Assessment of Blue Carbon Storage by Baja California (Mexico) Tidal Wetlands and Evidence for Wetland Stability in the Face of Anthropogenic and Climatic Impacts
Abstract
:1. Introduction
2. Materials and Methods
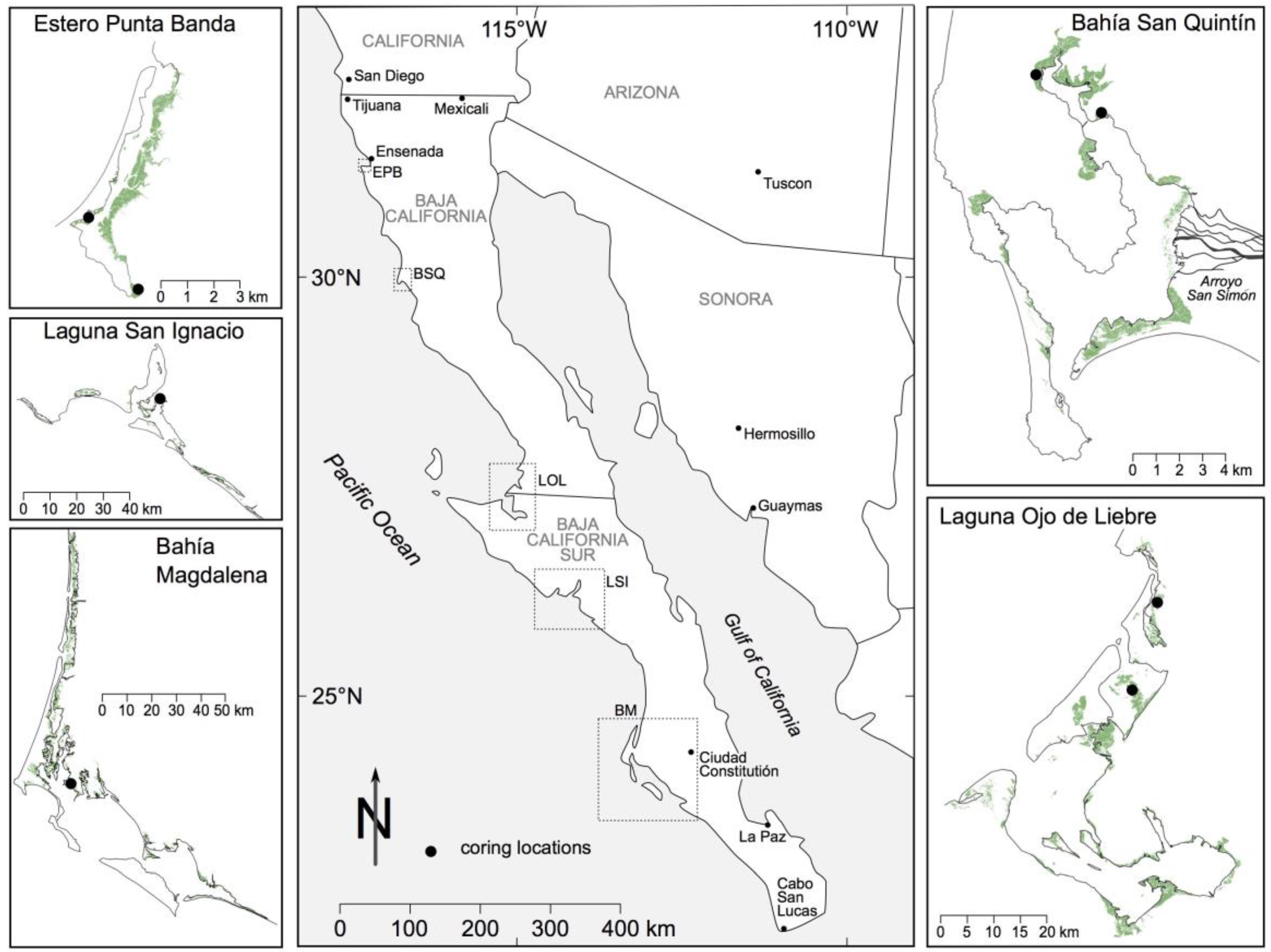
2.1. Environmental Setting
2.2. Carbon Storage
2.2.1. Remote Sensing
2.2.2. Sedimentary Analyses
2.2.3. Data Analysis
3. Results
3.1. Analysis of Aerial and High Resolution Satellite Imagery
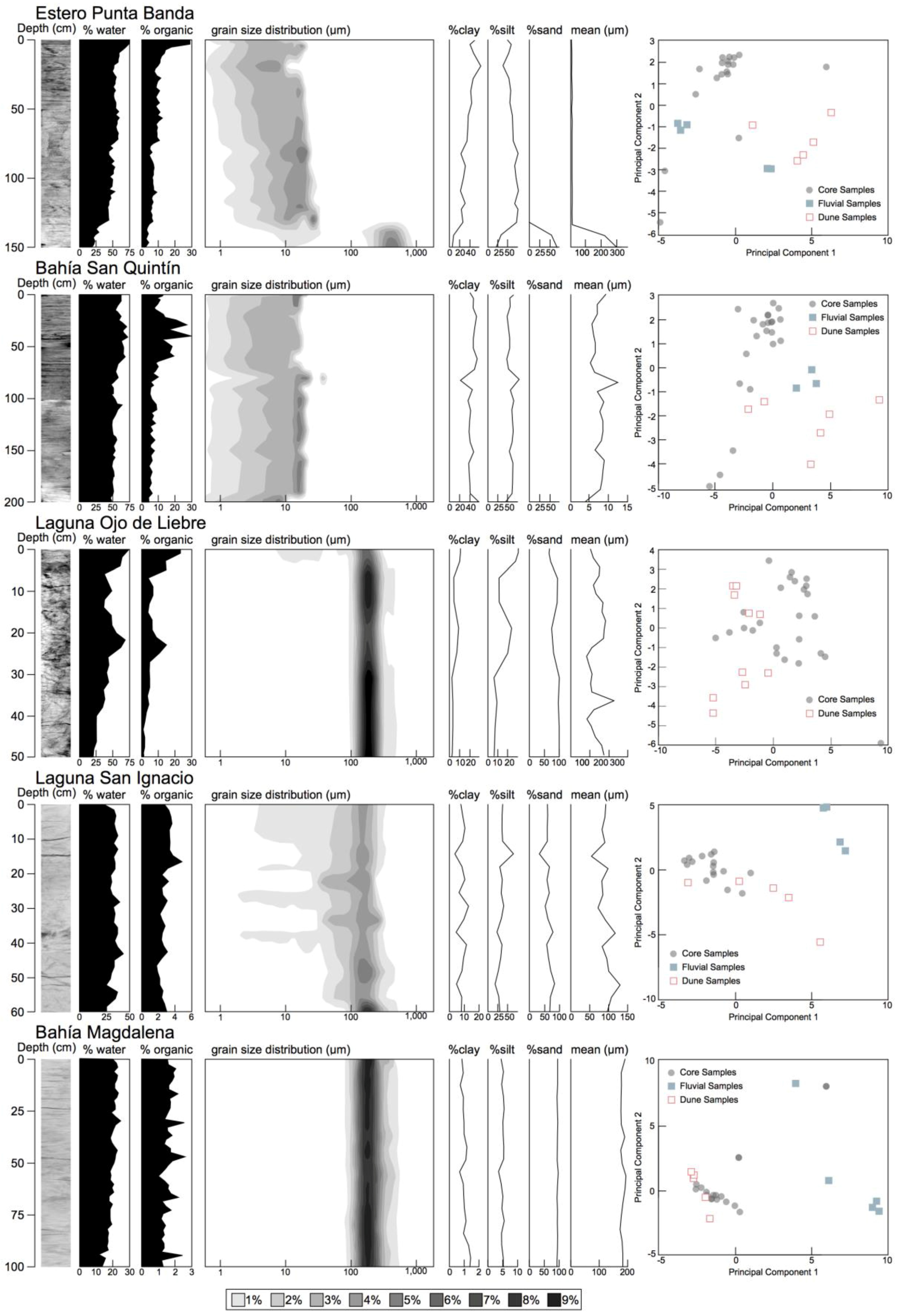
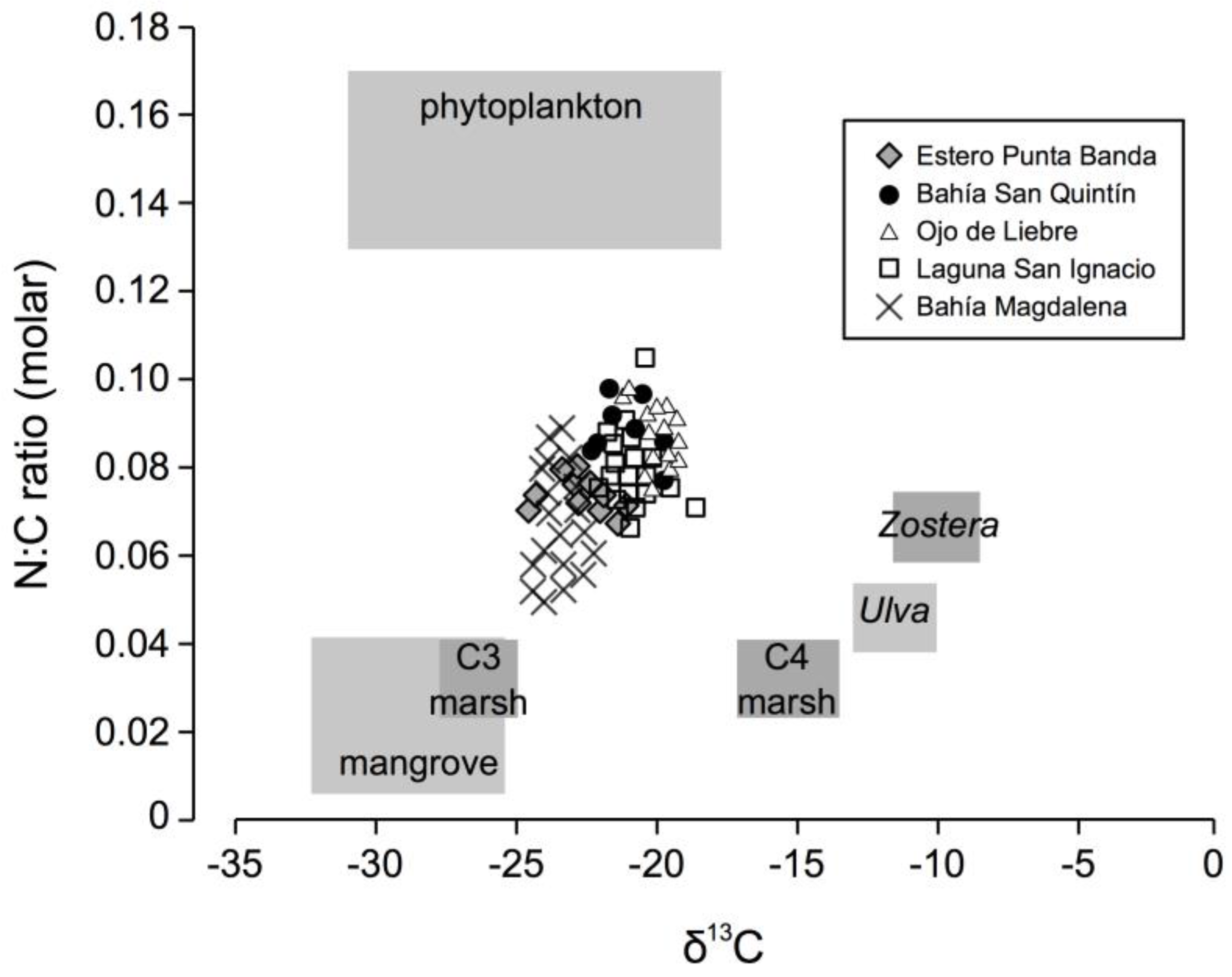
3.2. Sedimentary Analysis
4. Discussion
4.1. Carbon Storage
4.2. Wetland Stability
4.3. Future Anthropogenic Impacts
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Macleod, E.; Chmura, G.L.; Bouillon, S.; Salm, R.; Björk, M.; Duarte, C.M.; Lovelock, C.; Schlesinger, W.; Silliman, B.R. A blueprint for blue carbon: Toward an improved understanding of the role of vegetated coastal habitats in sequestering CO2. Front. Ecol. Environ. 2011, 9, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Schelske, C.L.; Odum, E.P. Mechanisms maintaining high productivity in Georgia estuaries. Proc. Gulf Caribb. Fish. Inst. 1961, 14, 75–80. [Google Scholar]
- Watson, E.B. Changing elevation, accretion, and tidal marsh plant assemblages in a South San Francisco Bay tidal marsh. Estuaries 2004, 27, 684–698. [Google Scholar] [CrossRef]
- Middelburg, J.J.; Nieuwenhuize, J.; Lubberts, R.K.; van de Plassche, O. Organic carbon isotope systematics of coastal marshes. Estuar. Coast. Shelf Sci. 1997, 45, 681–687. [Google Scholar] [CrossRef]
- Duarte, C.; Middelburg, J.J.; Caraco, N. Major role of marine vegetation on the oceanic carbon cycle. Biogeosciences 2005, 2, 1–8. [Google Scholar] [CrossRef]
- Hopkinson, C.S.; Cai, W.J.; Hu, X. Carbon sequestration in wetland dominated coastal systems—A global sink of rapidly diminishing magnitude. Curr. Opin. Environ. Sustain. 2012, 4, 186–194. [Google Scholar] [CrossRef]
- Moseman-Valtierra, S. Reconsidering the climatic role of marshes: Are they sinks or sources of greenhouse gases? In Marshes: Ecology, Management and Conservation; Abreu, D.C., de Borbon, S.L., Eds.; Nova Scientific Publishers: Hauppauge, NY, USA, 2012; pp. 1–48. [Google Scholar]
- Neubauer, S.C. On the challenges of modeling the net radiative forcing of wetlands: Reconsidering Mitsch et al. 2013. Landsc. Ecol. 2014, 29, 571–577. [Google Scholar] [CrossRef]
- Fenchel, T.; Blackburn, T.H. Bacteria and Mineral Cycling; Academic Press: London, UK, 1979. [Google Scholar]
- Morris, J.T.; Whiting, G.J. Emission of gaseous carbon dioxide from salt-marsh sediments and its relation to other carbon losses. Estuaries 1986, 9, 9–19. [Google Scholar] [CrossRef]
- Chmura, G.L.; Kellman, L.; Guntenspergen, G.R. The greenhouse gas flux and potential global warming feedbacks of a northern macrotidal and microtidal salt marsh. Environ. Res. Lett. 2011, 6, 044016. [Google Scholar] [CrossRef]
- Weston, N.B.; Neubauer, S.C.; Velinsky, D.J.; Vile, M.A. Net ecosystem carbon exchange and the greenhouse gas balance of tidal marshes along an estuarine salinity gradient. Biogeochemistry 2014, 120, 163–189. [Google Scholar] [CrossRef]
- Crooks, S.; Herr, D.; Tamelander, J.; Laffoley, D.; Vandever, J. Mitigating Climate Change through Restoration and Management of Coastal Wetlands and Near-Shore Marine Ecosystems: Challenges and Opportunities; World Bank: Washington, DC, USA, 2011. [Google Scholar]
- Nicholls, R.J.; Marinova, N.; Lowe, J.A.; Brown, S.; Vellinga, P.; De Gusmao, D.; Hinkel, J.; Tol, R.S.J. Sea-level rise and its possible impacts given a ‘beyond 4 C world’ in the twenty-first century. Philos. Trans. R. Soc. Lond. A 2011, 369, 161–181. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, M.L.; Blum, L.K. Enhanced decomposition offsets enhanced productivity and soil carbon accumulation in coastal wetlands responding to climate change. Biogeosciences 2011, 8, 987–993. [Google Scholar] [CrossRef]
- Bromberg, K.D.; Bertness, M.D. Reconstructing New England salt marsh losses using historical maps. Estuar. Coast. 2005, 28, 823–832. [Google Scholar] [CrossRef]
- Stein, E.D.; Dark, S.; Longcore, T.; Grossinger, R.; Hall, N.; Beland, M. Historical ecology as a tool for assessing landscape change and informing wetland restoration priorities. Wetlands 2010, 30, 589–601. [Google Scholar] [CrossRef]
- Dahl, T.E. Wetland Loses in the United States 1780s to 1980s; U.S. Department of the Interior, U.S. Fish and Wildlife Service: Washington, DC, USA, 1990.
- Landgrave, R.; Moreno-Casasola, P. Evaluación cuantitativa de la pérdida de humedales en México. Investig. Ambient. 2012, 4, 19–35. [Google Scholar]
- Ke, C.Q.; Zhang, D.; Wang, F.Q.; Chen, S.X.; Schmullius, C.; Boerner, W.M.; Wang, H. Analyzing coastal wetland change in the Yancheng national nature reserve, China. Reg. Environ. Chang. 2011, 11, 161–173. [Google Scholar] [CrossRef]
- Alongi, D.M.; Murdiyarso, D.; Fourqurean, J.W.; Kauffman, J.B.; Hutahaean, A.; Crooks, S.; Lovelock, C.E.; Howard, J.; Herr, D.; Fortes, M.; et al. Indonesia’s blue carbon: A globally significant and vulnerable sink for seagrass and mangrove carbon. Wetl. Ecol. Manag. 2016, 24, 3–13. [Google Scholar] [CrossRef]
- Ezcurra, P.; Ezcurra, E.; Garcillán, P.P.; Costa, M.T.; Aburto-Oropeza, O. Coastal landforms and accumulation of mangrove peat increase carbon sequestration and storage. Proc. Natl. Acad. Sci. USA 2016, 113, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- Schile, L.M.; Kauffman, J.B.; Crooks, S.; Fourqurean, J.W.; Glavan, J.; Megonigal, J.P. Limits on carbon sequestration in arid blue carbon ecosystems. Ecol. Appl. 2017, 27, 859–874. [Google Scholar] [CrossRef] [PubMed]
- López-Medellín, X.; Ezcurra, E.; González-Abraham, C.; Hak, J.; Santiago, L.S.; Sickman, J.O. Oceanographic anomalies and sea-level rise drive mangroves inland in the Pacific coast of Mexico. J. Veg. Sci. 2011, 22, 143–151. [Google Scholar] [CrossRef]
- Fagherazzi, S. The ephemeral life of a salt marsh. Geology 2013, 41, 943–944. [Google Scholar] [CrossRef]
- Watson, E.B.; Wigand, C.; Davey, E.W.; Andrews, H.M.; Bishop, J.; Raposa, K.B. Wetland loss patterns and inundation-productivity relationships prognosticate widespread salt marsh loss for southern New England. Estuar. Coast. 2017, 40, 662–681. [Google Scholar] [CrossRef]
- Weston, N.B. Declining sediments and rising seas: An unfortunate convergence for tidal wetlands. Estuar. Coast. 2014, 37, 1–23. [Google Scholar] [CrossRef]
- Holguin, G.; Gonzalez-Zamorano, P.; de-Bashan, L.E.; Mendoza, R.; Yoav Bashan, E.A. Mangrove health in an arid environment encroached by urban development—A case study. Sci. Total Environ. 2006, 353, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Peinado, M.; Alcaraz, F.; Delgadillo, J.; Cruz, M.; Alvarez, J.; Aguirre, J.L. The coastal salt marshes of California and Baja California. Plant Ecol. 1994, 110, 55–66. [Google Scholar]
- Aguila Ramírez, R.N.; Casas Valdez, M.; Ortega García, S.; Núñez López, R.A.; Cruz Ayala, M.B. Spatial and seasonal variation of macroalgal biomass in Laguna Ojo de Liebre, Baja California Sur, Mexico. Hydrobiologia 2003, 501, 207–214. [Google Scholar] [CrossRef]
- Bizzarro, J.J. A Review of the Physical and Biological Characteristics of the Bahia Magdalena Lagoon Complex (Baja CA Sur). Bull. South. CA. Acad. Sci. 2008, 107, 1–24. [Google Scholar] [CrossRef]
- Perez-Arteaga, A.; Jackson, S.F.; Carrera, E.; Gaston, K.J. Priority sites for wildfowl conservation in Mexico. Anim. Conserv. 2005, 8, 41–50. [Google Scholar] [CrossRef]
- Ward, D.H.; Morton, A.; Tibbitts, T.L.; Douglas, D.C.; Carrera-González, E. Long-term change in eelgrass distribution at Bahía San Quintín, Baja California, Mexico, using satellite imagery. Estuaries 2003, 26, 1529–1539. [Google Scholar] [CrossRef]
- Van Dyke, E.; Wasson, K. Historical ecology of a central California estuary: 150 years of habitat change. Estuaries 2005, 28, 173–189. [Google Scholar] [CrossRef]
- Tang, J.; Wang, L.; Myint, S.W. Improving urban classification through fuzzy supervised classification and spectral mixture analysis. Int. J. Remote Sens. 2007, 28, 4047–4063. [Google Scholar] [CrossRef]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Carriquiry, J.D.; Jorgensen, P.; Villaescusa, J.A.; Ibarra-Obando, S.E. Isotopic and elemental composition of marine macrophytes as biotracers of nutrient recycling within a coastal lagoon in Baja California, Mexico. Estuar. Coast. 2016, 39, 451–461. [Google Scholar] [CrossRef]
- Kwak, T.J.; Zedler, J.B. Food web analysis of southern California coastal wetlands using multiple stable isotopes. Oecologia 1997, 110, 262–277. [Google Scholar] [CrossRef] [PubMed]
- Cloern, J.E.; Canuel, E.A.; Harris, D. Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco Bay estuarine system. Limnol. Oceanogr. 2002, 47, 713–729. [Google Scholar] [CrossRef]
- Gonneea, M.E.; Payton, A.; Herrera-Silveira, J.A. Tracing organic matter sources and carbon burial in mangrove sediments over the past 160 years. Estuar. Coast. Shelf Sci. 2004, 61, 211–227. [Google Scholar] [CrossRef]
- Gray, A.B.; Pasternack, G.B.; Watson, E.B. Hydrogen peroxide treatment effects on the particle size distribution of alluvial and marsh sediments. Holocene 2010, 20, 293–301. [Google Scholar] [CrossRef]
- Folk, R.L. Petrology of Sedimentary Rocks; University of Texas: Austin, TX, USA, 1968. [Google Scholar]
- Xue, J.; Lee, C.; Wakeham, S.G.; Armstrong, R.A. Using principal components analysis (PCA) with cluster analysis to study the organic geochemistry of sinking particles in the ocean. Org. Geochem. 2011, 42, 356–367. [Google Scholar] [CrossRef]
- Vale, S.S.; Fuller, I.C.; Procter, J.N.; Basher, L.R.; Smith, I.E. Application of a confluence-based sediment-fingerprinting approach to a dynamic sedimentary catchment, New Zealand. Hydrol. Process. 2017, 30, 812–829. [Google Scholar] [CrossRef]
- Callaway, J.C.; Borgnis, E.L.; Turner, R.E.; Milan, C.S. Carbon sequestration and sediment accretion in San Francisco Bay tidal wetlands. Estuar. Coast. 2012, 35, 1163–1181. [Google Scholar] [CrossRef]
- Watson, E.B.; Hughes, B.B. Stable isotope analysis of estuarine biota of Elkhorn Slough. (unpublished data).
- Ibarra-Obando, S.E.; Solana-Arellano, E.; Poumian-Tapia, M. The role of Zostera marina in the carbon cycle of Bahía San Quintín, Baja California. In Carbono en Ecosistemas Acuáticos de México; Hernández de la Torre, B., Gaxiola-Castro, G., Eds.; Instituto Nacional de Ecología: Distrito Federal, México, 2007; pp. 201–213. [Google Scholar]
- Aburto-Oropeza, O.; Ezcurra, E.; Danemann, G.; Valdez, V.; Murray, J.; Sala, E. Mangroves in the Gulf of California increase fishery yields. Proc. Natl. Acad. Sci. USA 2008, 105, 10456–10459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouillon, S.; Connolly, R.M.; Lee, S.Y. Organic matter exchange and cycling in mangrove ecosystems: Recent insights from stable isotope studies. J. Sea Res. 2008, 59, 44–58. [Google Scholar] [CrossRef] [Green Version]
- Tue, N.Y.; Hamaoka, H.; Sogabe, A.; Quy, T.D.; Njuan, M.T.; Omori, K. The application of δ13C and C/N ratios as indicators of organic carbon storage and paleoenvironmental change of the mangrove ecosystem from Ba Lat Estuary, Red River, Vietnam. Environ. Earth Sci. 2011, 64, 1475–1486. [Google Scholar] [CrossRef]
- Ralison, O.A.; Borges, A.V.; Dehairs, F.; Middelburg, J.J.; Vouillon, S. Carbon biogeochemistry of the Betsiboka estuary (North-Western Madagascar). Stable Isot. Biogeosci. 2008, 39, 1649–1658. [Google Scholar] [CrossRef] [Green Version]
- Sollins, P.; Homann, P.; Caldwell, B.A. Stabilization and destabilization of soil organic matter: Mechanisms and controls. Geoderma 1996, 74, 65–105. [Google Scholar] [CrossRef]
- Kearney, M.S.; Rogers, A.S.; Townshend, J.R.G.; Rizzo, E.; Stutzer, D.; Stevenson, J.C.; Sundburg, K. Landsat imagery shows decline of coastal marshes in Chesapeake and Delaware Bays. EOS-Trans. Am. Geophys. Union 2002, 83, 173–178. [Google Scholar] [CrossRef]
- Smith, S.M. Multi-decadal changes in salt marshes of Cape Cod, MA: Photographic analyses of vegetation loss, species shifts, and geomorphic change. Northeast Nat. 2009, 16, 183–208. [Google Scholar] [CrossRef]
- Leonardi, N.; Ganju, N.K.; Fagherazzi, S. A linear relationship between wave power and erosion determines salt-marsh resilience to violent storms and hurricanes. Proc. Natl. Acad. Sci. USA 2011, 113, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, N.; Fagherazzi, S. Effect of local variability in erosional resistance on large-scale morphodynamic response of salt marshes to wind waves and extreme events. Geophys. Res. Lett. 2015, 42, 5872–5879. [Google Scholar] [CrossRef]
- Smith, J.A.M. The role of Phragmites australis in mediating inland salt marsh migration in a mid-Atlantic estuary. PLoS ONE 2013, 8, e65091. [Google Scholar] [CrossRef] [PubMed]
- Linhoss, A.; Kiker, G.; Shirley, M.; Frank, K. Sea-level rise, inundation and marsh migration: Simulating impacts on developed lands and environmental systems. J. Coast. Res. 2015, 31, 36–46. [Google Scholar] [CrossRef]
- Torio, D.D.; Chmura, G.L. Assessing coastal squeeze of tidal wetlands. J. Coast. Res. 2013, 29, 1049–1061. [Google Scholar] [CrossRef]
- Norcross, B. Surviving sandy: Long Beach Island and the greatest storm of the jersey shore. Bull. Am. Meteorol. Soc. 2015, 96, 466–467. [Google Scholar]
- Wilkinson, M.J.; Blaha, J.E.; Noli, D. A new lagoon on the Namibian coast of South Africa: Sand spit growth documented from STS-29 shuttle photography. Geocarta Int. 1989, 4, 63–66. [Google Scholar] [CrossRef]
- Fagherazzi, S.; Kirwan, M.L.; Mudd, S.M.; Guntenspergen, G.R.; Temmerman, S.; D’Alpaos, A.; van de Koppel, J.; Rybczyk, J.M.; Reyes, E.; Craft, C.; et al. Numerical models of salt marsh evolution: Ecological, geomorphic, and climatic factors. Rev. Geophys. 2012, 50, RG000359. [Google Scholar] [CrossRef]
- Morris, J.T.; Sundareshwar, P.V.; Nietch, C.T.; Kjerfve, B.; Cahoon, D.R. Responses of coastal wetlands to rising sea level. Ecology 2002, 83, 2869–2877. [Google Scholar] [CrossRef]
- Watson, E.B.; Oczkowski, A.J.; Wigand, C.; Hanson, A.R.; Davey, E.W.; Crosby, S.C.; Johnson, R.; Andrews, H.M. Nutrient enrichment and precipitation changes do not enhance resilience of salt marshes to sea level rise in the Northeastern U.S. Clim. Chang. 2014, 8, 501–509. [Google Scholar] [CrossRef]
- Donata, D.C.; Kauffman, J.B.; Murdiyarson, D.; Kurnianto, S.; Stidham, M.; Kanninen, M. Mangroves among the most carbon-rich forests in the tropics. Nat. Geosci. 2011, 4, 293–297. [Google Scholar] [CrossRef]
- Pendleton, L.; Donato, D.C.; Murray, B.C.; Crooks, S.; Jenkins, W.A.; Sifleet, S.; Craft, C.; Fourqurean, J.W.; Kauffman, J.B.; Marbà, N.; et al. Estimating global “blue carbon” emissions from conversion and degradation of vegetated coastal ecosystems. PLoS ONE 2012, e43542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eong, O.J. Mangroves—A carbon source and sink. Chemosphere 1993, 27, 1097–1107. [Google Scholar] [CrossRef]
- Morzaria-Luna, H.N.; Castillo-López, A.; Danemann, G.D.; Turk-Boyer, P. Conservation strategies for coastal wetlands in the Gulf of California, Mexico. Wetl. Ecol. Manag. 2014, 22, 267–288. [Google Scholar] [CrossRef]
- Travieso-Bello, A. La legislación aplicable a los sitios Ramsar en México. Lett. Juríd. 2009, 20, 1–14. [Google Scholar]
- Vidal Hernández, L.E. Implementation of Ramsar Strategic Plan in coastal wetlands of the Yucatán Peninsula. Lat. Am. J. Aquat. Res. 2017, 43, 845–855. [Google Scholar]
- Secratería de Medio Ambiente y Recuros Naturales [SEMARNAT]. Política Nacional de Humadales; SEMARNAT: Distrito Federal, México, 2014. [Google Scholar]
- Instituto Nacional de Ecología. Evaluación Preliminar de Las Tasas de Pérdida de la Superficie de Manglar en México; Instituto Nacional de Ecología: Distrito Federal, México, 2005. [Google Scholar]
- Ortega-Rubio, A.; Lluch-Cota, D.; Castellanos-Vera, A. Salt production at San Ignacio Lagoon: A sustainable development project? Int. J. Sust. Dev. World 2001, 8, 155–165. [Google Scholar] [CrossRef]
- Ibarra-Obando, S.E.; Escofet, A. Industrial development effects on the ecology of a Pacific Mexican estuary. Environ. Conserv. 1987, 14, 135–141. [Google Scholar] [CrossRef]
- Ibarra-Obando, S.E.; Poumian-Tapia, M. The effect of tidal exclusion on salt marsh vegetation in Baja California, Mexico. Wetl. Ecol. Manag. 1991, 1, 131–148. [Google Scholar] [CrossRef]
- Ibarra-Obando, S.E.; Poumian-Tapia, M.; Morzaria-Luna, H.N. Long-term effects of tidal exclusion on salt marsh plain species at Estero de Punta Banda, Baja California. Estuar. Coast. 2010, 33, 753–768. [Google Scholar] [CrossRef]

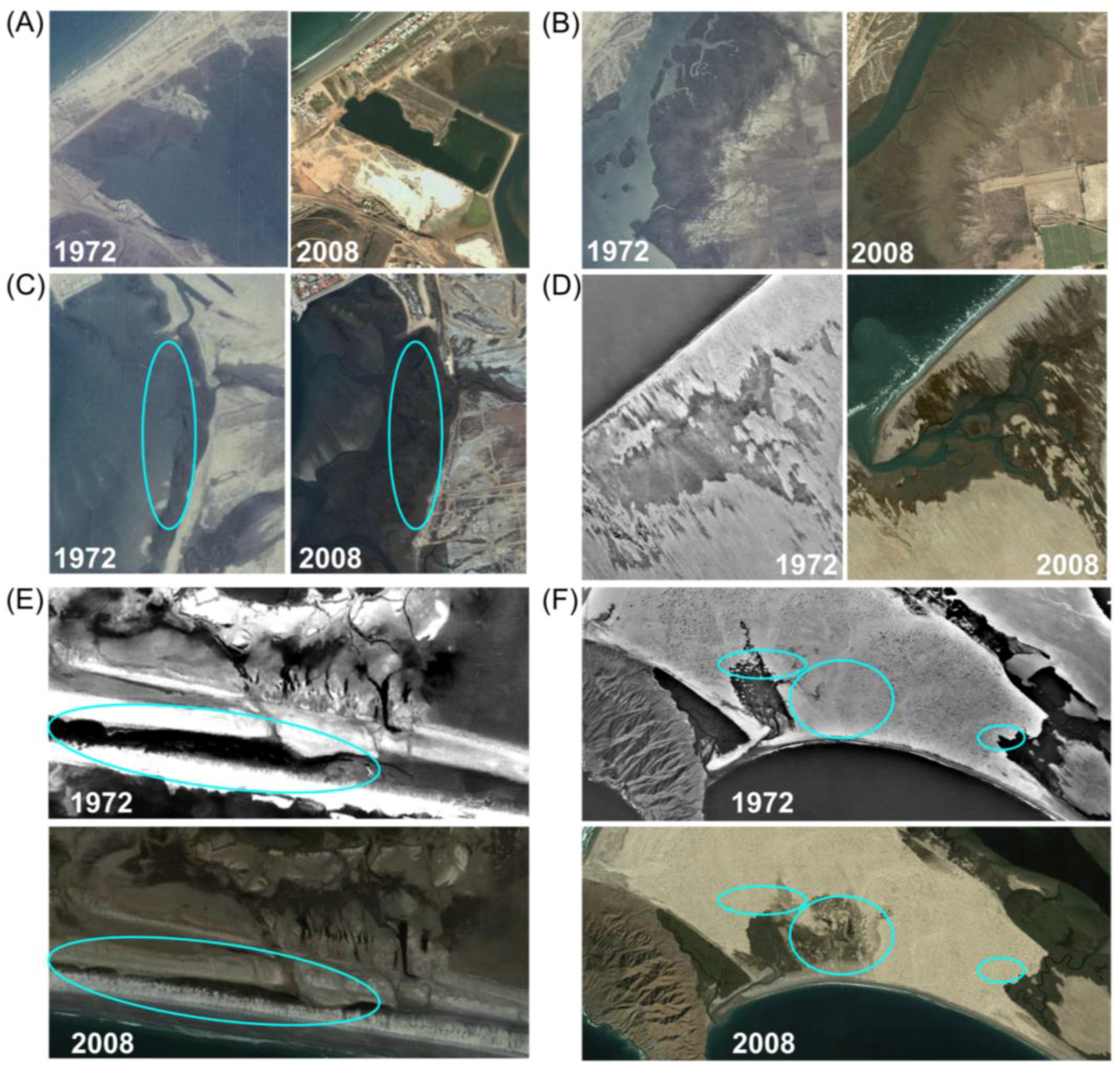
| 1972 (ha) | 2008 (ha) | Change (%) | Key Drivers of Change | |
|---|---|---|---|---|
| Estero Punta Banda | 309 | 264 | −14.5 | Burial by alluvium (−) |
| Recruitment on tidal flats (+) | ||||
| Coastal development (−) | ||||
| Inlet dimension shifts (+) | ||||
| Bahía San Quintín | 746 | 822 | 10.20% | Recruitment on tidal flats (+) |
| Burial by alluvium (−) | ||||
| Barrier transgression (−) | ||||
| New inlet (−) | ||||
| Laguna Ojo de Liebre | 9448 | 11,026 | 16.70% | Barrier breach/lagoon formation (+) |
| Burial by dunes (−) | ||||
| Transgression (+) | ||||
| Laguna San Ignacio | 4690 | 4379 | −6.63% | Burial by dunes (−) |
| Barrier transgression (−) | ||||
| Bahía Magdalena | 22,246 | 22,600 | 0.69% | Transgression (+) |
| Burial by dunes (−) | ||||
| Total | 37,638 | 39,091 | 3.86% |
| EPB | BSQ | LOL | LSI | BM | |
|---|---|---|---|---|---|
| 1972 | −1.0% | 8.3% | −6.2% | −4.5% | −1.4% |
| 2008 | −2.9% | −6.7% | −3.4% | −9.9% | −0.8% |
| Bulk Density g cc−1 | Clay% | Silt% | Sand% | Graphic Mean (μm) | Graphic SD (Φ) | Organic% | Organic C mg cc−1 | |
|---|---|---|---|---|---|---|---|---|
| Estero Punta Banda | 0.81 | 35 | 64 | 0 | 7.5 | 1.4 | 8.6 | 23 |
| −0.3 | −11 | −11 | 0 | −2 | −0.23 | −4.6 | −5.3 | |
| Bahía San Quintín | 0.63 | 43 | 57 | 0 | 6.3 | 1.4 | 9.2 | 29.3 |
| −0.13 | −7.2 | −7.2 | 0 | −1.4 | −0.077 | −5.4 | −2.8 | |
| Laguna Ojo de Liebre | 0.96 | 6 | 16 | 78 | 130 | 2.2 | 6.4 | 28 |
| −0.36 | −2.9 | −9 | −12 | −35 | −0.6 | −5.5 | −7.7 | |
| Laguna San Ignacio | 1.2 | 8.3 | 34 | 58 | 92 | 2.6 | 2.8 | 4.87 |
| −0.13 | −2.4 | −9.5 | −9.6 | −17 | −0.25 | −0.63 | −0.33 | |
| Bahía Magdalena | 1.6 | 1 | 4.9 | 94 | 180 | 0.76 | 1.6 | 6.68 |
| −0.077 | −0.19 | −0.51 | −0.62 | −6.2 | −0.035 | −0.36 | −0.11 |
| Location (N/W) | 2008 Wetlands (ha) | Soil Organic C mg cc−1 | Organic C g Lagoon−1 | Storage g CO2eq year−1 | |
|---|---|---|---|---|---|
| Estero Punta Banda | 31.70°, 116.62°- | 264 | 23 | 3.04 × 1010 | 7.49 × 108 |
| 31.76°, 116.65° | |||||
| Bahía San Quintín | 30.35°, 115.92°- | 822 | 29.3 | 1.20 × 1011 | 2.20 × 109 |
| 30.52°, 116.03° | |||||
| Laguna Ojo de Liebre 1 | 27.42°, 113.88°- | 11,026 | 28 | 1.54 × 1012 | 2.44 × 1010 |
| 28.25°, 114.40° | |||||
| Laguna San Ignacio 1 | 26.40°, 112.75°- | 4378 | 4.87 | 1.07 × 1011 | 5.37 × 109 |
| 26.98°, 113.72° | |||||
| Bahía Magdalena 1 | 24.50°, 111.85°- | 22,600 | 6.68 | 7.54 × 1011 | 2.26 × 1010 |
| 24.80°, 112.15° | |||||
| Total | 39,091 | 2.55 × 1012 | 5.53 × 1010 |
| Correlation with Organic C Density | r | p |
|---|---|---|
| % clay | 0.629 | <0.001 |
| % silt | 0.374 | 0.006 |
| % sand | −0.525 | <0.001 |
| median grain size (μm) | −0.362 | 0.007 |
| inclusive graphic mean (φ) | 0.557 | <0.001 |
| inclusive graphic standard deviation | −0.254 | 0.046 |
| temperature | −0.493 | <0.001 |
| precipitation | 0.571 | <0.001 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watson, E.B.; Hinojosa Corona, A. Assessment of Blue Carbon Storage by Baja California (Mexico) Tidal Wetlands and Evidence for Wetland Stability in the Face of Anthropogenic and Climatic Impacts. Sensors 2018, 18, 32. https://doi.org/10.3390/s18010032
Watson EB, Hinojosa Corona A. Assessment of Blue Carbon Storage by Baja California (Mexico) Tidal Wetlands and Evidence for Wetland Stability in the Face of Anthropogenic and Climatic Impacts. Sensors. 2018; 18(1):32. https://doi.org/10.3390/s18010032
Chicago/Turabian StyleWatson, Elizabeth Burke, and Alejandro Hinojosa Corona. 2018. "Assessment of Blue Carbon Storage by Baja California (Mexico) Tidal Wetlands and Evidence for Wetland Stability in the Face of Anthropogenic and Climatic Impacts" Sensors 18, no. 1: 32. https://doi.org/10.3390/s18010032





