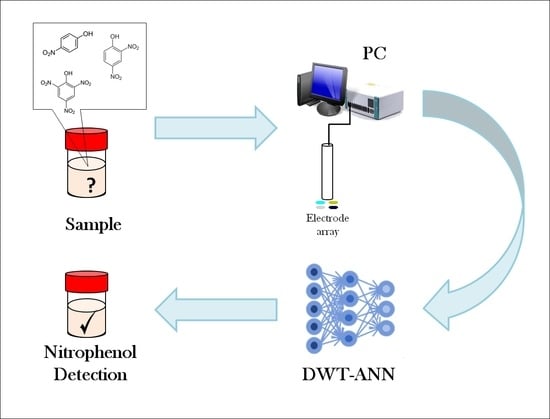
A Voltammetric Electronic Tongue for the Resolution of Ternary Nitrophenol Mixtures
Abstract
:1. Introduction
2. Experimental
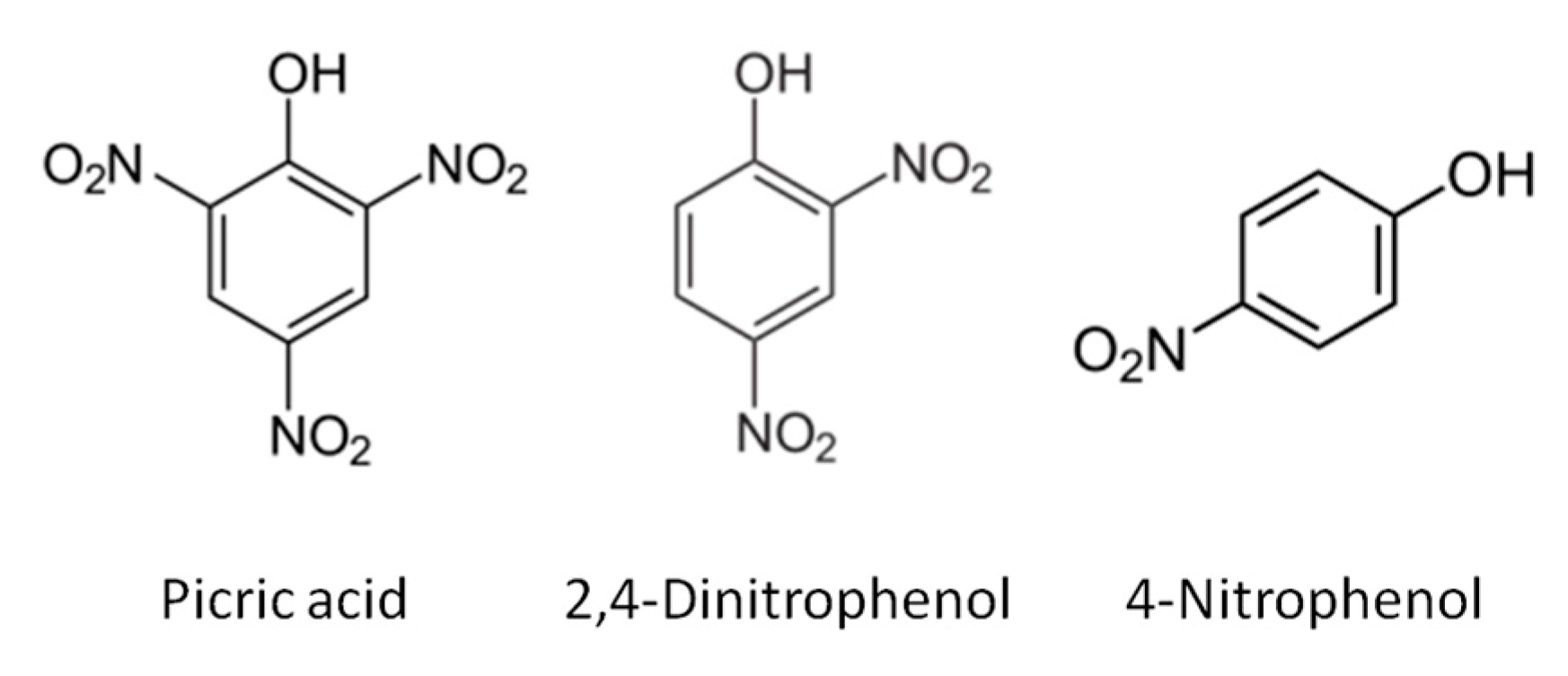
2.1. Reagents
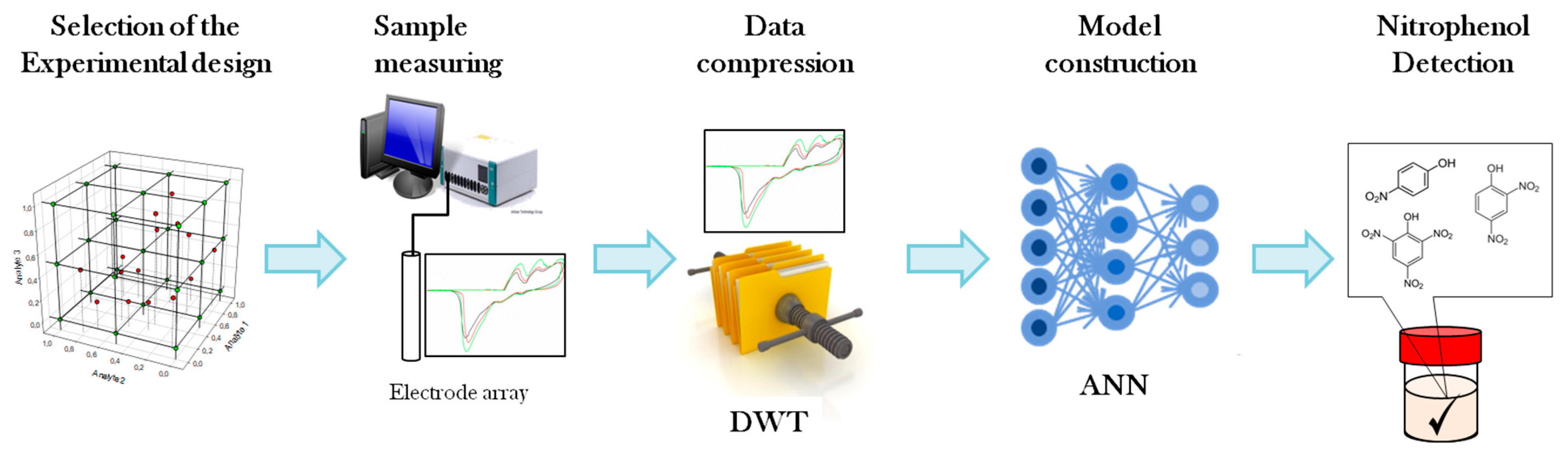
2.2. Electronic Tongue
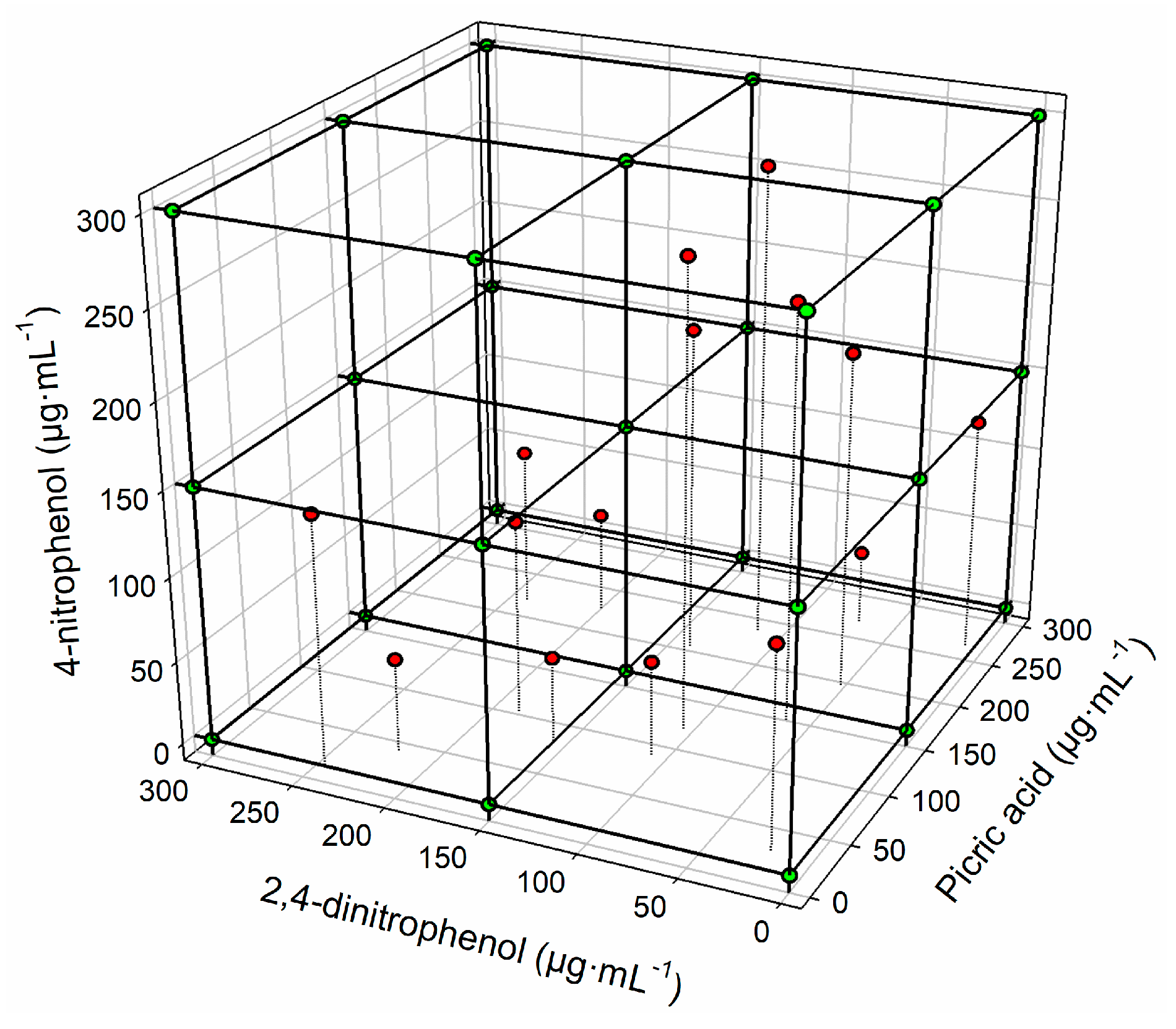
2.3. Sample Preparation
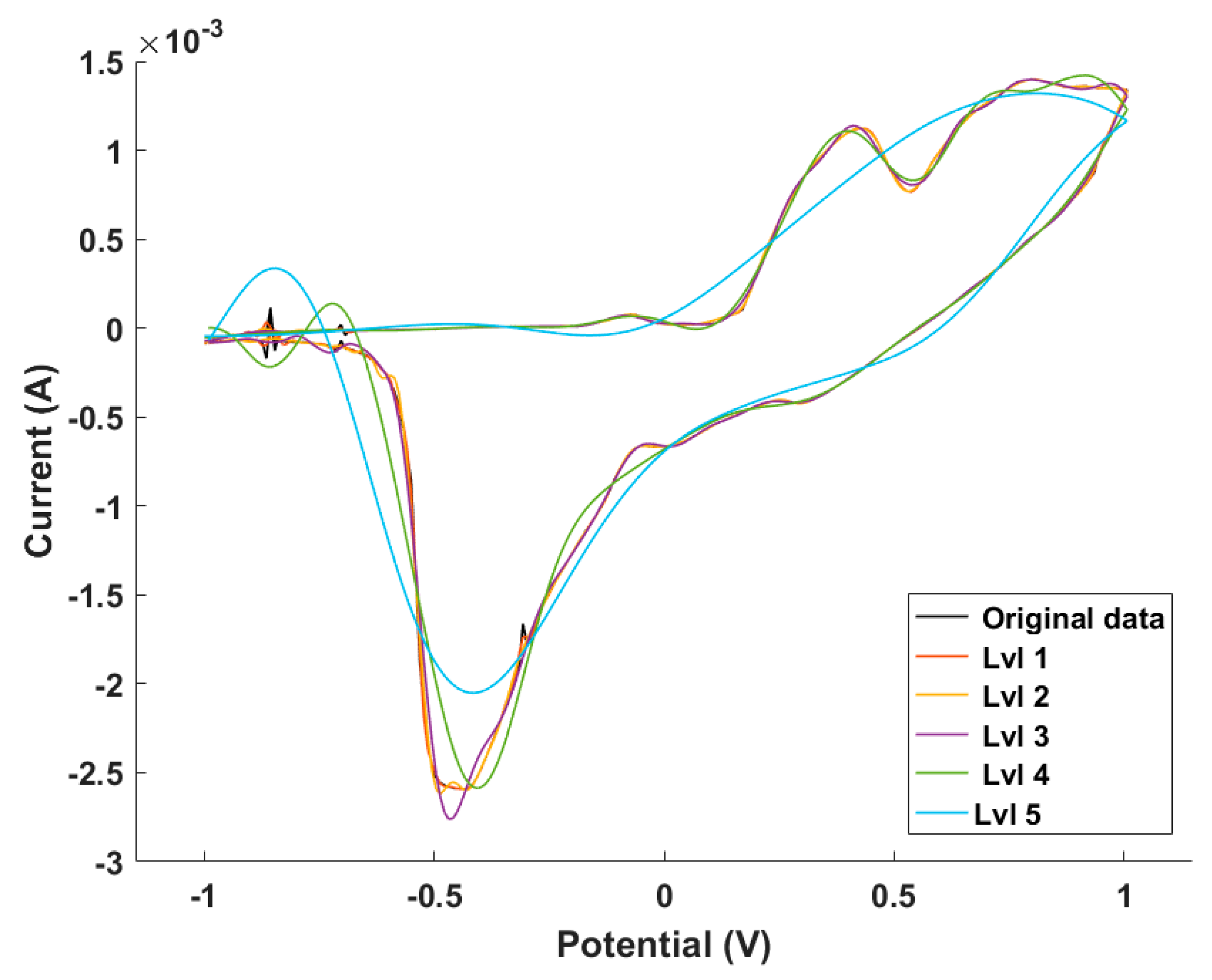
2.4. Data Processing
3. Results and Discussion
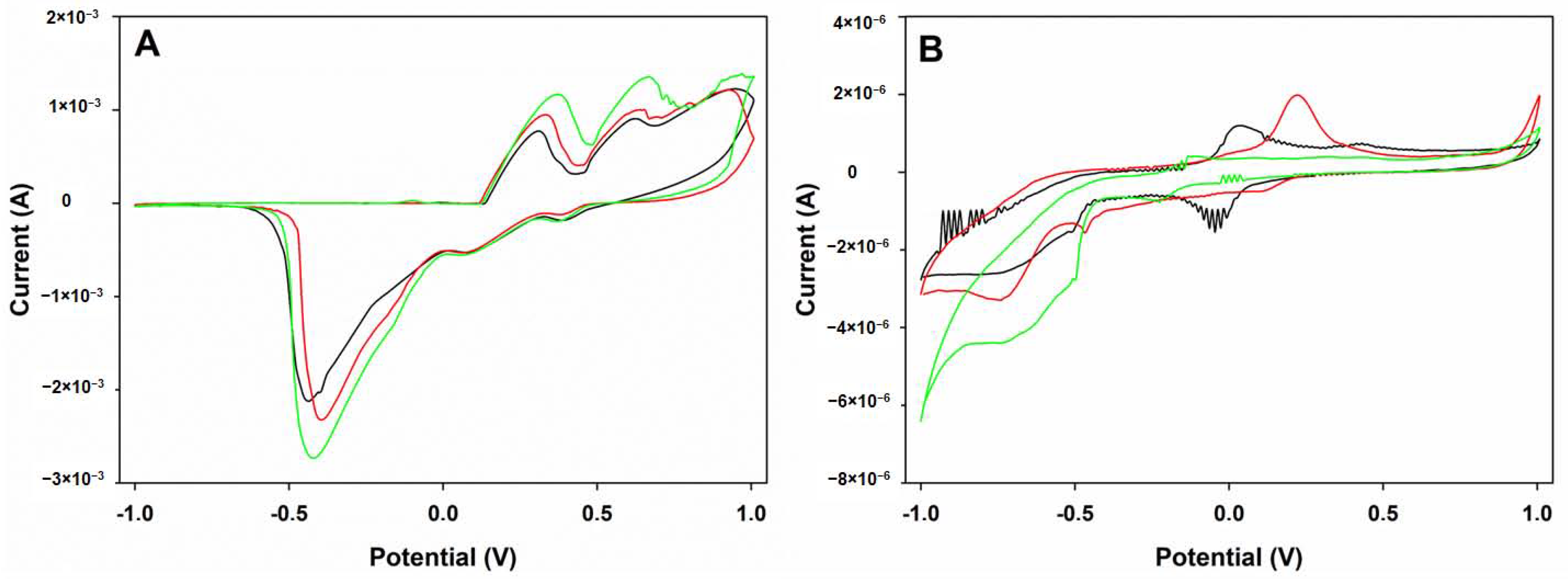
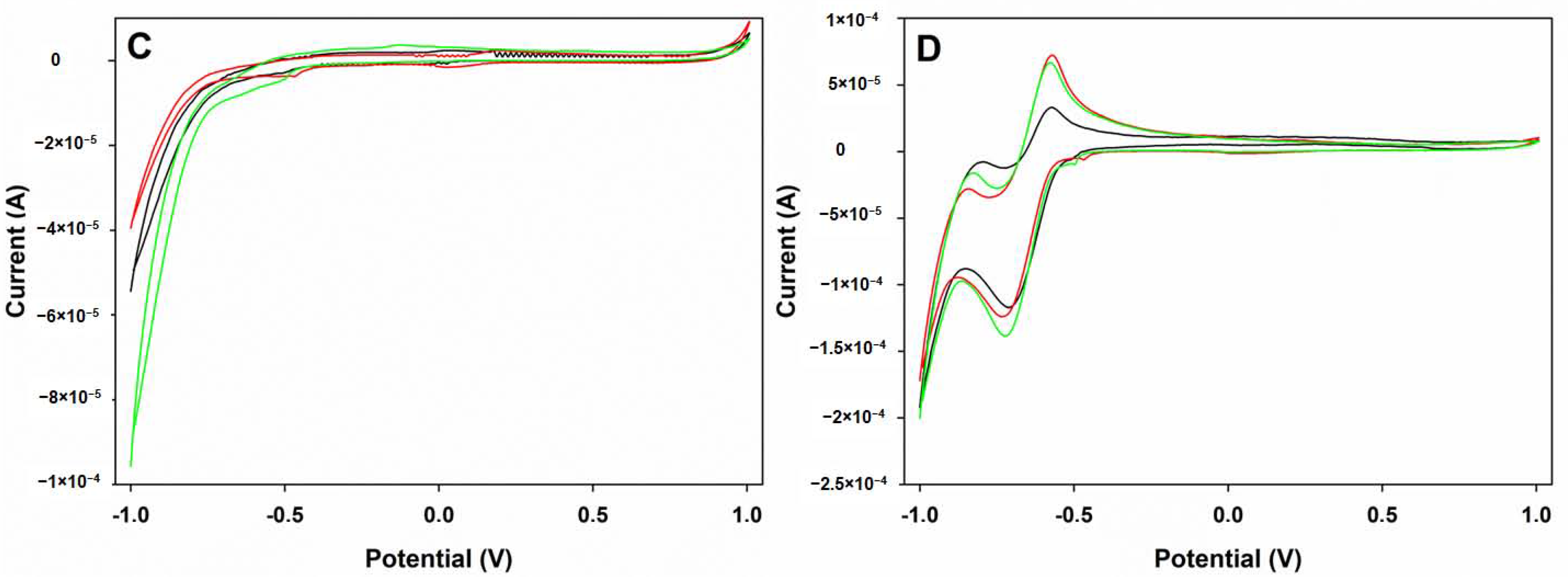
3.1. Voltammetric Array Response
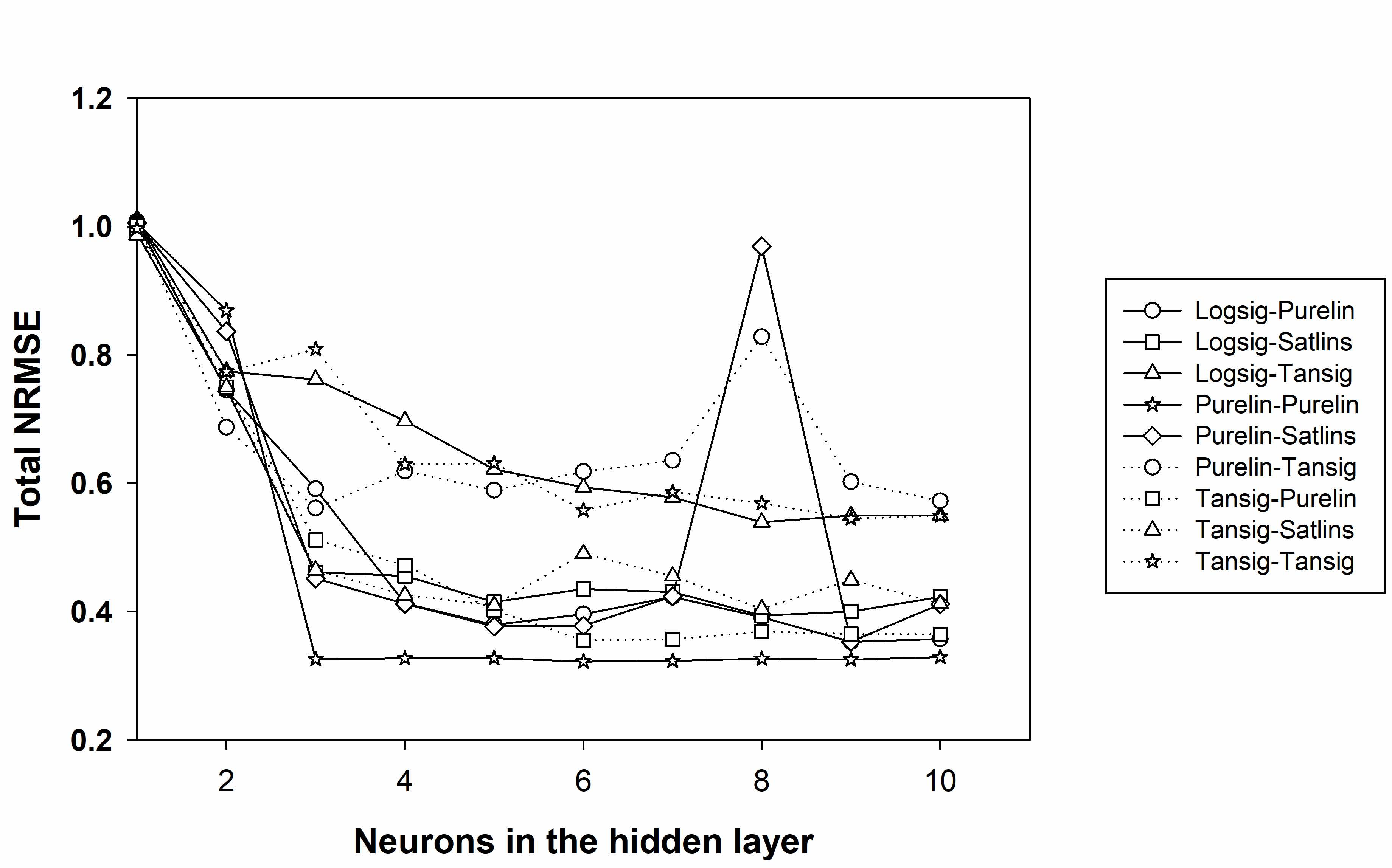
3.2. ANN Model Design
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- US Environmental Protection Agency. 4-Nitrophenol, Health and Environmental Effects Profile No. 135; US Environmental Protection Agency: Washington, DC, USA, 1980.
- Council of the European Union. Council Directive 76/464/EEC. Official J. L 1976, 129, 23–29. [Google Scholar]
- Gogate, P.R.; Pandit, A.B. A review of imperative technologies for wastewater treatment I: Oxidation technologies at ambient conditions. Adv. Environ. Res. 2004, 8, 501–551. [Google Scholar] [CrossRef]
- Yao, C.; Sun, H.; Fu, H.-F.; Tan, Z.-C. Sensitive simultaneous determination of nitrophenol isomers at poly(p-aminobenzene sulfonic acid) film modified graphite electrode. Electrochim. Acta 2015, 156, 163–170. [Google Scholar] [CrossRef]
- Thirumalraj, B.; Rajkumar, C.; Chen, S.-M.; Lin, K.-Y. Determination of 4-nitrophenol in water by use of a screen-printed carbon electrode modified with chitosan-crafted ZnO nanoneedles. J. Colloid Interface Sci. 2017, 499, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wu, Z.; Yang, H.; Deng, L.; Chen, X. Reduced graphene oxide-cyclodextrin-chitosan electrochemical sensor: Effective and simultaneous determination of o- and p-nitrophenols. Sens. Actuators B Chem. 2017, 25, 446–454. [Google Scholar] [CrossRef]
- Hryniewicz, B.M.; Orth, E.S.; Vidotti, M. Enzymeless PEDOT-based electrochemical sensor for the detection of nitrophenols and organophosphates. Sens. Actuators B Chem. 2018, 25, 570–578. [Google Scholar] [CrossRef]
- Del Valle, M. Electronic tongues employing electrochemical sensors. Electroanalysis 2010, 22, 1539–1555. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Wilson, D.; Abbas, M.N.; Radwan, A.L.A.; del Valle, M. Potentiometric electronic tongue to resolve mixtures of sulfide and perchlorate anions. Sensors 2011, 11, 3214–3226. [Google Scholar] [CrossRef] [PubMed]
- Gutés, A.; Ibanez, A.; Céspedes, F.; Alegret, S.; del Valle, M. Simultaneous determination of phenolic compounds by means of an automated voltammetric “electronic tongue”. Anal. Bioanal. Chem. 2005, 382, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Mimendia, A.; Gutierrez, J.M.; Opalski, L.J.; Ciosek, P.; Wróblewski, W.; Del Valle, M. SIA System employing the transient response from a potentiometric sensor array—Correction of a saline matrix effect. Talanta 2010, 82, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Barón, L.; Cartas, R.; Merkoçi, A.; Alegret, S.; Gutiérrez, J.M.; Leija, L.; Hernandez, P.R.; Muñoz, R.; del Valle, M. Data compression for a voltammetric electronic tongue modelled with artificial neural networks. Anal. Lett. 2005, 38, 2189–2206. [Google Scholar] [CrossRef]
- Del Valle, M. Sensor arrays and electronic tongue systems. Int. J. Electrochem. 2012, 2012, 986025. [Google Scholar] [CrossRef]
- Del Valle, M. Bioinspired sensor systems. Sensors 2011, 11, 10180–10186. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Kokot, S. Does chemometrics enhance the performance of electroanalysis? Anal. Chim. Acta 2008, 626, 130–146. [Google Scholar] [CrossRef] [PubMed]
- Cetó, X.; Gutiérrez, J.M.; Gutiérrez, M.; Céspedes, F.; Capdevila, J.; Mínguez, S.; Jiménez-Jorquera, C.; Del Valle, M. Determination of total polyphenol index in wines employing a voltammetric electronic tongue. Anal. Chim. Acta 2012, 732, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Parra, V.; Arrieta, Á.A.; Fernández-Escudero, J.-A.; Rodríguez-Méndez, M.L.; De Saja, J.A. Electronic tongue based on chemically modified electrodes and voltammetry for the detection of adulterations in wines. Sens. Actuators B Chem. 2006, 118, 448–453. [Google Scholar] [CrossRef]
- Cetó, X.; González-Calabuig, A.; Capdevila, J.; Puig-Pujol, A.; del Valle, M. Instrumental measurement of wine sensory descriptors using a voltammetric electronic tongue. Sens. Actuators B Chem. 2015, 207, 1053–1059. [Google Scholar] [CrossRef]
- Cetó, X.; González-Calabuig, A.; del Valle, M. Use of a bioelectronic tongue for the monitoring of the photodegradation of phenolic compounds. Electroanalysis 2015, 27, 225–233. [Google Scholar] [CrossRef]
- Cetó, X.; O’Mahony, A.M.; Wang, J.; del Valle, M. Simultaneous identification and quantification of nitro-containing explosives by advanced chemometric data treatment of cyclic voltammetry at screen-printed electrodes. Talanta 2013, 107, 270–276. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, R.M.; Mello, C.; Kubota, L.T. Simultaneous determination of phenol isomers in binary mixtures by differential pulse voltammetry using carbon fibre electrode and neural network with pruning as a multivariate calibration tool. Anal. Chim. Acta 2000, 420, 109–121. [Google Scholar] [CrossRef]
- Zeravik, J.; Hlavacek, A.; Lacina, K.; Skládal, P. State of the art in the field of electronic and bioelectronic tongues–towards the analysis of wines. Electroanalysis 2009, 21, 2509–2520. [Google Scholar] [CrossRef]
- Esteban, M.; Ariño, C.; Díaz-Cruz, J. Chemometrics for the analysis of voltammetric data. TrAC Trends in Anal. Chem. 2006, 25, 86–92. [Google Scholar] [CrossRef]
- Mallat, S.G. A theory for multiresolution signal decomposition: The wavelet representation. IEEE Trans. Pattern Anal. Mach. Intell. 1989, 11, 674–693. [Google Scholar] [CrossRef]
- Alegret, S.; Alonso, J.; Bartroli, J.; Céspedes, F.; MartinezFabregas, E.; del Valle, M. Amperometric biosensors based on bulk-modified epoxy graphite biocomposites. Sens. Mater. 1996, 8, 147–153. [Google Scholar]
- Gutés, A.; Calvo, D.; Céspedes, F.; del Valle, M. Automatic sequential injection analysis electronic tongue with integrated reference electrode for the determination of ascorbic acid, uric acid and paracetamol. Microchim. Acta 2007, 157, 1–6. [Google Scholar] [CrossRef]
- Cetó, X.; Gutiérrez, J.M.; Moreno-Barón, L.; Alegret, S.; del Valle, M. Voltammetric electronic tongue in the analysis of cava wines. Electroanalysis 2011, 23, 72–78. [Google Scholar] [CrossRef]
- Cetó, X.; Céspedes, F.; Pividori, M.I.; Gutiérrez, J.M.; del Valle, M. Resolution of phenolic antioxidant mixtures employing a voltammetric bio-electronic tongue. Analyst 2012, 137, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Enache, T.A.; Oliveira-Brett, A.M. Phenol and para-substituted phenols electrochemical oxidation pathways. J. Electroanal. Chem. 2011, 655, 9–16. [Google Scholar] [CrossRef]
- El Mhammedi, M.; Achak, M.; Bakasse, M.; Chtaini, A. Electrochemical determination of para-nitrophenol at apatite-modified carbon paste electrode: Application in river water samples. J. Hazard. Mater. 2009, 163, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Despagne, F.; Massart, D.L. Neural networks in multivariate calibration. Analyst 1998, 123, 157R–178R. [Google Scholar] [CrossRef] [PubMed]
- Hanrahan, G. Computational neural networks driving complex analytical problem solving. Anal. Chem. 2010, 82, 4307–4313. [Google Scholar] [CrossRef] [PubMed]
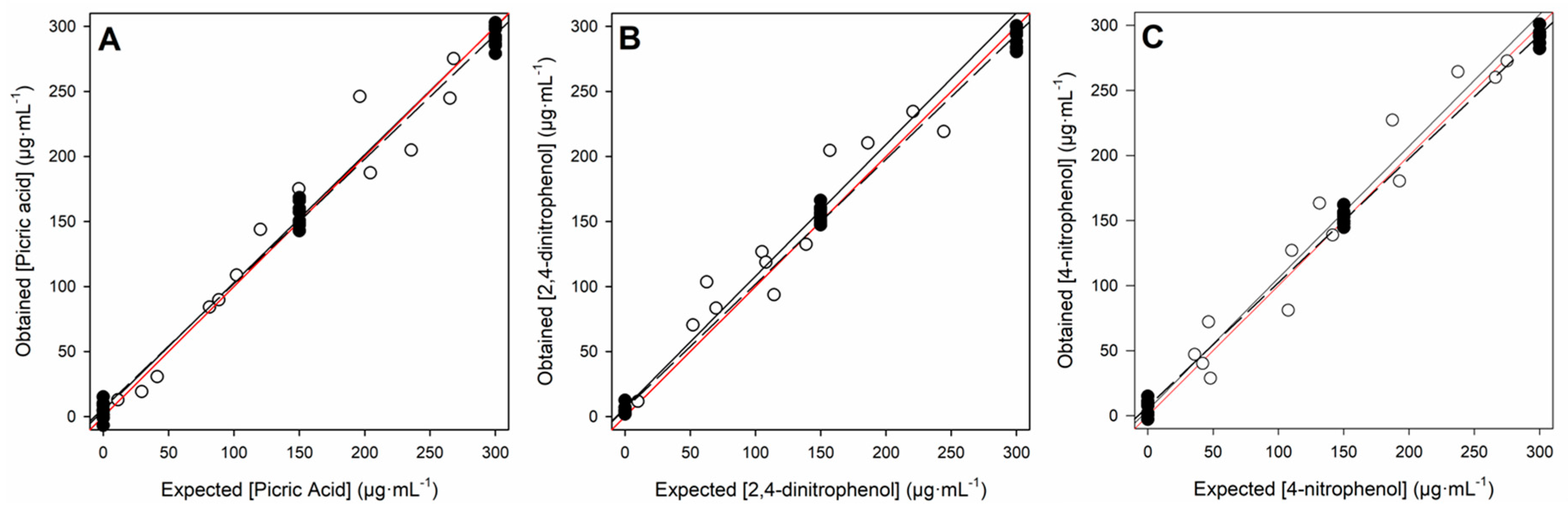
| Set | Analyte | r | Slope | Intercept (mg·L−1) | NRMSE | Total NRMSE |
|---|---|---|---|---|---|---|
| Training Set | picric acid | 0.998 | 0.959 ± 0.027 | 6.3 ± 5.2 | 0.032 | 0.030 |
| 2,4-dinitrophenol | 0.998 | 0.961 ± 0.027 | 5.8 ± 10.4 | 0.032 | ||
| 4-nitrophenol | 0.998 | 0.952 ± 0.021 | 7.1 ± 4.0 | 0.029 | ||
| Testing Set | picric acid | 0.983 | 0.983 ± 0.161 | 4.6 ± 26.0 | 0.073 | 0.076 |
| 2,4-dinitrophenol | 0.948 | 1.012 ± 0.222 | 6.7 ± 30.2 | 0.087 | ||
| 4-nitrophenol | 0.973 | 1.013 ± 0.160 | 4.4 ± 26.0 | 0.073 |
| Sample | Expected Concentration (μg·mL−1) | Single Sensor Concentration (μg·mL−1) | ET Concentration (μg·mL−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| picric acid | 2,4-dinitro-phenol | 4-nitro-phenol | picric acid | 2,4-dinitro-phenol | 4-nitro-phenol | picric acid | 2,4-dinitro-phenol | 4-nitro-phenol | |
| 1 | 102 | 186 | 107 | 275 | 151 | 110 | 109 | 210 | 81 |
| 2 | 11.1 | 244 | 141 | 392 | 222 | 26 | 12.9 | 219 | 139 |
| 3 | 265 | 9.9 | 131 | 208 | 67 | 8.1 | 245 | 11.7 | 163 |
| 4 | 204 | 138 | 187 | 362 | 196 | 73 | 187 | 132 | 227 |
| 5 | 81 | 157 | 42 | 270 | 148 | 49 | 84 | 205 | 40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Calabuig, A.; Cetó, X.; Del Valle, M. A Voltammetric Electronic Tongue for the Resolution of Ternary Nitrophenol Mixtures. Sensors 2018, 18, 216. https://doi.org/10.3390/s18010216
González-Calabuig A, Cetó X, Del Valle M. A Voltammetric Electronic Tongue for the Resolution of Ternary Nitrophenol Mixtures. Sensors. 2018; 18(1):216. https://doi.org/10.3390/s18010216
Chicago/Turabian StyleGonzález-Calabuig, Andreu, Xavier Cetó, and Manel Del Valle. 2018. "A Voltammetric Electronic Tongue for the Resolution of Ternary Nitrophenol Mixtures" Sensors 18, no. 1: 216. https://doi.org/10.3390/s18010216