Technol. 2001, 12, R53. [Google Scholar] [CrossRef]
- Mello, L.D.; Kubota, L.T. Review of the use of biosensors as analytical tools in the food and drink industries. Food Chem. 2002, 77, 237–256. [Google Scholar] [CrossRef]
- Goldsmith, S.J. Radioimmunoassay: Review of basic principles. Semin. Nucl. Med. 1975, 5, 125–152. [Google Scholar] [CrossRef]
- O’Sullivan, T.D.; Heitz, R.T.; Parashurama, N.; Barkin, D.B.; Wooley, B.A.; Gambhir, S.S.; Harris, J.S.; Levi, O. Real-time, continuous, fluorescence sensing in a freely-moving subject with an implanted hybrid VCSEL/CMOS biosensor. Biomed. Opt. Express 2013, 4, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Suni, I.I.; Bever, C.S.; Hammock, B.D. Impedance biosensors: Applications to sustainability and remaining technical challenges. ACS Sustain. Chem. Eng. 2014, 2, 1649–1655. [Google Scholar] [CrossRef] [PubMed]
- Kussrow, A.; Enders, C.S.; Bornhop, D.J. Interferometric methods for label-free molecular interaction studies. Anal. Chem. 2011, 84, 779–792. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface plasmon resonance sensors for detection of chemical and biological species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.H.; Ho, H.P.; Kong, S.K.; Kabashin, A.V. Phase-sensitive surface plasmon resonance biosensors: Methodology, instrumentation and applications. Ann. Phys. 2012, 524, 637–662. [Google Scholar] [CrossRef]
- Svedendahl, M.; Verre, R.; Käll, M. Refractometric biosensing based on optical phase flips in sparse and short-range-ordered nanoplasmonic layers. Light Sci. Appl. 2014, 3, e220. [Google Scholar] [CrossRef]
- Chou, C.; Wu, H.T.; Huang, Y.C.; Chen, Y.L.; Kuo, W.C. Characteristics of a paired surface plasma waves biosensor. Opt. Express 2006, 14, 4307–4315. [Google Scholar] [CrossRef] [PubMed]
- Nizamov, S.; Mirsky, V.M. Self-referencing SPR-biosensors based on penetration difference of evanescent waves. Biosens. Bioelectron. 2011, 28, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Arwin, H. Spectroscopic ellipsometry and biology: Recent developments and challenges. Thin Solid Films 1998, 313, 764–774. [Google Scholar] [CrossRef]
- Elwing, H. Protein absorption and ellipsometry in biomaterial research. Biomaterials 1998, 19, 397–406. [Google Scholar] [CrossRef]
- Sun, H.; Qi, C.; Niu, Y.; Kang, T.; Wei, Y.; Jin, G.; Zhu, W. Detection of cytomegalovirus antibodies using a biosensor based on imaging ellipsometry. PLoS ONE 2015, 10, e0136253. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Pan, F.; Cowsill, B.; Lu, J.R.; Garcia-Gancedo, L.; Flewitt, A.J.; Luo, J. Interfacial immobilization of monoclonal antibody and detection of human prostate-specific antigen. Langmuir 2011, 27, 7654–7662. [Google Scholar] [CrossRef] [PubMed]
- Svensson, O.; Arnebrant, T. Antibody–antigen interaction on polystyrene: An in situ ellipsometric study. J. Colloid Interface Sci. 2012, 368, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, Y.; Wang, C.; Zhu, W.; Ma, H.; Jin, G. Detection of alpha-fetoprotein through biological signal amplification by biosensor based on imaging ellipsometry. Thin Solid Films 2011, 519, 2763–2767. [Google Scholar] [CrossRef]
- Jin, G. Development of biosensor based on imaging ellipsometry. Phys. Status Solidi A 2008, 205, 810–816. [Google Scholar] [CrossRef]
- Wang, Z.H.; Jin, G. A label-free multisensing immunosensor based on imaging ellipsometry. Anal. Chem. 2003, 75, 6119–6123. [Google Scholar] [CrossRef] [PubMed]
- Bombarová, K.; Chlpík, J.; Cirák, J. Surface plasmon resonance ellipsometry based biosensor for the investigation of biomolecular interactions. Mater. Today Proc. 2015, 2, 70–76. [Google Scholar] [CrossRef]
- Li, K.W.; Wang, Z.B.; Wang, L.M.; Zhang, R. 45° double-drive photoelastic modulation. J. Opt. Soc. Am. A 2016, 33, 2041–2046. [Google Scholar] [CrossRef]
- Li, K.W.; Wang, L.M.; Zhang, R.; Wang, Z.B. Modulation axis performs circular motion in a 45° dual-drive symmetric photoelastic modulator. Rev. Sci. Instrum. 2016, 87, 123103. [Google Scholar] [CrossRef] [PubMed]
- Li, K.W.; Zhang, R.; Jing, N.; Chen, Y.; Zhang, M.; Wang, L.M.; Wang, Z.B. Fast and full range measurements of ellipsometric parameters using a 45° dual-drive symmetric photoelastic modulator. Opt. Express 2017, 25, 5725–5733. [Google Scholar] [CrossRef]
- Fujiwara, H. Spectroscopic Ellipsometry: Principles and Applications; John Wiley and Sons, Ltd.: Tokyo, Japan, 2007; pp. 36–39. ISBN 978-0-470-01608-4. [Google Scholar]
- Vörös, J. The density and refractive index of adsorbing protein layers. Biophys. J. 2004, 87, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Chien, F.C.; Chen, S.J. Direct determination of the refractive index and thickness of a biolayer based on coupled waveguide-surface plasmon resonance mode. Opt. Lett. 2006, 31, 187–189. [Google Scholar] [CrossRef] [PubMed]
- Aspnes, D.E.; Studna, A.A. Dielectric functions and optical parameters of Si, Ge, GaP, GaAs, GaSb, InP, InAs, and InSb from 1.5 to 6.0 eV. Phys. Rev. B 1983, 27, 985–1009. [Google Scholar] [CrossRef]
- Azzam, R.M. Complex reflection coefficients of p-and s-polarized light at the pseudo-Brewster angle of a dielectric-conductor interface. J. Opt. Soc. Am. A 2013, 30, 1975–1979. [Google Scholar] [CrossRef] [PubMed]
- De Feijter, J.; Benjamins, D.J.; Veer, F.A. Ellipsometry as a tool to study the adsorption behavior of synthetic and biopolymers at the air-water interface. Biopolymers 1978, 17, 1759–1772. [Google Scholar] [CrossRef]
- Zhao, H.; Brown, P.H.; Schuck, P. On the distribution of protein refractive index increments. Biophys. J. 2011, 100, 2309–2317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, Y.; Wu, Q.; Ma, P.; Zhang, H.; Wang, Y.; Song, D. Enhancing sensitivity of surface plasmon resonance biosensor by Ag nanocubes/chitosan composite for the detection of mouse IgG. Talanta 2016, 146, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Bolduc, O.R.; Pelletier, J.N.; Masson, J.F. SPR biosensing in crude serum using ultralow fouling binary patterned peptide SAM. Anal. Chem. 2010, 82, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Darain, F.; Gan, K.L.; Tjin, S.C. Antibody immobilization on to polystyrene substrate—On-chip immunoassay for horse igg based on fluorescence. Biomed. Microdevices 2009, 11, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kannan, P.; Zhang, L.; Chen, T.; Kim, D.H. Concave gold nanoparticle-based highly sensitive electrochemical IgG immunobiosensor for the detection of antibody–antigen interactions. RSC Adv. 2015, 5, 58478–58484. [Google Scholar] [CrossRef]
- Wilson, M.S. Electrochemical immunosensors for the simultaneous detection of two tumor markers. Anal. Chem. 2005, 77, 1496–1502. [Google Scholar] [CrossRef] [PubMed]
- Zangooie, S.; Bjorklund, R.; Arwin, H. Protein adsorption in thermally oxidized porous silicon layers. Thin Solid Films 1998, 313, 825–830. [Google Scholar] [CrossRef]

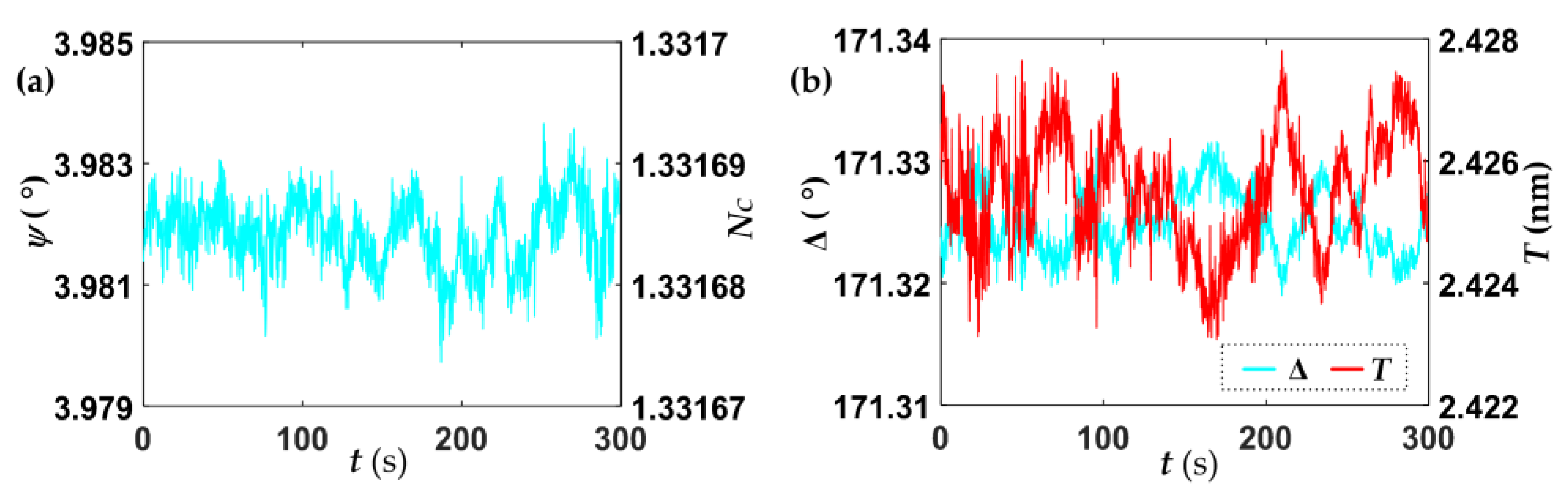
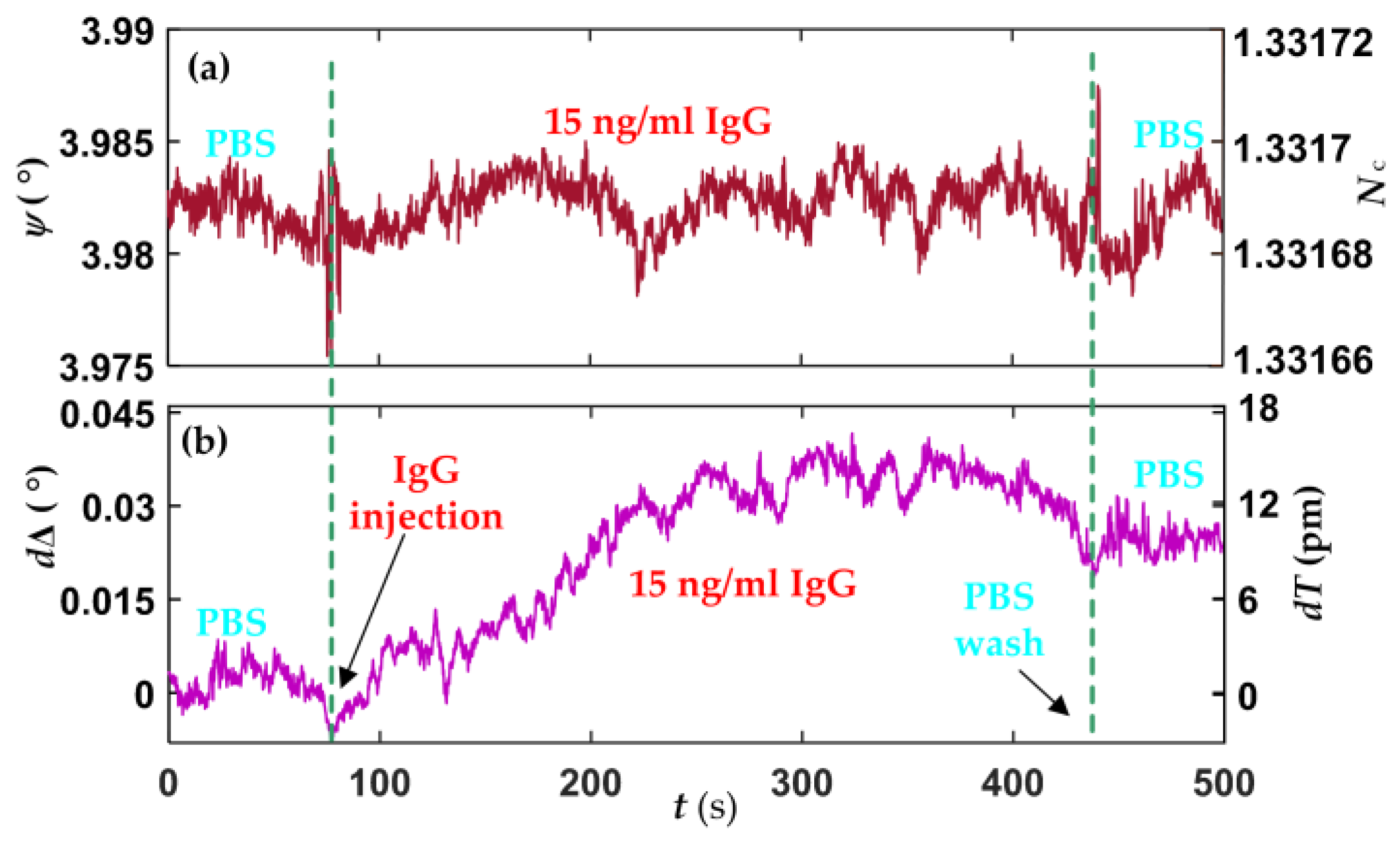
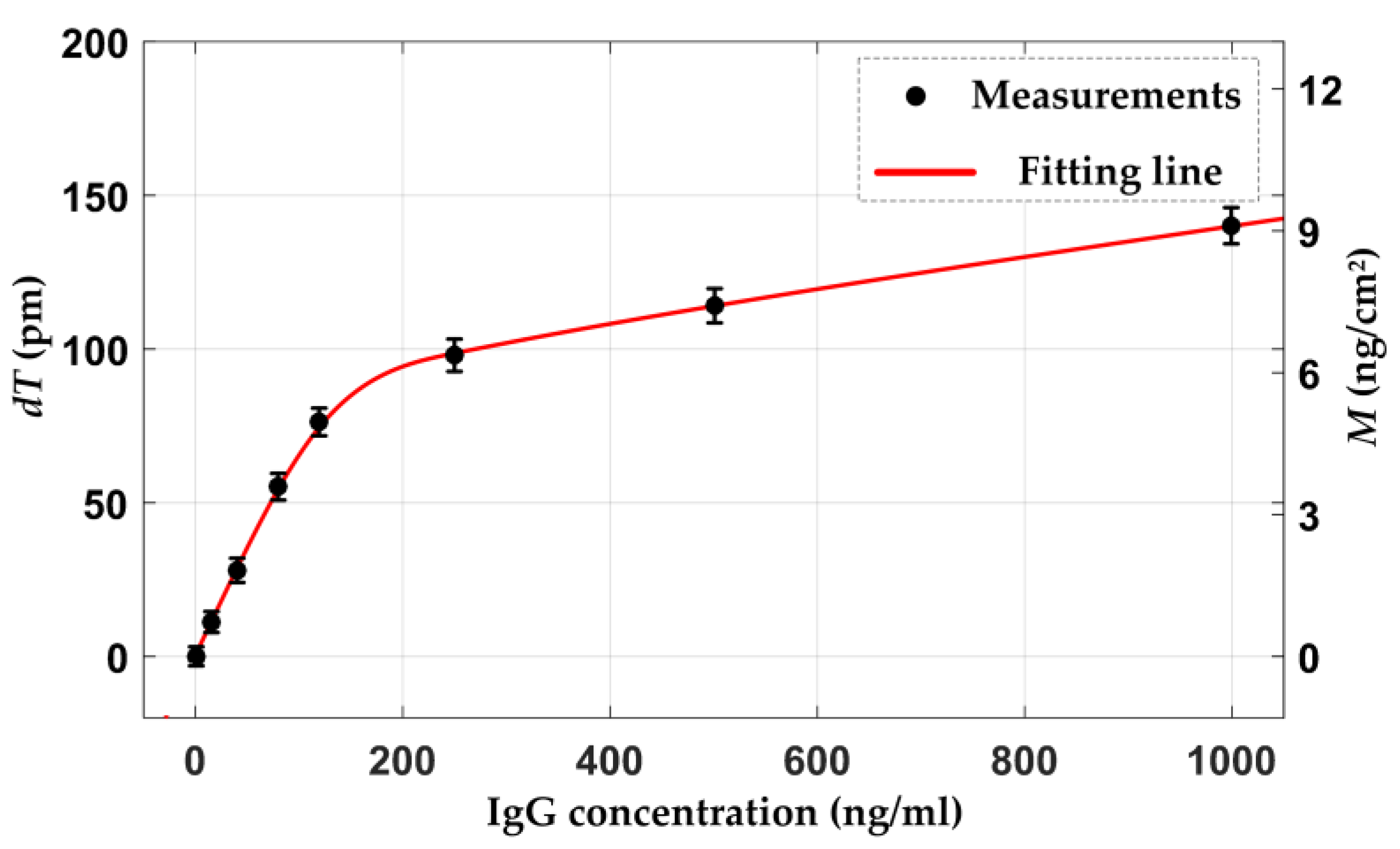
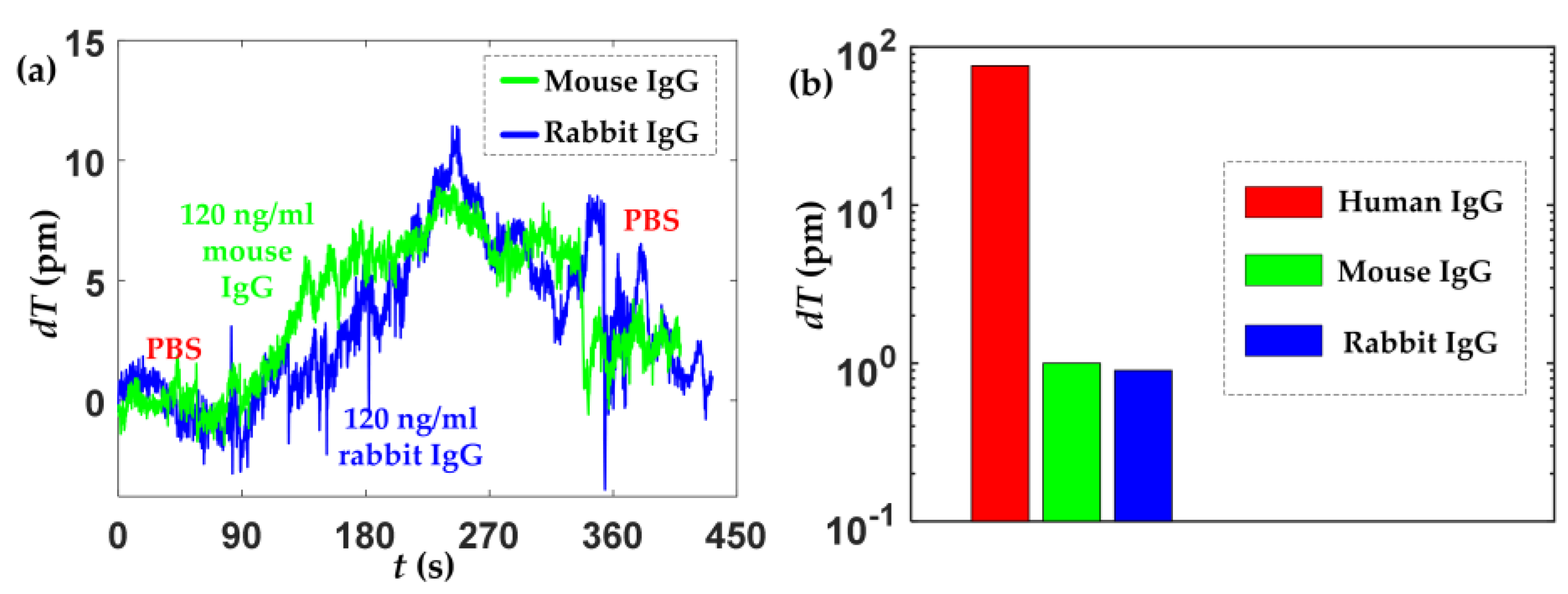
| Parameters | Mean Value | Standard Deviation |
|---|---|---|
| Ψ (°) | 3.982 | 0.001 |
| ∆ (°) | 171.325 | 0.003 |
| NC | 1.331687 | 6 × 10−6 |
| T (nm) | 2.425 | 0.001 |
| Method | Measurement Strategy | Analyte | Detection Limit | Response Time Level | References |
|---|---|---|---|---|---|
| SPR | AgNCs 1 + chitosan | Mouse IgG | 0.6 µg/mL | <1 min | [36] |
| Petide SAM 2 | Human IgG | 0.45 ng/mL (3 pM) | <1 min | [37] | |
| PSPW 3 | Mouse IgG | 10 pg/mL | <1 s | [15] | |
| Fluorescence | Fluorescence microsope | Horse IgG | 0.71 µg/mL | 25 min | [38] |
| Electrochemistry | CAuNCs 4 | Rabbit IgG | 5 ng/mL | <1 s | [39] |
| ELISA 5 | Goat IgG | 1 ng/mL | 2–5 min | [40] | |
| Ellipsometry | RCE 6 + Porous silicon | Albumin | - | <10 s | [41] |
| Imaging | AFP 7 | 5 ng/mL | <1 s | [22] | |
| SPRE | SPR + Ellipsometry | β-Cyclodextrins | 1 pg/mL (1 pM) | 2 s | [25] |
| Our platform | 45° dual-drive symmetric PEM + Bare Si | Human IgG | 15 ng/mL | 1 ms | Present work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Wang, S.; Wang, L.; Yu, H.; Jing, N.; Xue, R.; Wang, Z. Fast and Sensitive Ellipsometry-Based Biosensing. Sensors 2018, 18, 15. https://doi.org/10.3390/s18010015
Li K, Wang S, Wang L, Yu H, Jing N, Xue R, Wang Z. Fast and Sensitive Ellipsometry-Based Biosensing. Sensors. 2018; 18(1):15. https://doi.org/10.3390/s18010015
Chicago/Turabian StyleLi, Kewu, Shuang Wang, Liming Wang, Hui Yu, Ning Jing, Rui Xue, and Zhibin Wang. 2018. "Fast and Sensitive Ellipsometry-Based Biosensing" Sensors 18, no. 1: 15. https://doi.org/10.3390/s18010015





