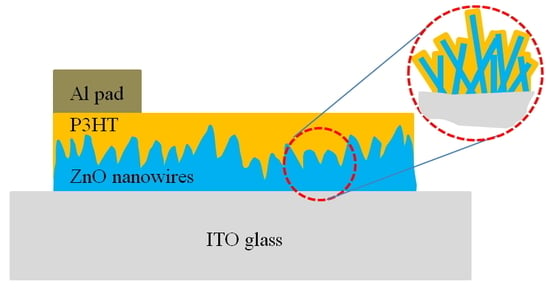
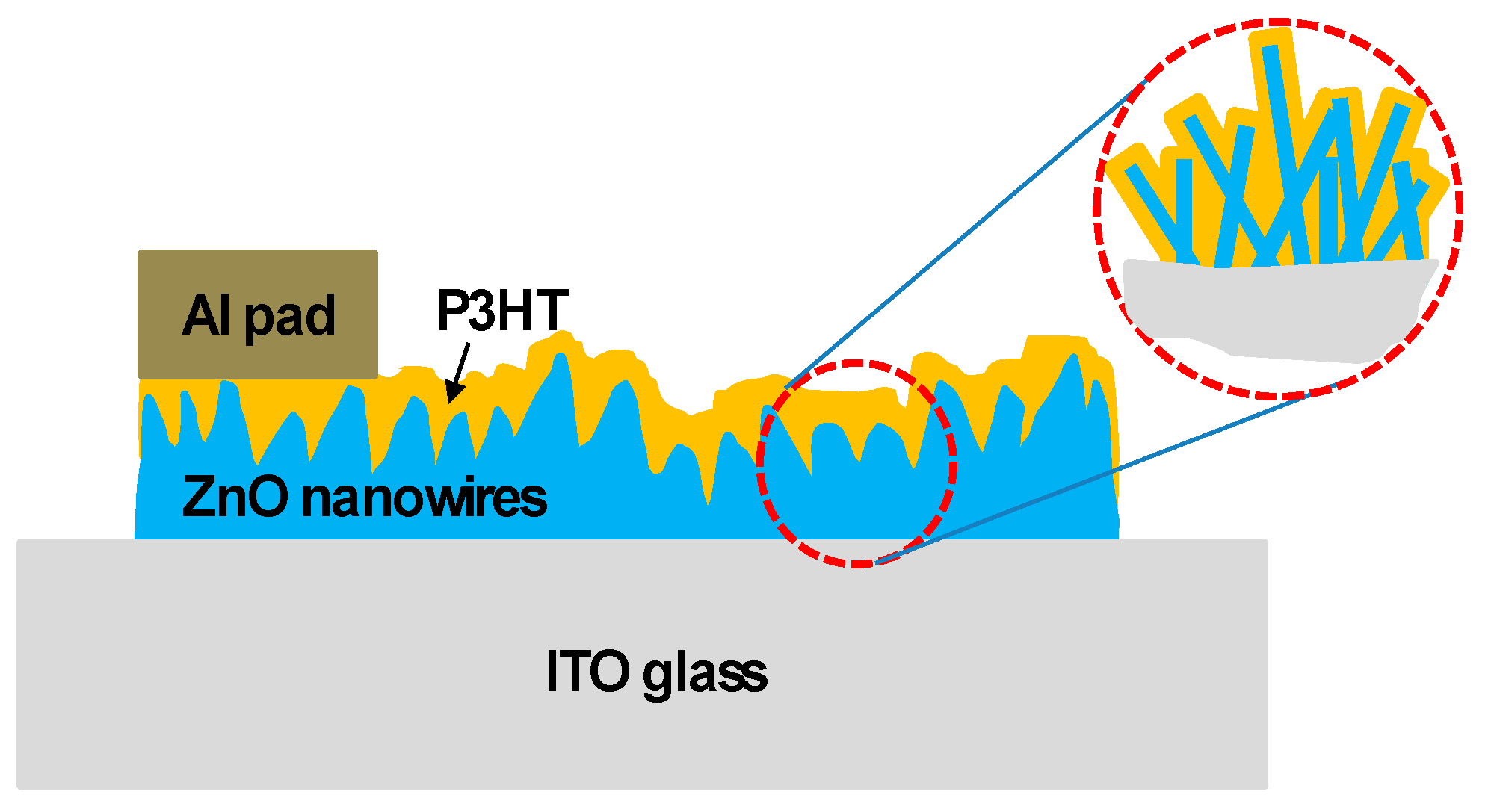
Fabrication of a P3HT-ZnO Nanowires Gas Sensor Detecting Ammonia Gas
Abstract
:1. Introduction
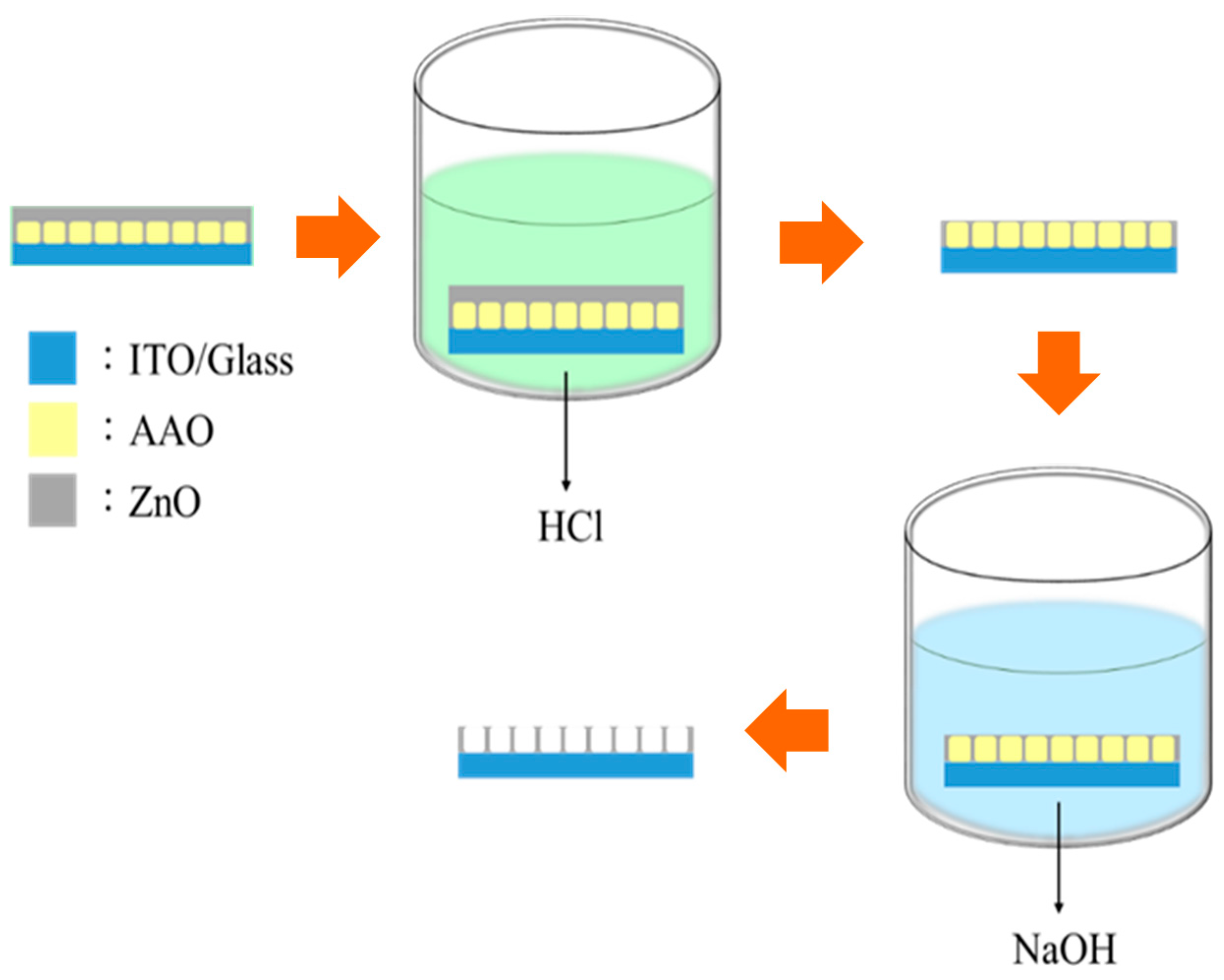
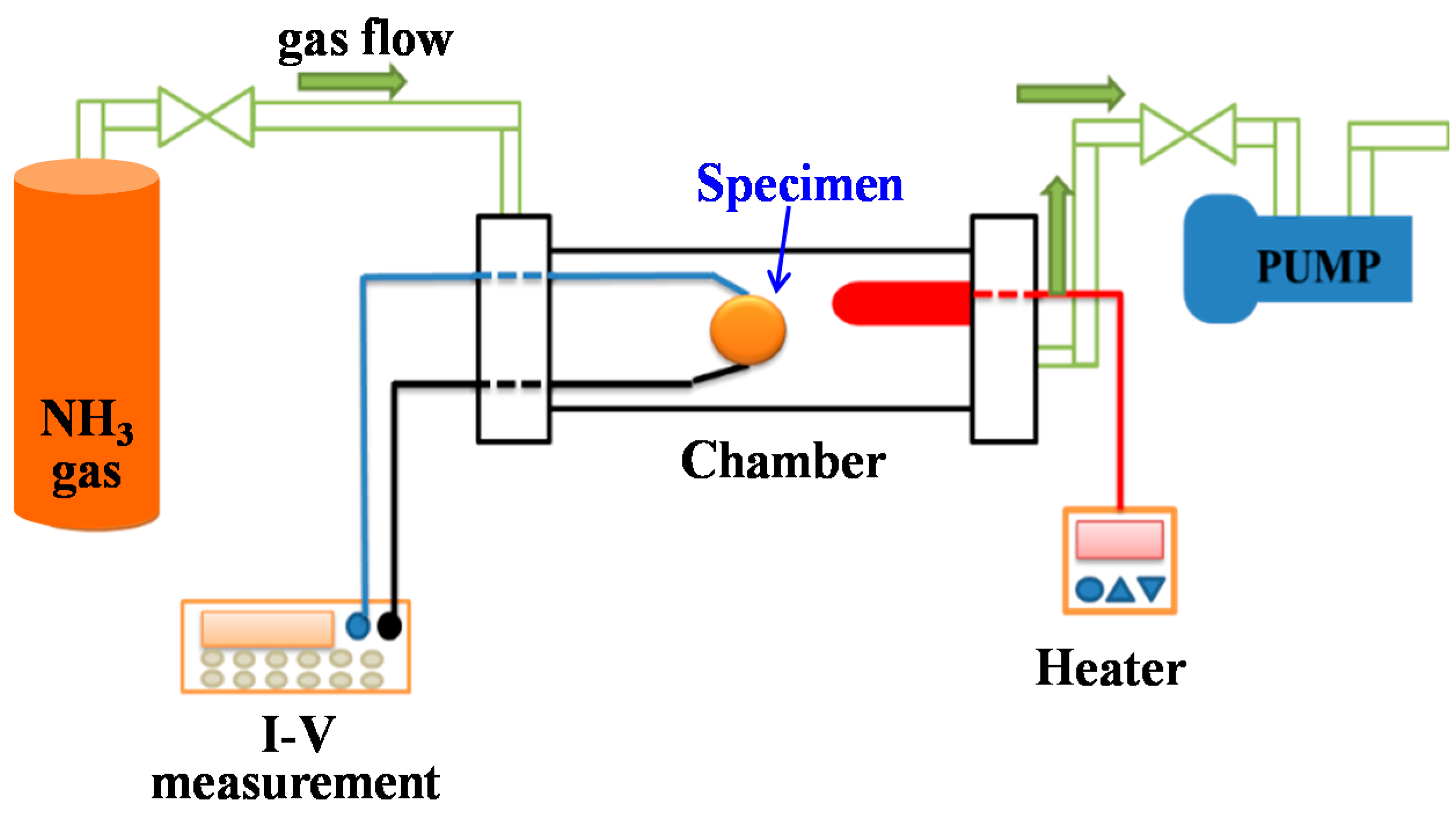
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. Metal oxide catalyst assisted SnO2 thin film based SO2 gas sensor. Sens. Actuators B Chem. 2016, 224, 282–289. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Yao, M.S.; Tang, W.X.; Wang, G.E.; Nath, B.; Xu, G. MOF thin film-coated metal oxide nanowire array: Significantly improved chemiresistor sensor performance. Adv. Mater. 2016, 28, 5229–5234. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.G.; Huang, C.W.; Chen, J.H.; Li, Y.H. Fabrication of a miniature zinc aluminum oxide nanowire array gas sensor and application for environmental monitoring. Int. J. Photoenergy 2014, 2014, 515268. [Google Scholar] [CrossRef]
- Kuo, C.G.; Huang, J.J.; Chen, J.H.; Zeng, R.J. Fabrication of high array zinc-indium oxide nanowires and a nanowire gas sensor. Sens. Mater. 2017, 29, 533–538. [Google Scholar]
- Xia, Y.; Wang, J.; Xu, J.L.; Li, X.; Xie, D.; Xiang, L.; Komarneni, S. Confined formation of ultrathin ZnO nanorods/reduced graphene oxide mesoporous nanocomposites for high-performance room-temperature NO2 sensors. ACS Appl. Mater. Interfances 2016, 8, 35454–35463. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.; Yang, D.; Park, J.; Kim, S.; Cho, I.; Yang, H.H.; Cho, M.; Mousavi, S.; Choi, K.H.; Park, I. Micropatterning of metal oxide nanofibers by electrohydrodynamic (EHD) printing towards highly integrated and multiplexed gas sensor applications. Sens. Actuators B Chem. 2017, 250, 574–583. [Google Scholar] [CrossRef]
- Saxena, V.; Aswal, D.; Kaur, M.; Koiry, S.; Gupta, S.; Yakhmi, J. Enhanced NO2 selectivity of hybrid poly(3-hexylthiophene): ZnO-nanowire thin films. Appl. Phys. Lett. 2007, 90, 043516. [Google Scholar] [CrossRef]
- Jeong, S.H.; Lee, J.Y.; Lim, B.; Lee, J.; Noh, Y.Y. Diketopyrrolopyrrole-based conjugated polymer for printed organic field-effect transistors and gas sensors. Dyes Pigment. 2017, 140, 244–249. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, W.; Zhuang, X.; Tang, Y.; Yu, J. Thickness modulation on semiconductor towards high performance gas sensors based on organic thin film transistors. Mater. Sci. Eng. B 2017, 226, 107–113. [Google Scholar] [CrossRef]
- Chu, P.H.; Wang, G.; Fu, B.; Choi, D.; Park, J.O.; Srinivasarao, M.; Reichmanis, E. Synergistic effect of regioregular and regiorandom poly(3-hexylthiophene) blends for high performance flexible organic field effect transistors. Adv. Electron. Mater. 2016, 2, 1500384. [Google Scholar] [CrossRef]
- Assadi, A.; Gustafsson, G.; Willander, M.; Svensson, C.; Inganäs, O. Determination of field-effect mobility of poly(3-hexylthiophene) upon exposure to NH3 gas. Synthetic Met. 1990, 37, 123–130. [Google Scholar] [CrossRef]
- Fukuda, H.; Ise, M.; Kogure, T.; Takano, N. Gas sensors based on poly-3-hexylthiophene thin-film transistors. Thin Solid Films 2004, 464, 441–444. [Google Scholar] [CrossRef]
- Jeong, J.W.; Lee, Y.D.; Kim, Y.M.; Park, Y.W.; Choi, J.H.; Park, T.H. The response characteristics of a gas sensor based on poly-3-hexylithiophene thin-film transistors. Sens. Actuators B Chem. 2010, 146, 40–45. [Google Scholar] [CrossRef]
- Hsu, W.F.; Kuo, C.G.; Chao, Y.C.; Lee, J.F.; Yang, C.F.; Juang, F.R. Growth of ZnO nano-wire arrays using AAO template and atomic-layer deposition method. In Proceedings of the International Conference on Applied System Innovation (ICASI), Okinawa, Japan, 26–30 May 2016. [Google Scholar]
- Chu, S.Z.; Wada, K.; Inoue, S.; Isogai, M.; Yasumori, A. Fabrication of ideally ordered nanoporous alumina films and integrated alumina nanotubule arrays by high-field anodization. Adv. Mater. 2005, 17, 2115–2119. [Google Scholar] [CrossRef]
- Roslyakov, I.V.; Koshkodaev, D.S.; Eliseev, A.A.; Hermida-Merino, D.; Petukhov, A.V.; Napolskii, K.S. Crystallography-induced correlations in pore ordering of anodic alumina films. J. Phys. Chem. C 2016, 120, 19698–19704. [Google Scholar] [CrossRef]
- Napolskii, K.S.; Roslyakov, I.V.; Romanchuk, A.Y.; Kapitanova, O.O.; Mankevich, A.S.; Lebedev, V.A.; Eliseev, A.A. Origin of long-range orientational pore ordering in anodic films on aluminium. J. Mater. Chem. 2012, 22, 11922–11926. [Google Scholar] [CrossRef]
- Pashchanka, M.; Schneider, J.J. Self-ordering regimes of porous anodic alumina layers formed in highly diluted sulfuric acid electrolytes. J. Phys. Chem. C 2016, 120, 14590–14596. [Google Scholar] [CrossRef]
- Nayar, P.; Khanna, A.; Kabiraj, D.; Abhilash, S.R.; Beake, B.D.; Losset, Y.; Chen, B. Structural, optical and mechanical properties of amorphous and crystalline alumina thin films. Thin Solid Films 2014, 568, 19–24. [Google Scholar] [CrossRef]
- Geng, Y.; Zhao, T.; Lian, G.; Cui, X.; Liu, Y.; Liu, J.; Wang, Q.; Cui, D. A positive synergetic effect observed in the P3HT-SnO2 composite semiconductor: The striking increase of carrier mobility. RSC Adv. 2016, 6, 2387–2393. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, Q.; Xie, G.; Su, Y.; Zhao, K.; Du, H.; Jiang, Y. Gas sensors based on polyaniline/zinc oxide hybrid film for ammonia detection at room temperature. Chem. Phys. Lett. 2016, 665, 147–152. [Google Scholar] [CrossRef]
- Shimamoto, C.; Hirata, I.; Katsu, K. Breath and blood ammonia in liver cirrhosis. Hepato Gastroenterol. 2000, 47, 443–445. [Google Scholar]
- Zan, H.W.; Li, C.H.; Yeh, C.C.; Dai, M.Z.; Meng, H.F.; Tsai, C.C. Room-temperature-operated sensitive hybrid gas sensor based on amorphous indium gallium zinc oxide thin-film transistors. Appl. Phys. Lett. 2011, 98, 253503. [Google Scholar] [CrossRef]
- Tai, H.; Yuan, Z.; Zheng, W.; Ye, Z.; Liu, C.; Du, X. ZnO nanoparticles/reduced graphene oxide bilayer thin films for improved NH3-sensing performances at room temperature. Nanoscale Res. Lett. 2016, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Jiang, C.; Sun, Y.E. Room-temperature high-performance ammonia gas sensor based on layer-by-layer self-assembled molybdenum disulfide/zinc oxide nanocomposite film. J. Alloys Compd. 2017, 698, 476–483. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, W.; Strout, T.; Schempf, A.; Zhang, H.; Li, B.; Cui, J.; Lei, Y. Ammonia gas sensor using polypyrrole-coated TiO2/ZnO nanofibers. Electroanalysis 2009, 21, 1432–1438. [Google Scholar] [CrossRef]
- Tang, H.; Yan, M.; Zhang, H.; Li, S.; Ma, X.; Wang, M.; Yang, D. A selective NH3 gas sensor based on Fe2O3-ZnO nanocomposites at room temperature. Sens. Actuators B Chem. 2006, 114, 910–915. [Google Scholar] [CrossRef]
- Lamdhade, G.T.; Raulkar, K.B.; Yawale, S.S.; Yawale, S.P. Fabrication of multilayer SnO2-ZnO-PPy sensor for ammonia gas detection. Indian J. Phys. 2015, 89, 1025–1030. [Google Scholar] [CrossRef]
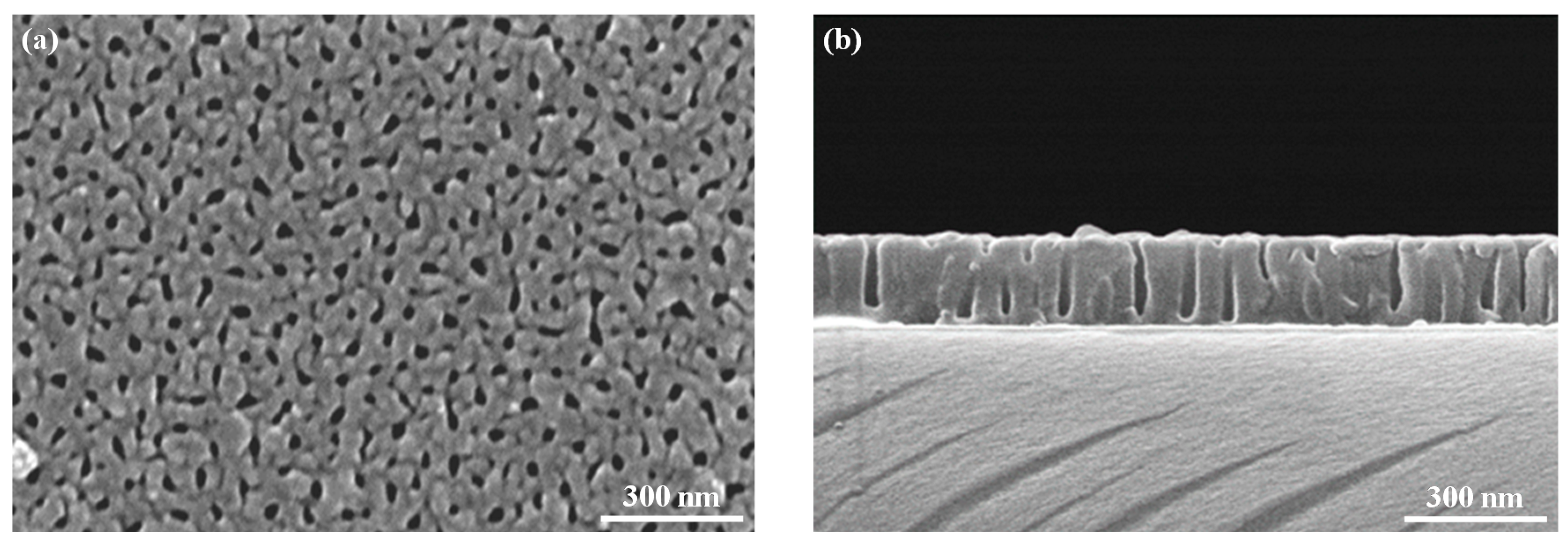
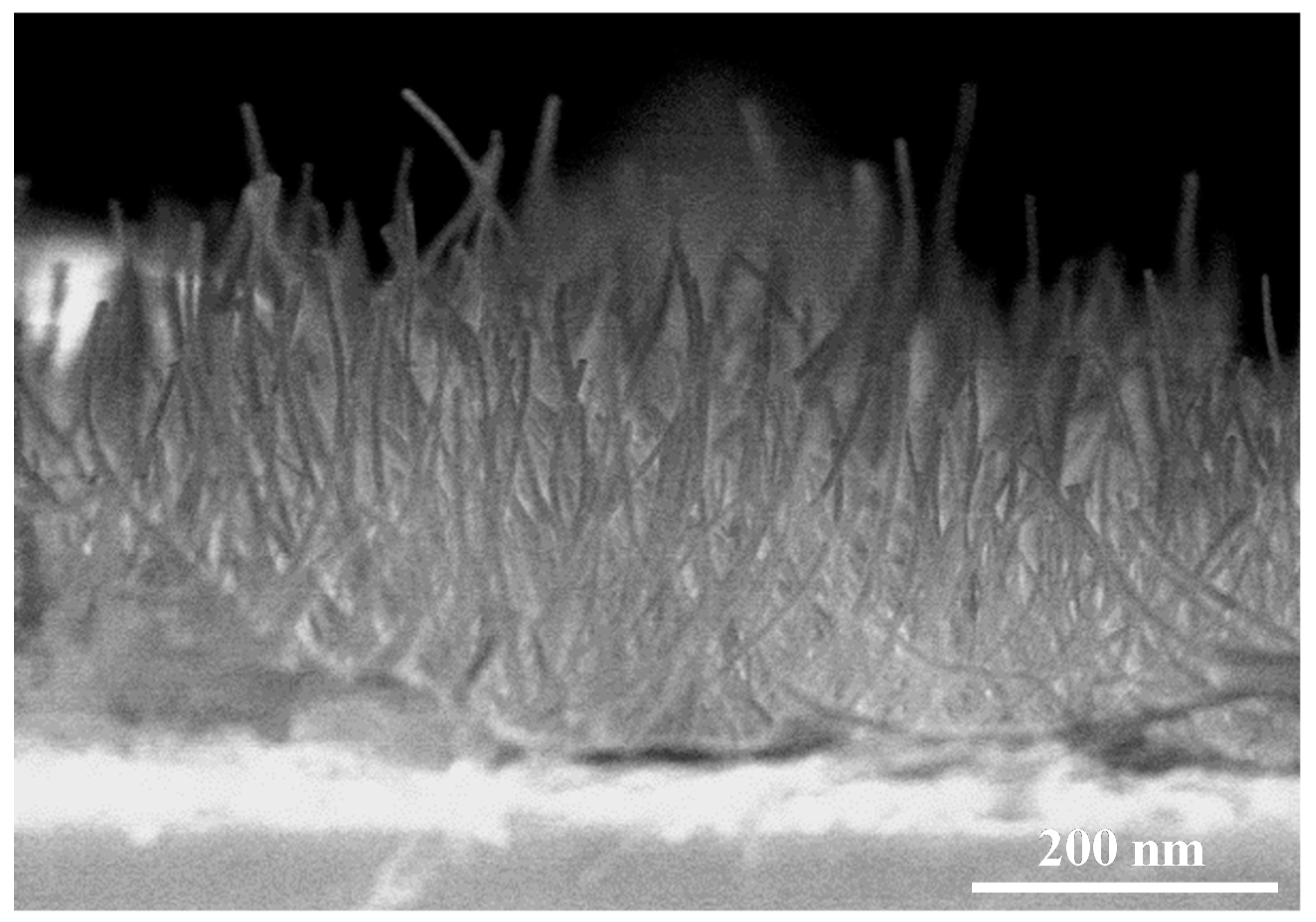
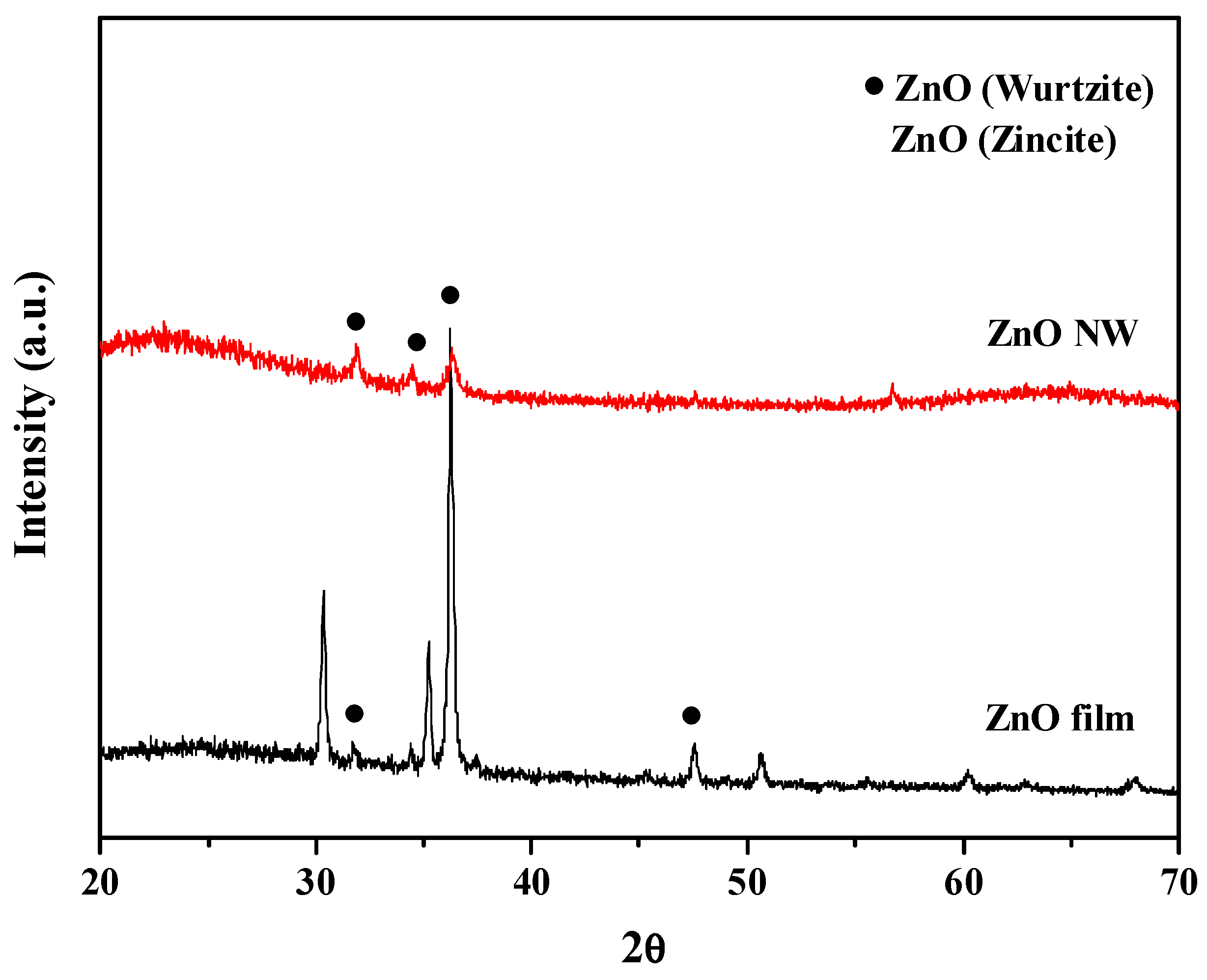
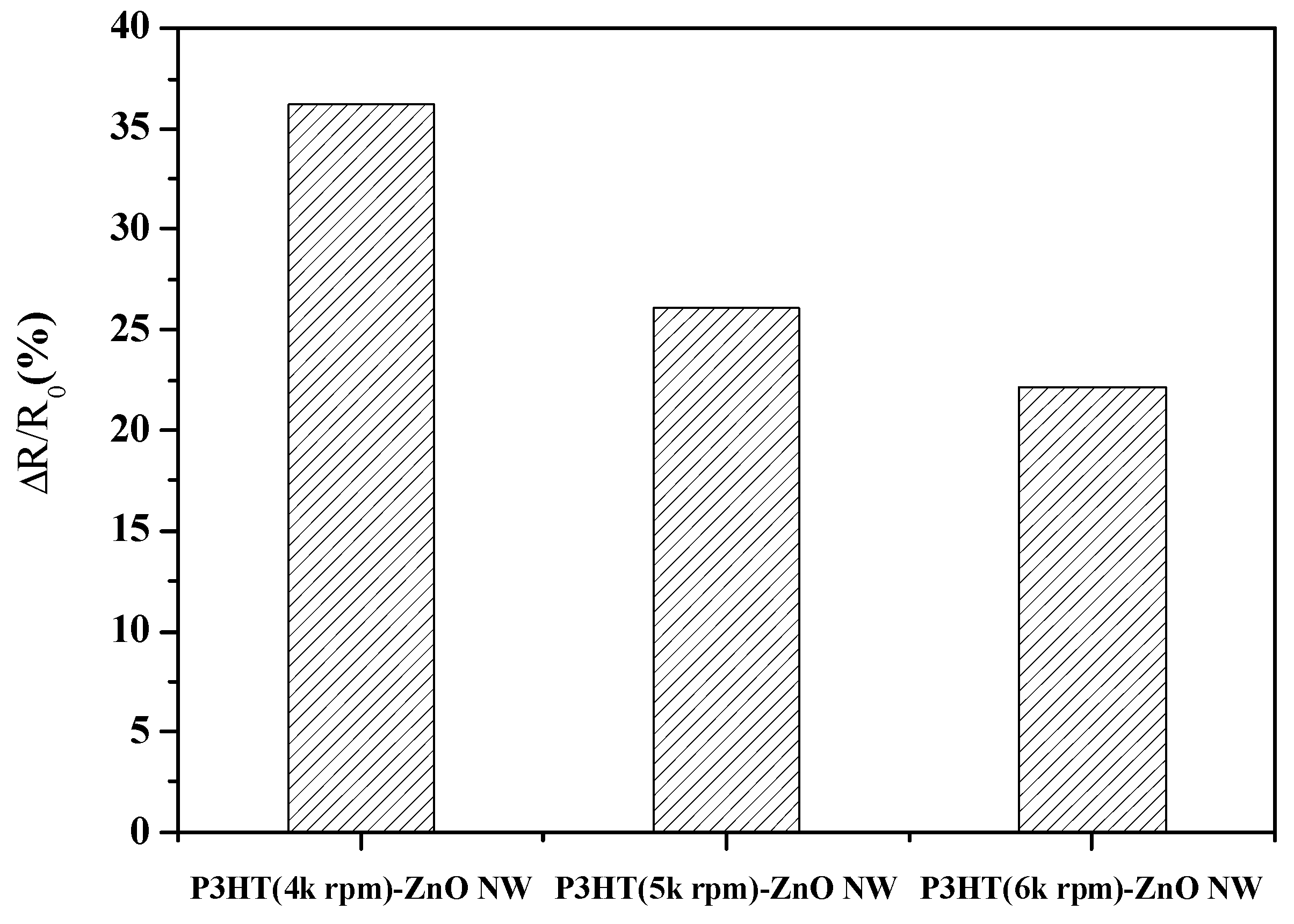
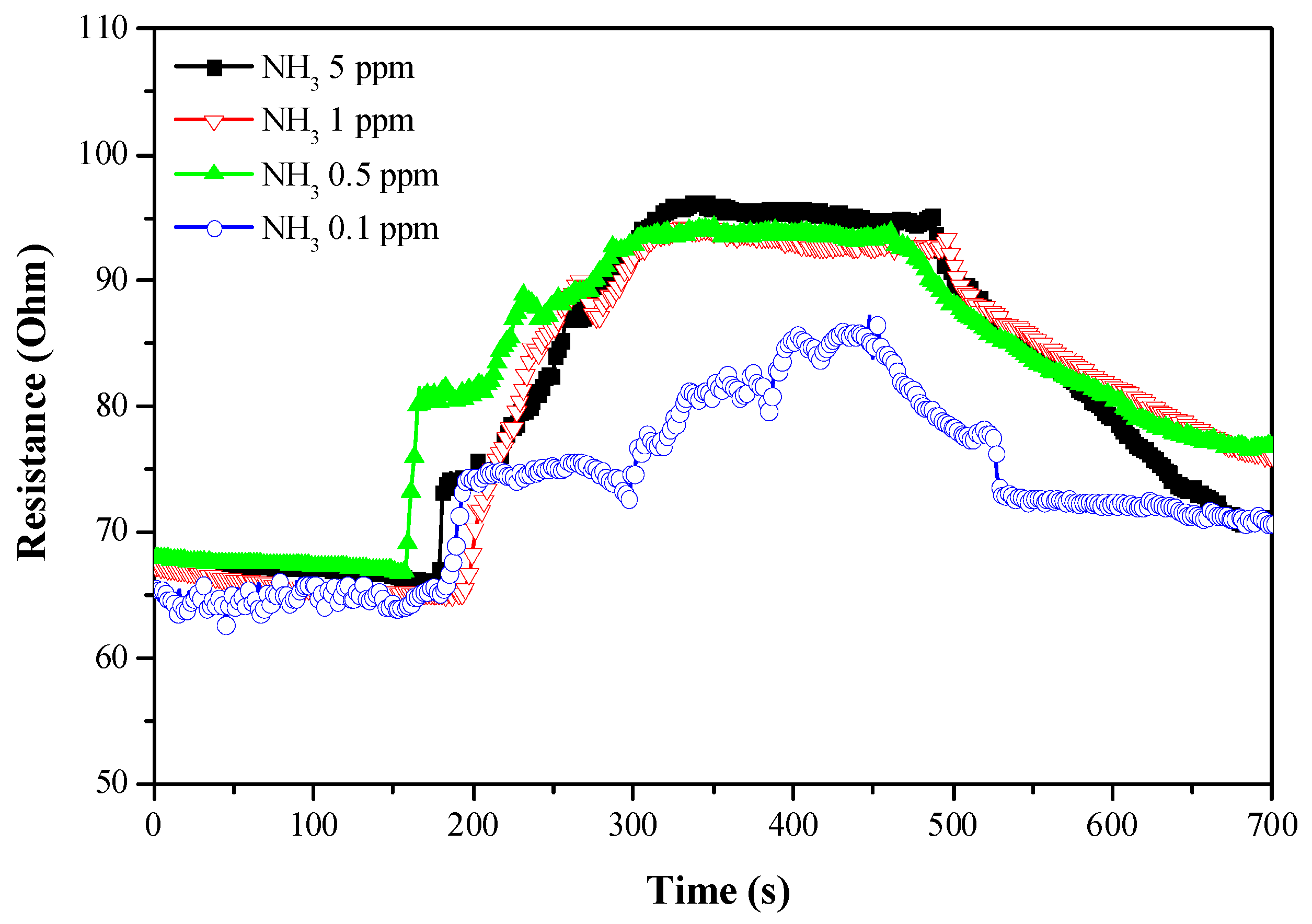
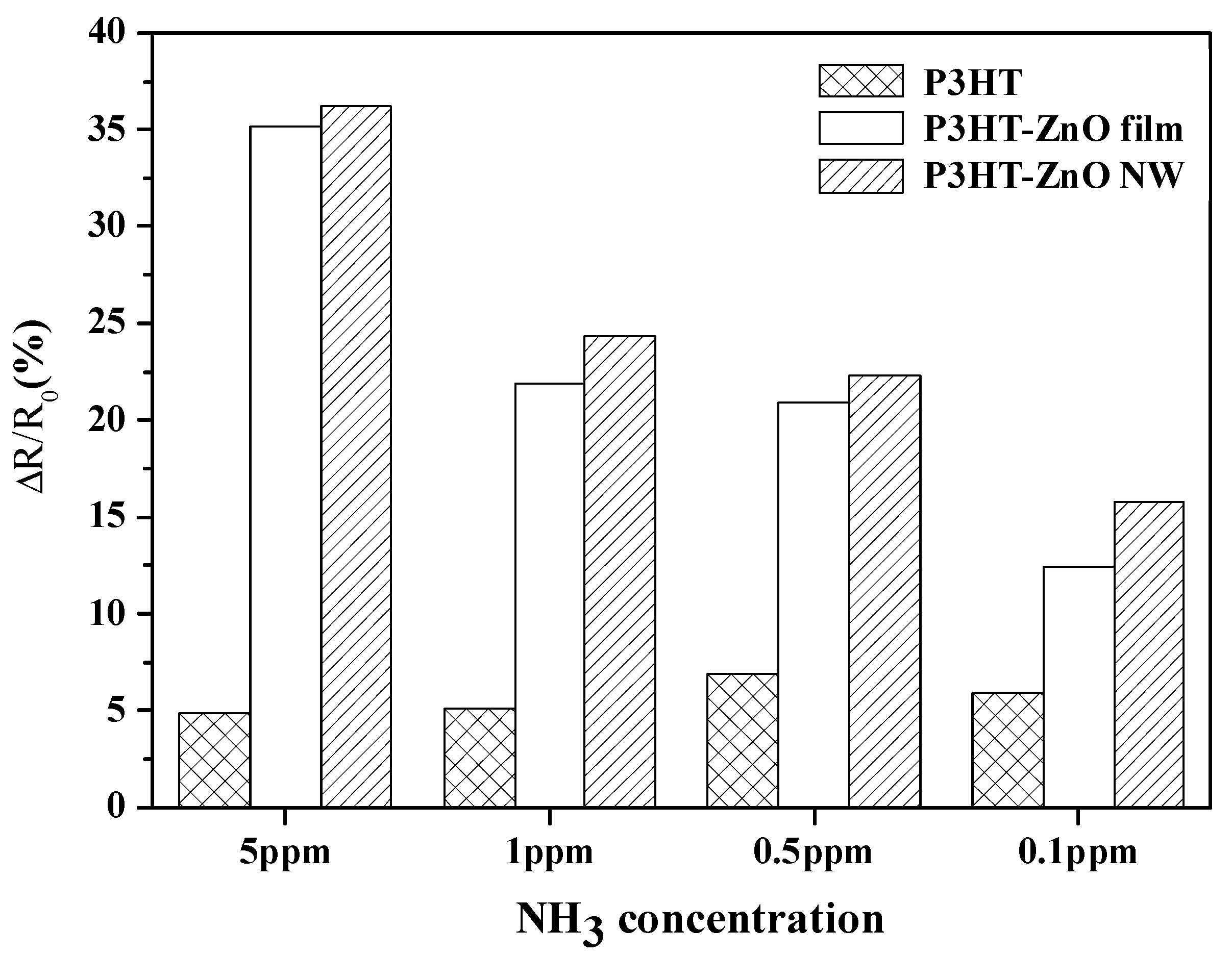
| Sensing Materials | Working Temperature | Concentration (ppm) | Maximum Sensitivity ** (per ppm) | Ref. |
|---|---|---|---|---|
| rGO*-ZnO bilayer thin film | RT | 50 | 0.0206 | [27] |
| MoS2-ZnO nanocomposite | RT | 0.25~100 | 0.0292 | [28] |
| PPy coated TiO2-ZnO nanofiber | RT | 0.5~450 | 0.2323 | [29] |
| Fe2O3-ZnO nanocomposite | RT | 0.4 | 25,000 | [30] |
| PANI-ZnO hybrid film | RT | 10~50 | 0.0302 | [24] |
| SnO2-ZnO-PPy multilayer | RT | 30~70 | 0.0239 | [31] |
| P3HT-ZnO nanowires | RT | 0.1~5 | 11.5762 | This work |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, C.-G.; Chen, J.-H.; Chao, Y.-C.; Chen, P.-L. Fabrication of a P3HT-ZnO Nanowires Gas Sensor Detecting Ammonia Gas. Sensors 2018, 18, 37. https://doi.org/10.3390/s18010037
Kuo C-G, Chen J-H, Chao Y-C, Chen P-L. Fabrication of a P3HT-ZnO Nanowires Gas Sensor Detecting Ammonia Gas. Sensors. 2018; 18(1):37. https://doi.org/10.3390/s18010037
Chicago/Turabian StyleKuo, Chin-Guo, Jung-Hsuan Chen, Yi-Chieh Chao, and Po-Lin Chen. 2018. "Fabrication of a P3HT-ZnO Nanowires Gas Sensor Detecting Ammonia Gas" Sensors 18, no. 1: 37. https://doi.org/10.3390/s18010037