A Prototype Sensor for In Situ Sensing of Fine Particulate Matter and Volatile Organic Compounds
Abstract
:1. Introduction
2. Materials and Methods
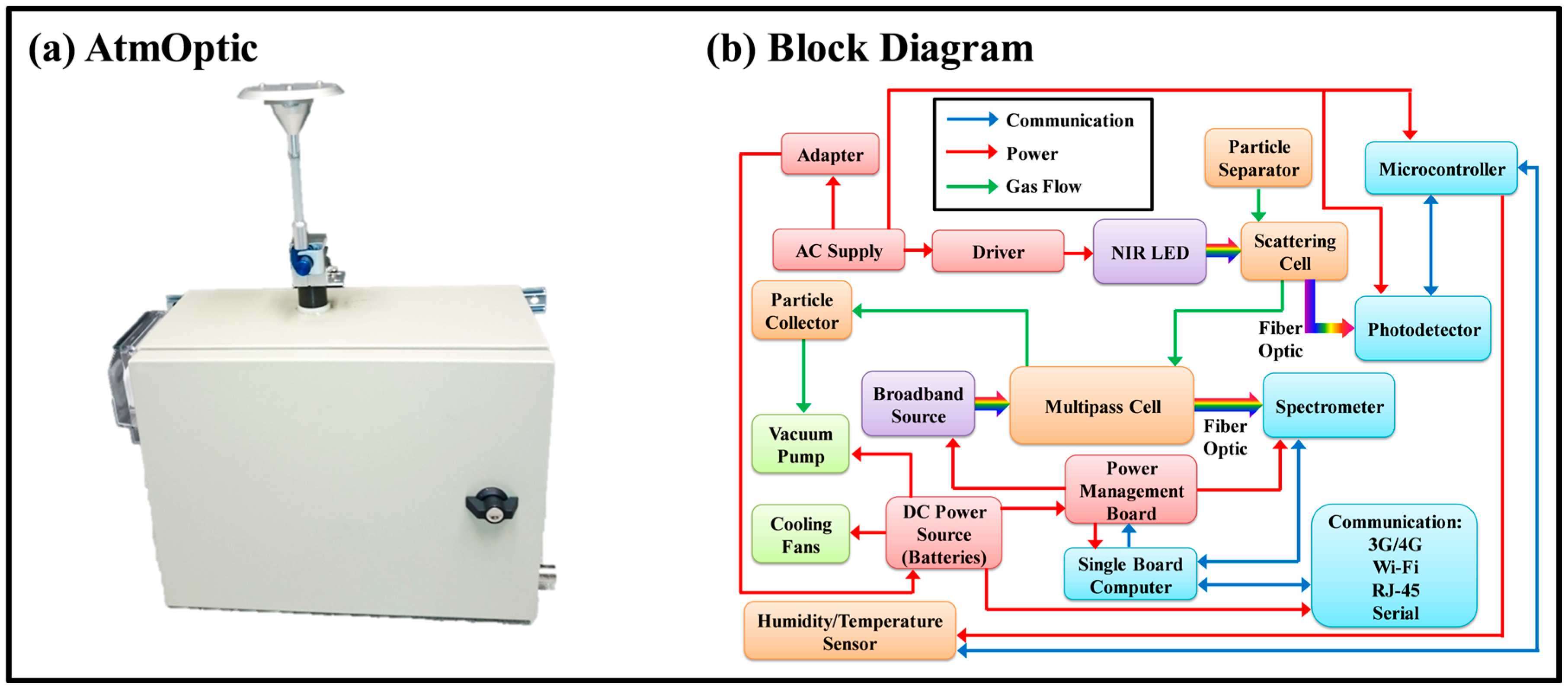
2.1. Instrument
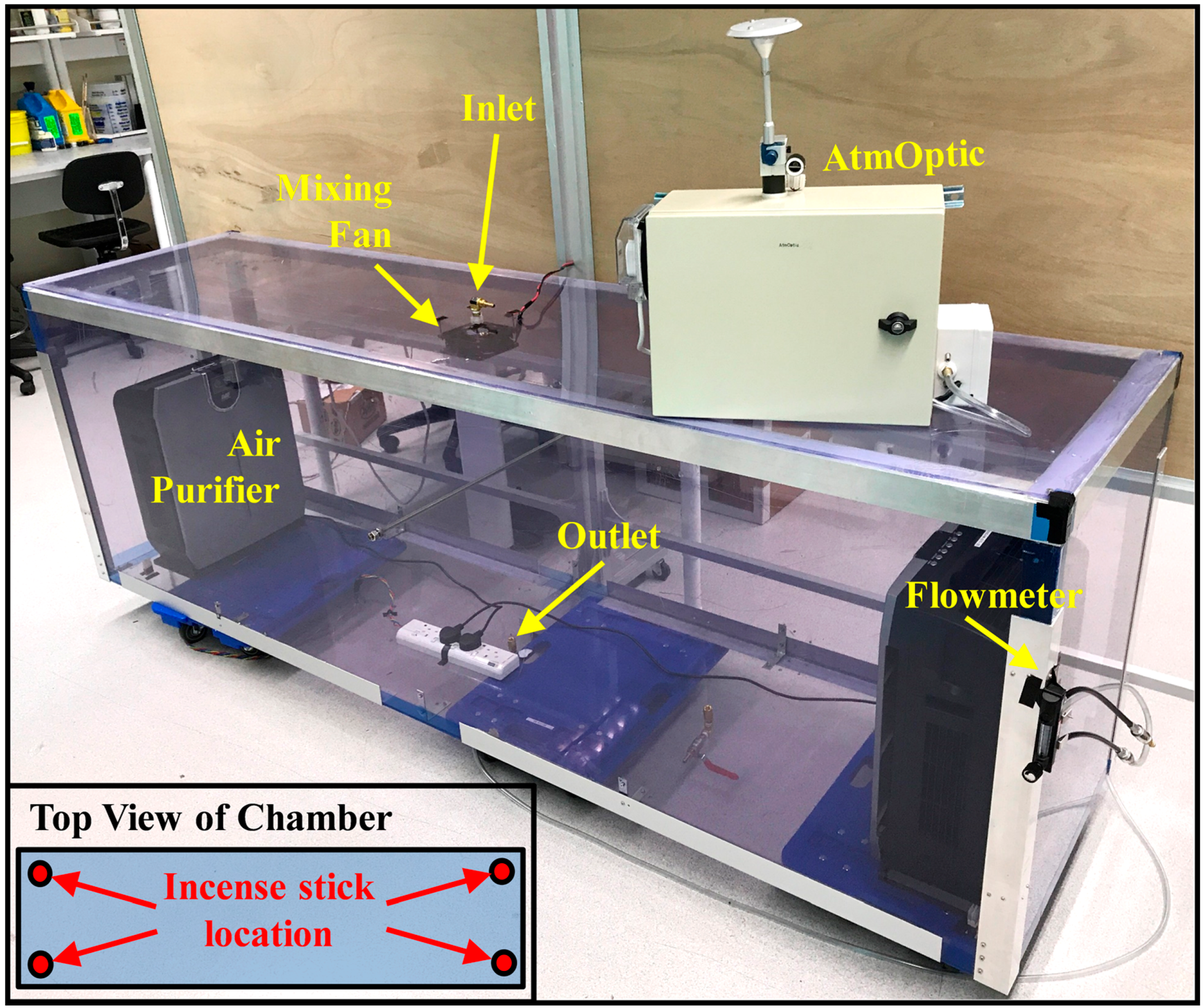
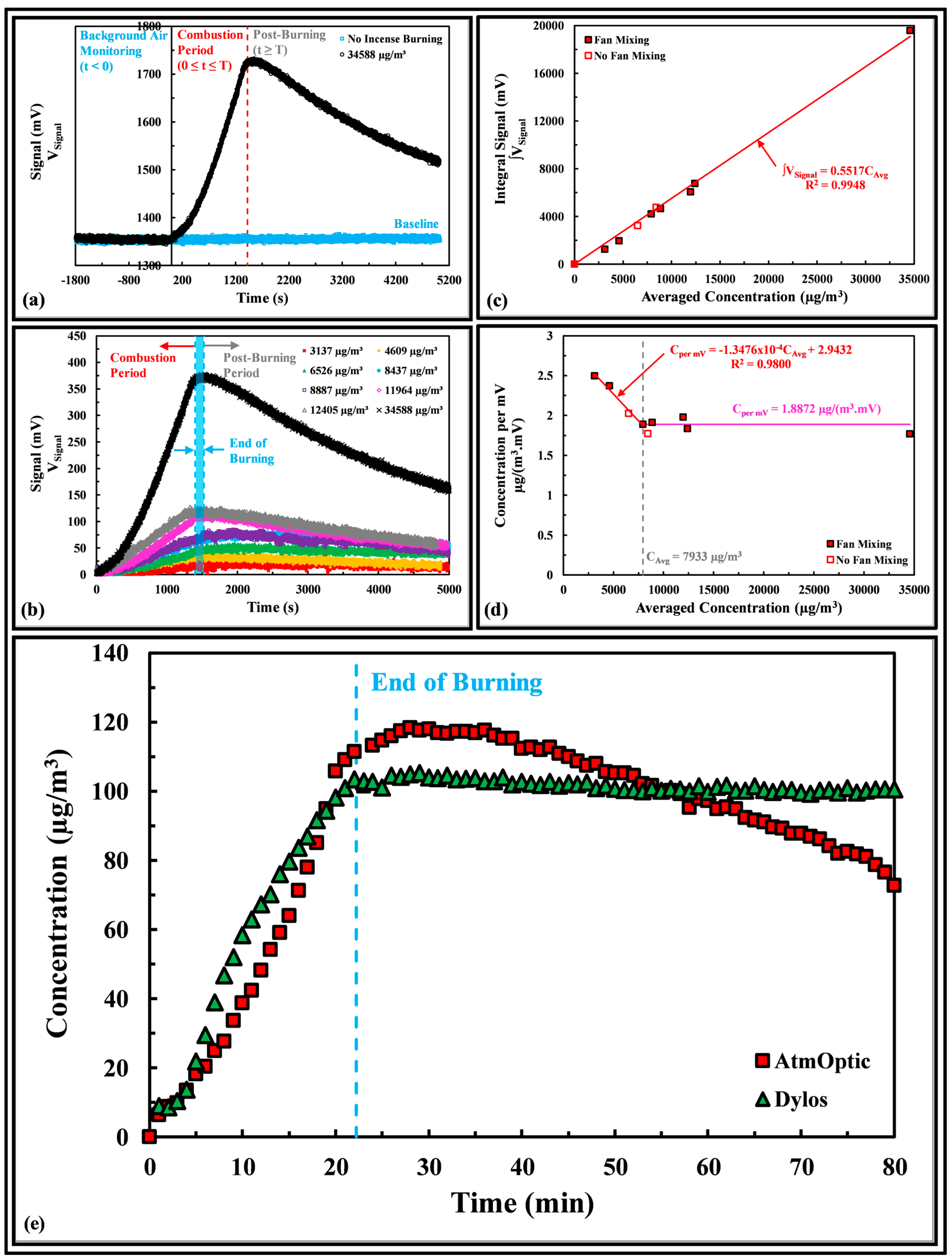
2.2. Fine Particulate Matter
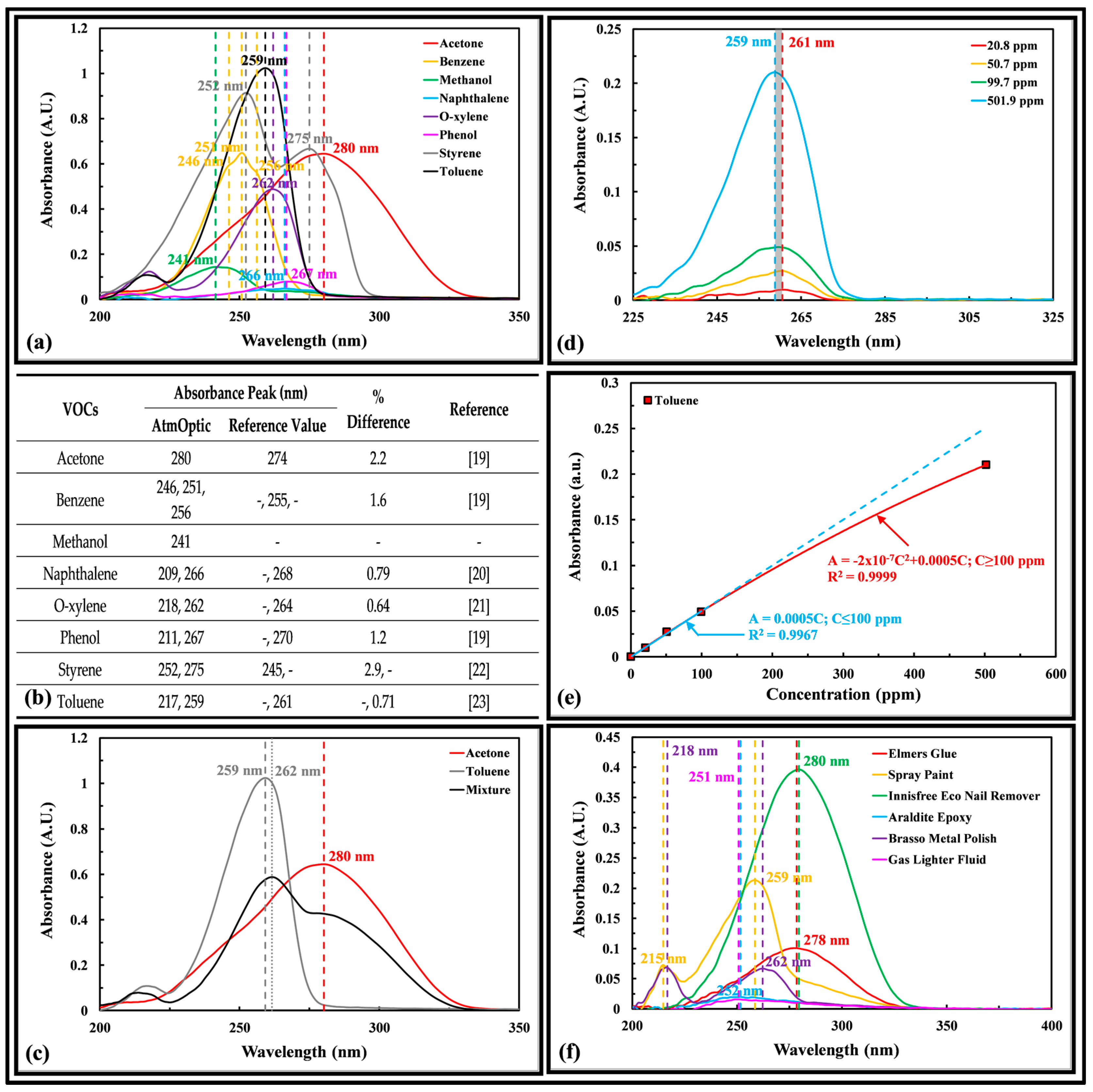
2.3. Volatile Organic Compounds
2.3.1. Laboratory Chemicals
2.3.2. Household Products
3. Results and Discussion
3.1. Fine Particulate Matter
3.2. Volatile Organic Compounds
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization (WHO). New Release: World Health Assembly Closes, Passing Resolutions on Air Pollution and Epilepsy. 2015. Available online: http://www.who.int/mediacentre/news/releases/2015/wha-26-may-2015/en/ (assessed on 15 August 2017).
- U.S. Environmental Protection Agency (USEPA). National-Scale Air Toxics Assessment, and Summary of Results for the 2011 National-Scale Assessment. 2015. Available online: http://www.epa.gov/national-air-toxics-assessment/2011-national-air-toxics-assessment (assessed on 15 August 2017).
- World Health Organization (WHO). WHO Guidelines for Indoor Air Quality: Selected Pollutants; World Health Organization Regional Office for Europe: Copenhagen, Denmark, 2010. [Google Scholar]
- Williams, R.; Kilaru, V.; Snyder, E.; Kaufman, A.; Dye, T.; Rutter, A.; Russell, A.; Hafner, H. Air Sensor Guidebook; EPA/600/R-14/159; U.S. Environmental Protection Agency: Washington, DC, USA, 2014.
- Aleixandre, M.; Gerboles, M. Review of small commercial sensors for indicative monitoring of ambient gas. Chem. Eng. Trans. 2012, 30, 169–174. [Google Scholar]
- MacDonell, M.; Raymond, M.; Wyker, D.; Finster, M.; Chang, Y.-S.; Raymond, T.; Temple, B.; Scofield, M. Mobile Sensors and Applications for Air Pollutants; EPA/600/R-14/051; U.S. Environmental Protection Agency: Washington, DC, USA, 2013.
- Snyder, E.; Watkins, T.H.; Solomon, P.A.; Thoma, E.D.; Williams, R.; Hagler, G.S.W.; Shelow, D.; Hindin, D.A.; Kilaru, V.J.; Peter, W. The changing paradigm of air pollution monitoring. Environ. Sci. Technol. 2013, 47, 11369–11377. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Morawska, L.; Martani, C.; Biskos, G.; Neophytou, M.; Sabatino, S.D.; Bell, M.; Norford, L.; Britter, R. The rise of low-cost sensing for managing air pollution in cities. Environ. Int. 2015, 75, 199–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.; Kaufman, A.; Garvey, S. Next Generation Air Monitor (NGAM) VOC Sensor Evaluation Report; EPA/600/R-15/122; U.S. Environmental Protection Agency: Washington, DC, USA, 2015.
- Liu, W.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A survey on gas sensing technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Ng, C.L.; Hemond, H.F.; Senft-Grupp, S. Highly Compact Multi-Optical-Junction Optical Flowcell and Flexibly Deployable Optical Sensing Assemblies and Systems for In-Situ Real-Time Spectroscopic Measurements. Patent Corporation Treaty Application PCT/SG2012/000142, 20 April 2012. [Google Scholar]
- Ng, C.L.; Senft-Grupp, S.; Hemond, H.F. A multi-platform optical sensor for in situ sensing of water chemistry. Limnol. Oceanogr. Methods 2012, 10, 978–990. [Google Scholar] [CrossRef]
- See, S.W.; Balasubramanian, R. Characterization of fine particle emissions from incense burning. Build. Environ. 2011, 46, 1074–1080. [Google Scholar] [CrossRef]
- Tittarelli, A.; Borgini, A.; Bertoldi, M.; De Saeger, E.; Ruprecht, A.; Stefanoni, R.; Tagliabue, G.; Contiero, P.; Crosignani, P. Estimation of Particle Mass Concentration in Ambient Air Using a Particle Counter. Atmos. Environ. 2008, 42, 8543–8548. [Google Scholar] [CrossRef]
- Lee, J.Y.; Shin, H.J.; Bae, S.Y.; Kim, Y.P.; Kang, C.-H. Seasonal Variations of Particle Size Distributions of PAHs at Seoul, South Korea. Air Qual. Atmos. Health 2008, 1, 57–68. [Google Scholar] [CrossRef]
- Air Quality Sensor Network for Philadelphia—Data Validation. Available online: http://www.fijnstofmeter.com/documentatie/Data-Validation.pdf (accessed on 13 September 2017).
- Day, D.E.; Malm, W.C.; Kreidenwais, S.M. Aerosol Light Scattering Measurements as a Function of Relative Humidity. J. Air Waste Manag. Assoc. 2000, 50, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: Berlin, Germany, 2006. [Google Scholar]
- Kshitij Education India. Effect of Conjucation on λmax. Available online: http://www.kshitij-iitjee.com/effect-of-conjugation (accessed on 15 August 2017).
- NIST Chemistry Webbook, SRD 69. Naphthalene. Available online: http://webbook.nist.gov/cgi/cbook.cgi?Name=naphthalene&Units=SI&cTG=on&cUV=on (accessed on 15 August 2017).
- Camou, S.; Tamechika, E.; Horiuchi, T. Portable Sensor for Determining Benzene Concentration from Airborne Liquid Samples with High Accuracy. NTT Tech. Rev. 2012, 10. Available online: https://www.ntt-review.jp/archive/ntttechnical.php?contents=ntr201202fa7.html (accessed on 15 August 2017).
- NIST Chemistry Webbook, SRB69. Styrene. Available online: http://webbook.nist.gov/cgi/cbook.cgi?Name=styrene&Units=SI&cTG=on&cUV=on (accessed on 15 August 2017).
- NIST Chemistry Webbook, SRD 69. Toluene. Available online: http://webbook.nist.gov/cgi/cbook.cgi?Name=toluene&Units=SI&cTG=on&cUV=on (accessed on 15 August 2017).
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, C.-L.; Kai, F.-M.; Tee, M.-H.; Tan, N.; Hemond, H.F. A Prototype Sensor for In Situ Sensing of Fine Particulate Matter and Volatile Organic Compounds. Sensors 2018, 18, 265. https://doi.org/10.3390/s18010265
Ng C-L, Kai F-M, Tee M-H, Tan N, Hemond HF. A Prototype Sensor for In Situ Sensing of Fine Particulate Matter and Volatile Organic Compounds. Sensors. 2018; 18(1):265. https://doi.org/10.3390/s18010265
Chicago/Turabian StyleNg, Chee-Loon, Fuu-Ming Kai, Ming-Hui Tee, Nicholas Tan, and Harold F. Hemond. 2018. "A Prototype Sensor for In Situ Sensing of Fine Particulate Matter and Volatile Organic Compounds" Sensors 18, no. 1: 265. https://doi.org/10.3390/s18010265



