Screen-Printed Electrode Modified by Bismuth /Fe3O4 Nanoparticle/Ionic Liquid Composite Using Internal Standard Normalization for Accurate Determination of Cd(II) in Soil
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals and Reagents
2.2. Preparation of Fe3O4/ILSPE
2.3. Sample Preparation
2.4. Electrochemical Measurement Procedure
3. Results
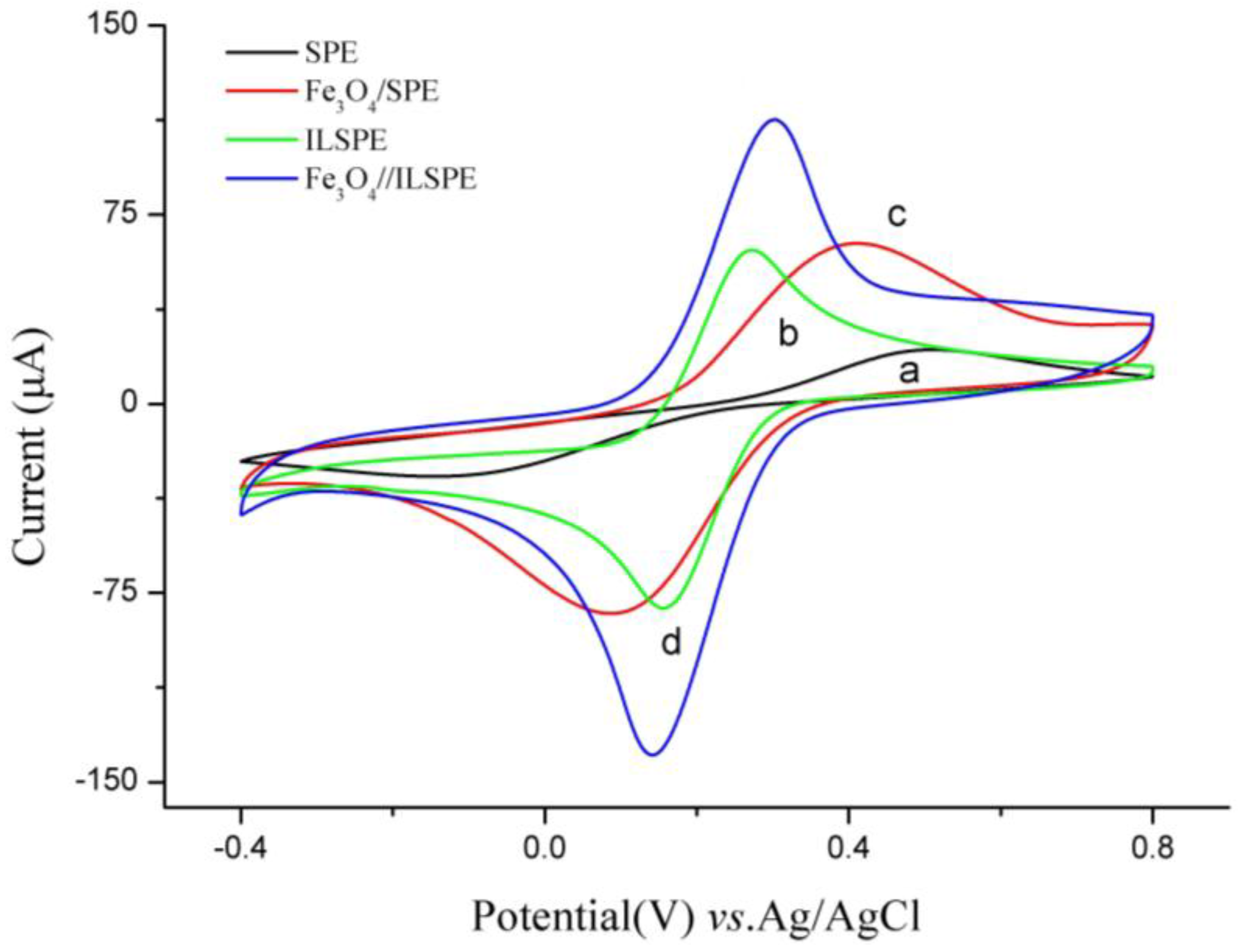
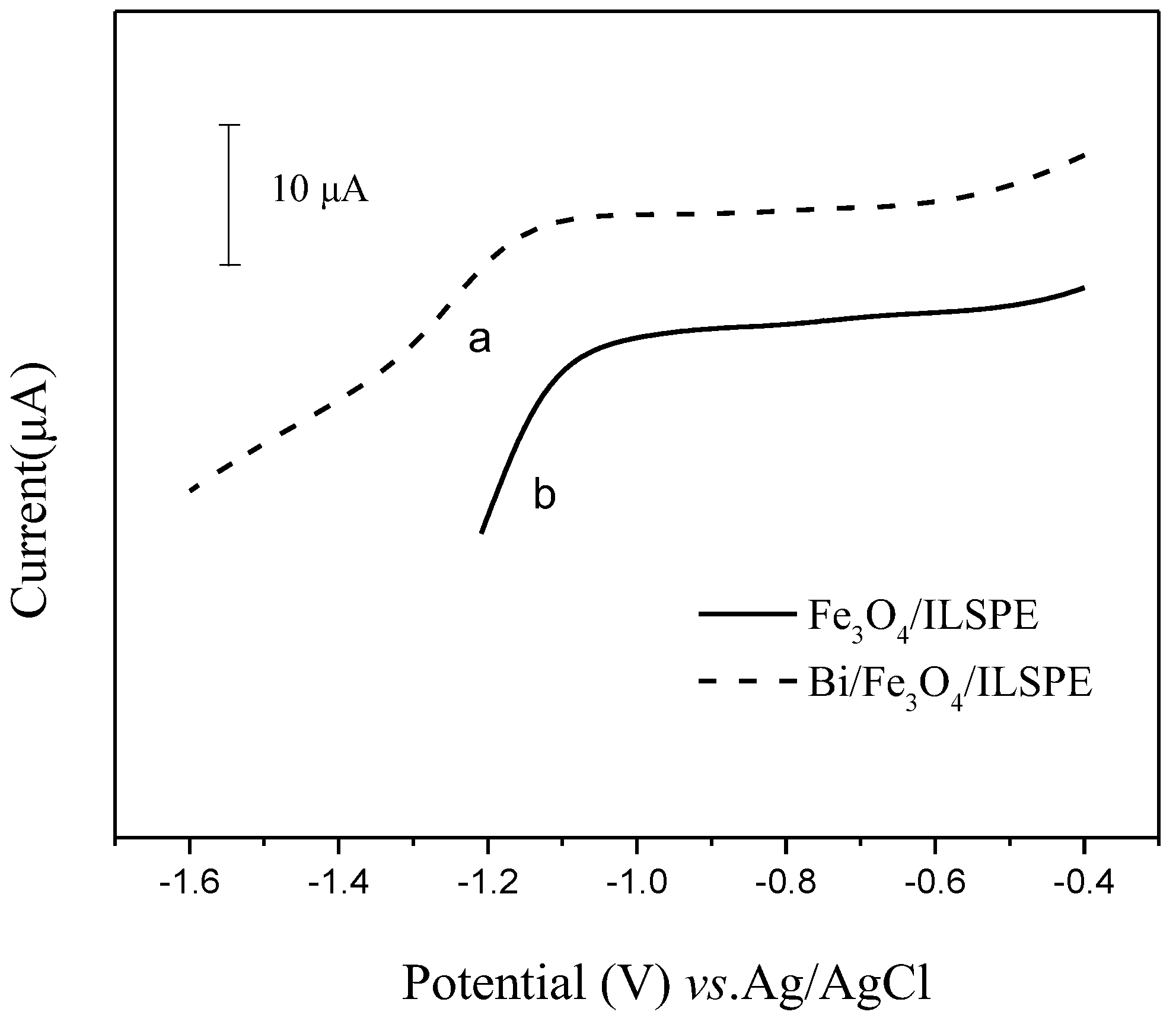
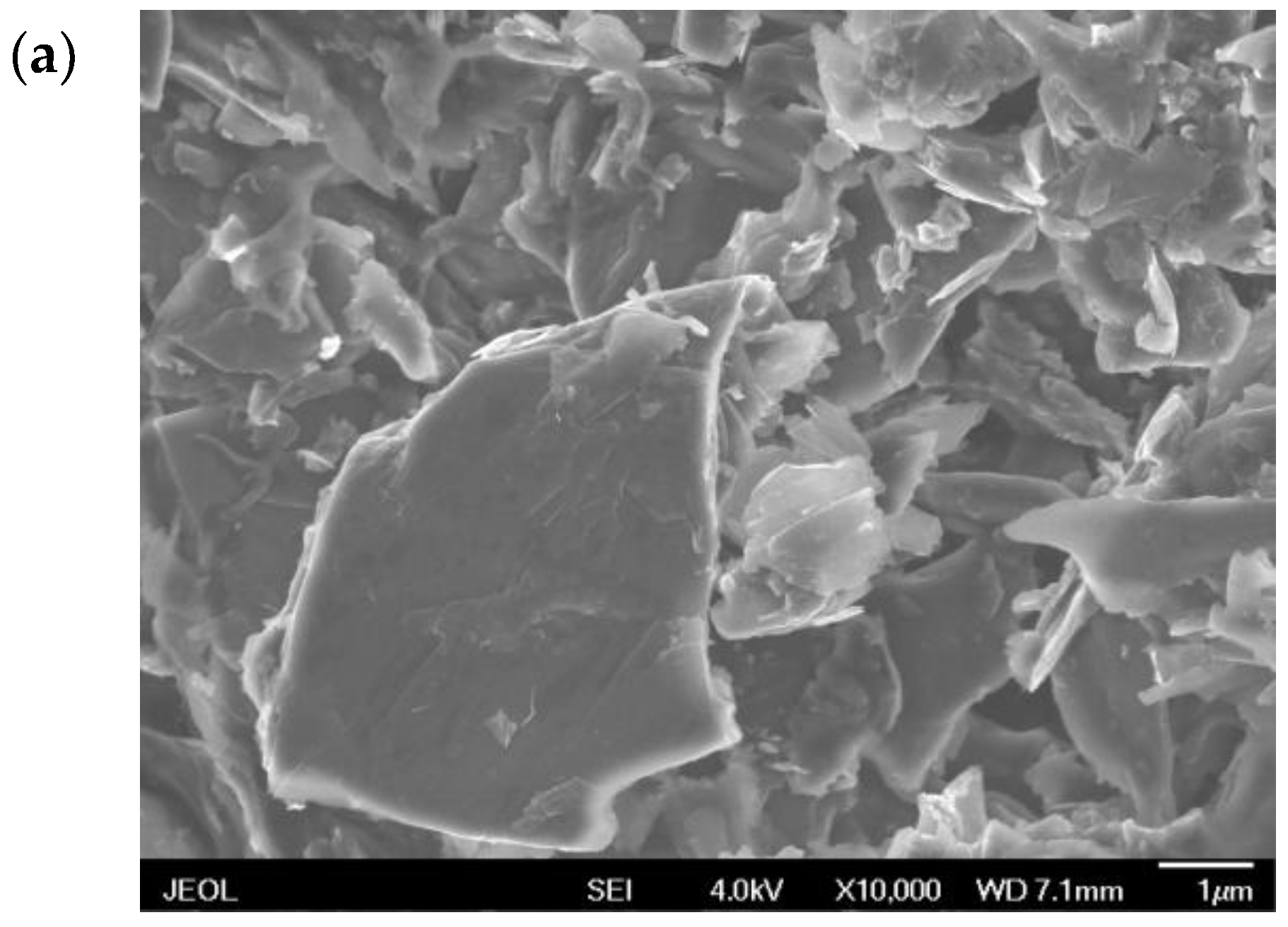
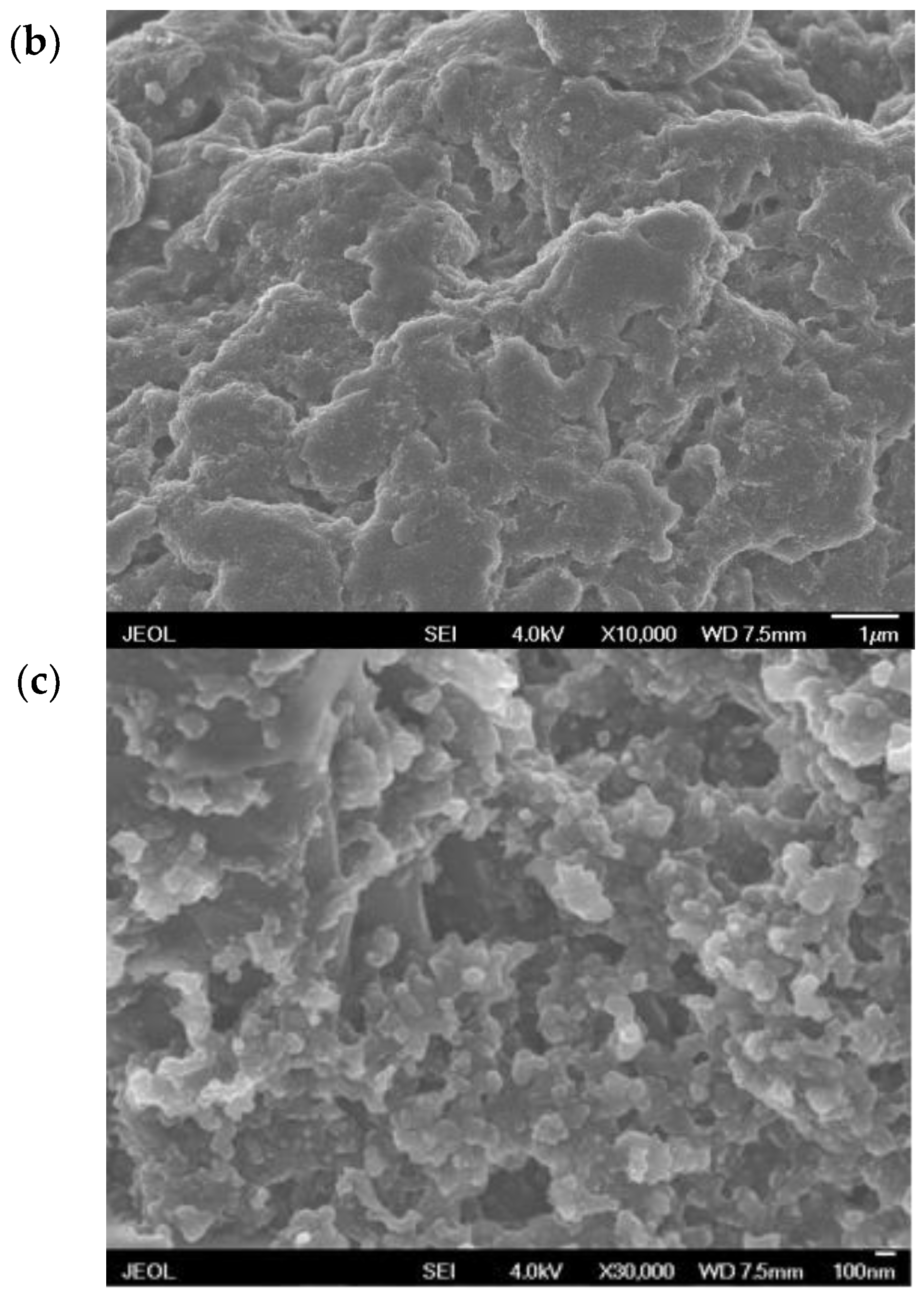
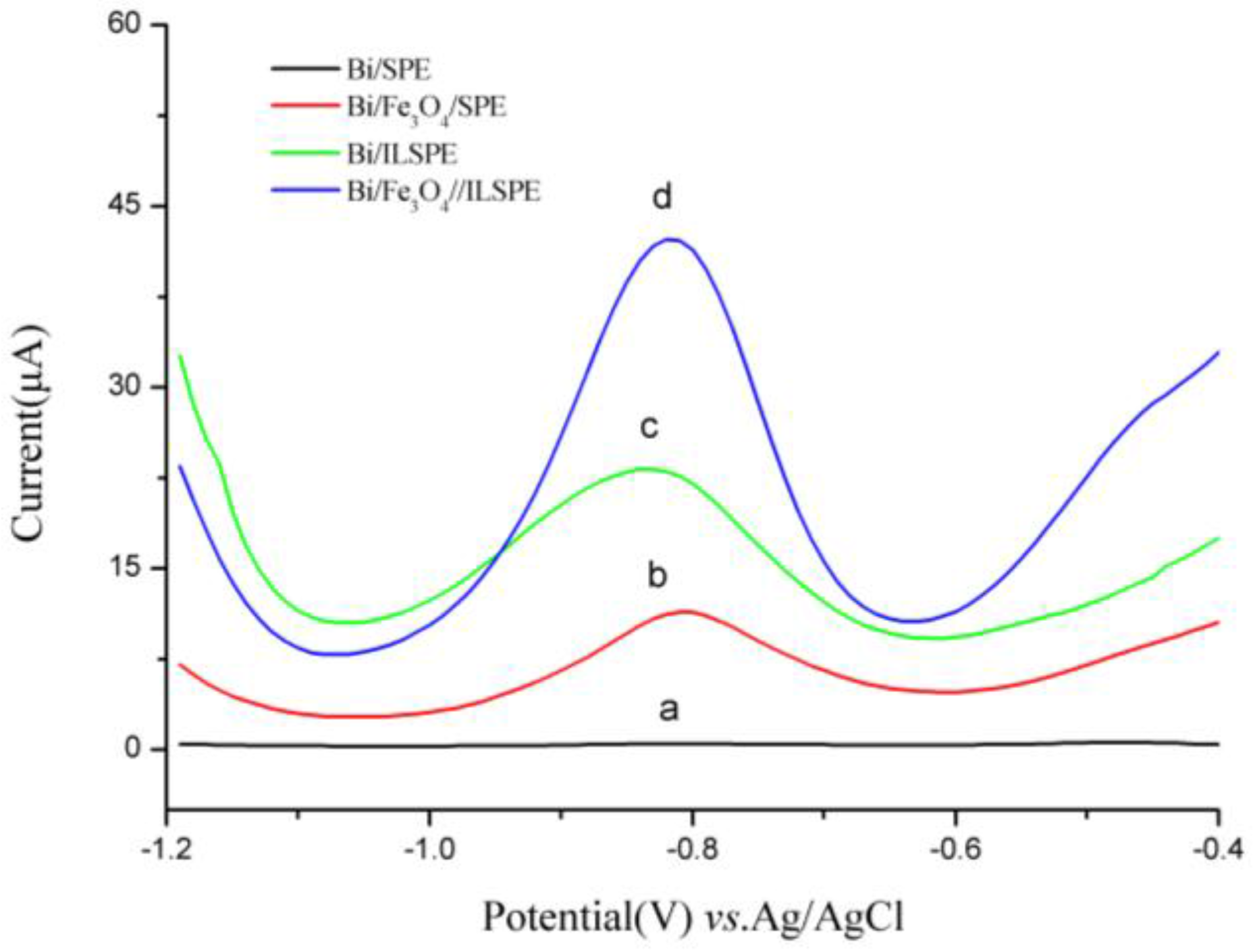
3.1. Characteristics
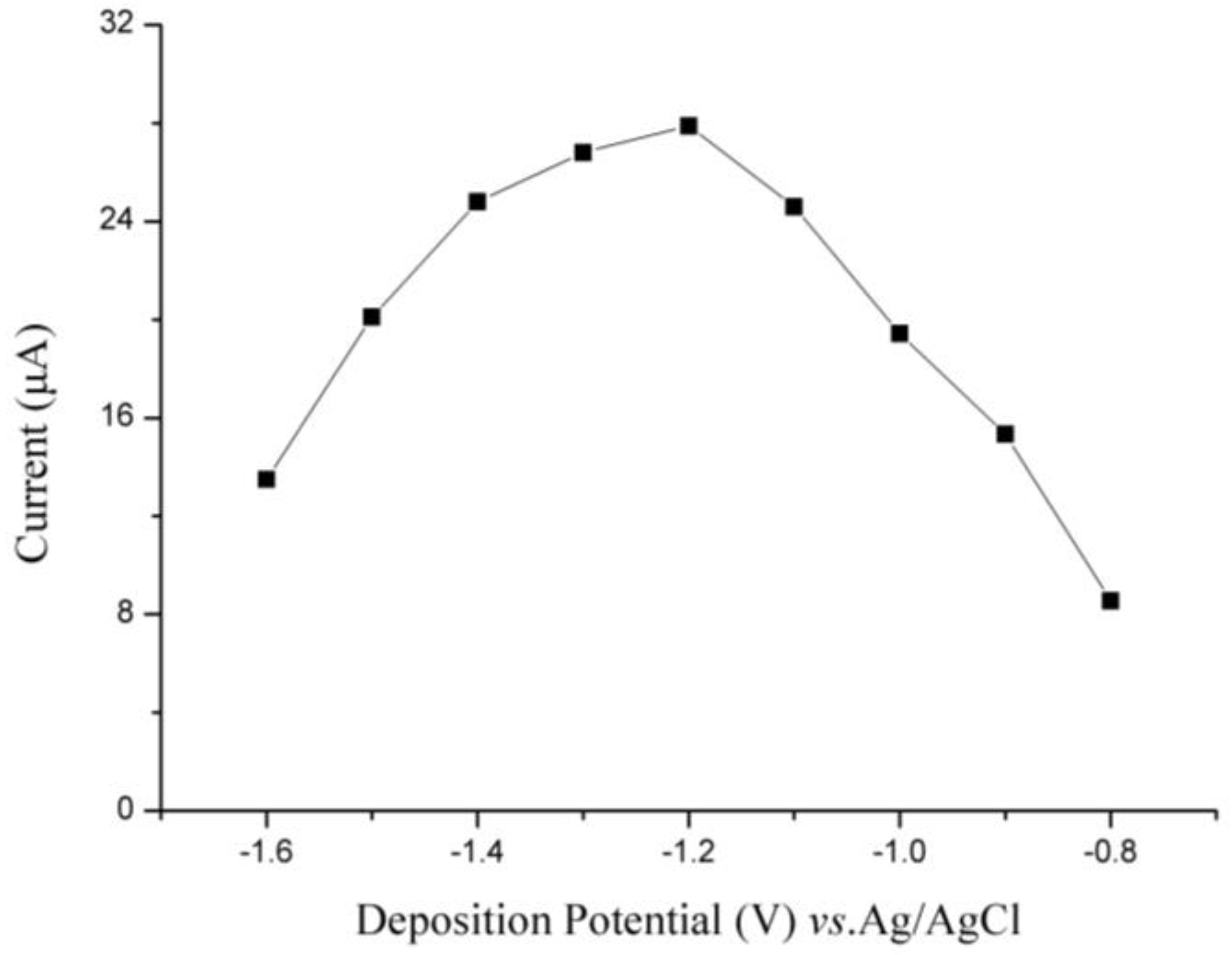
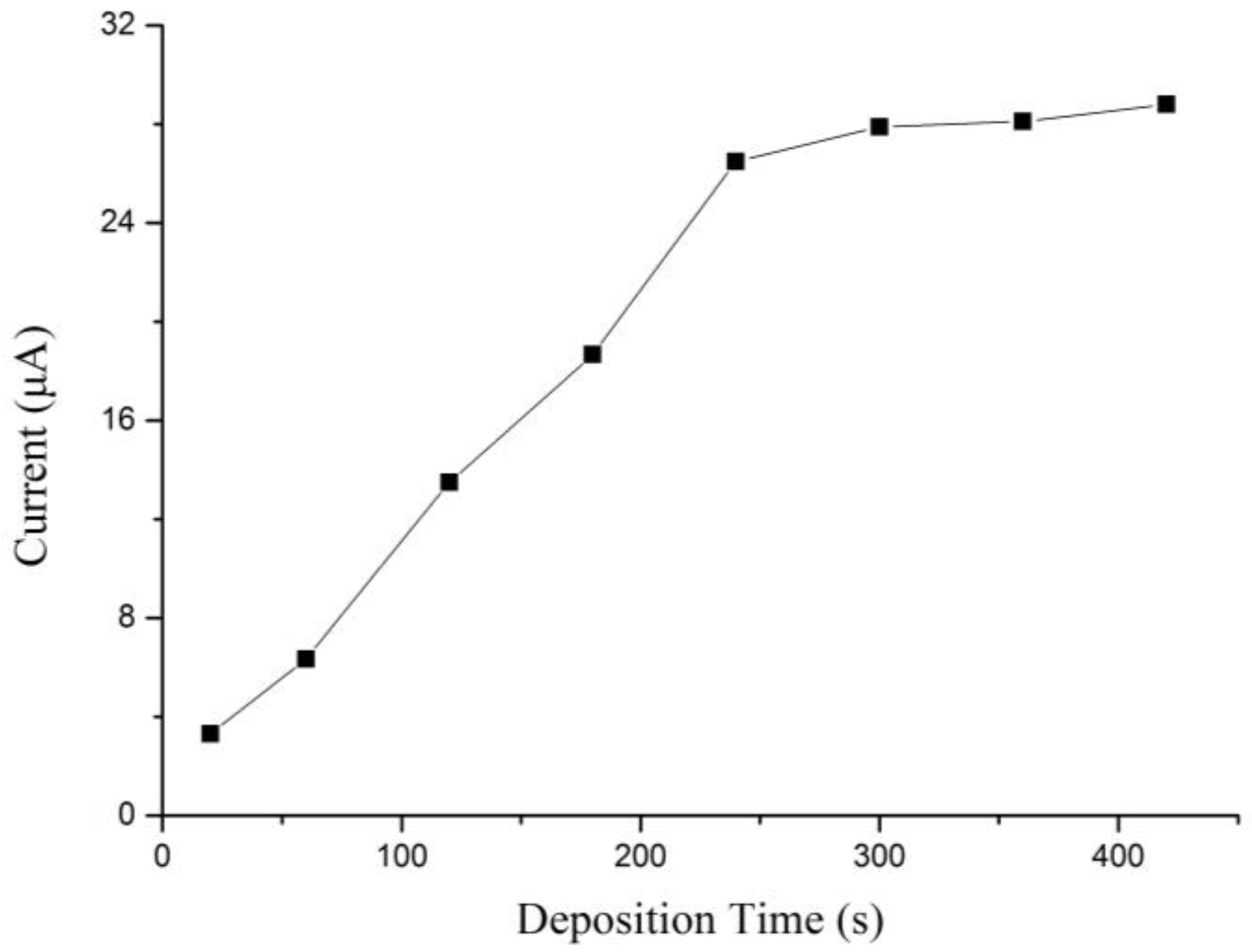
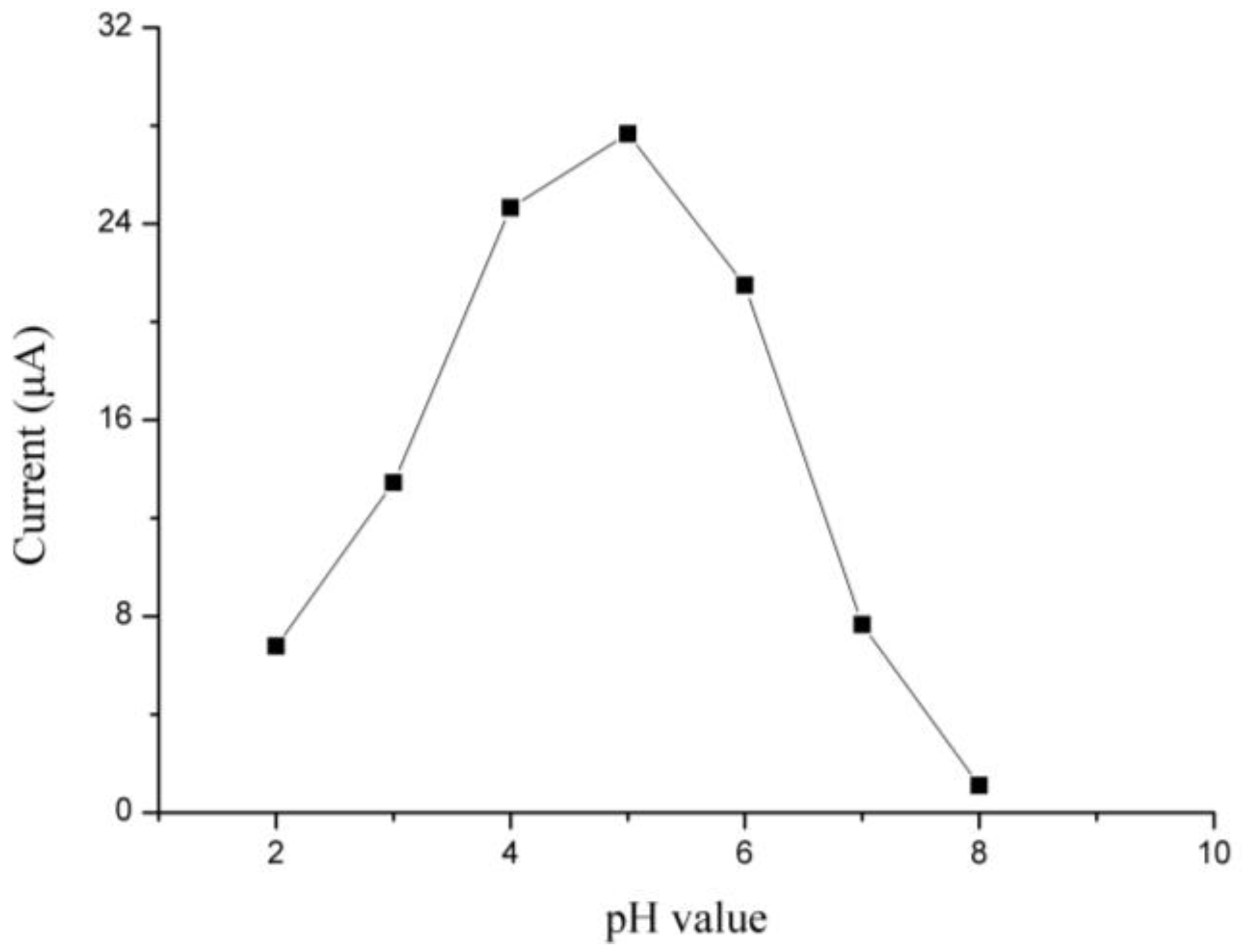
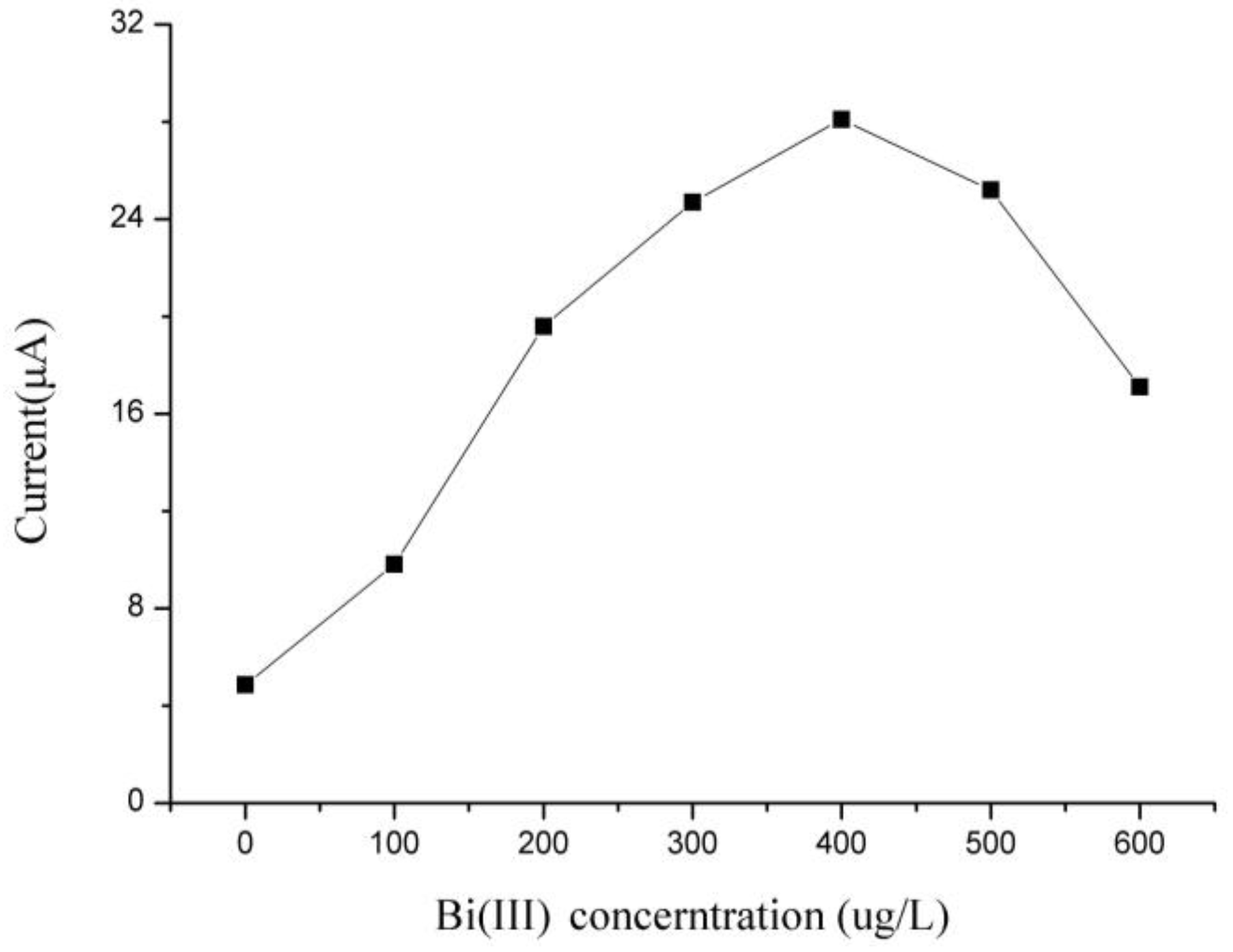
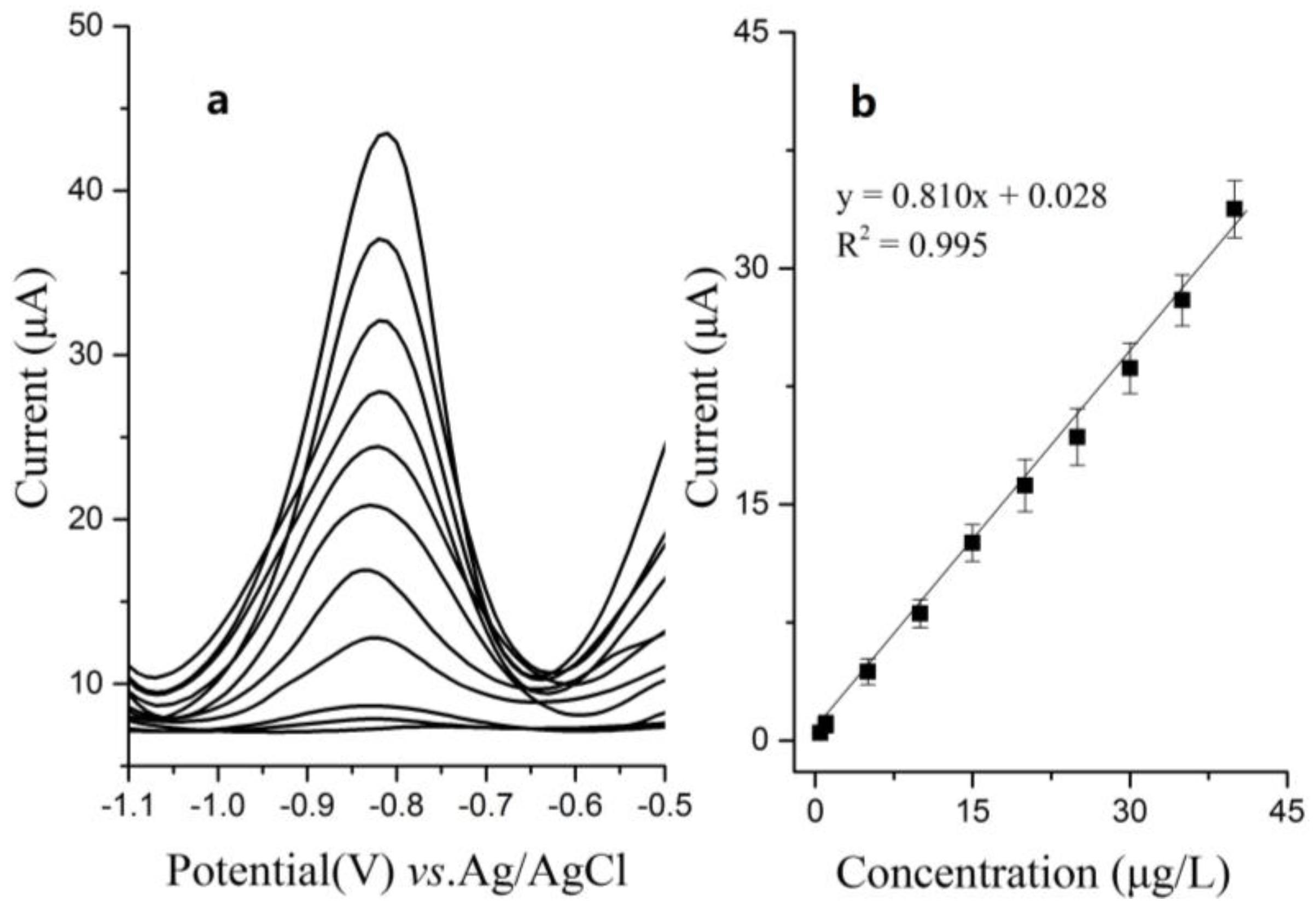
3.2. Optimization of Experimental Parameters
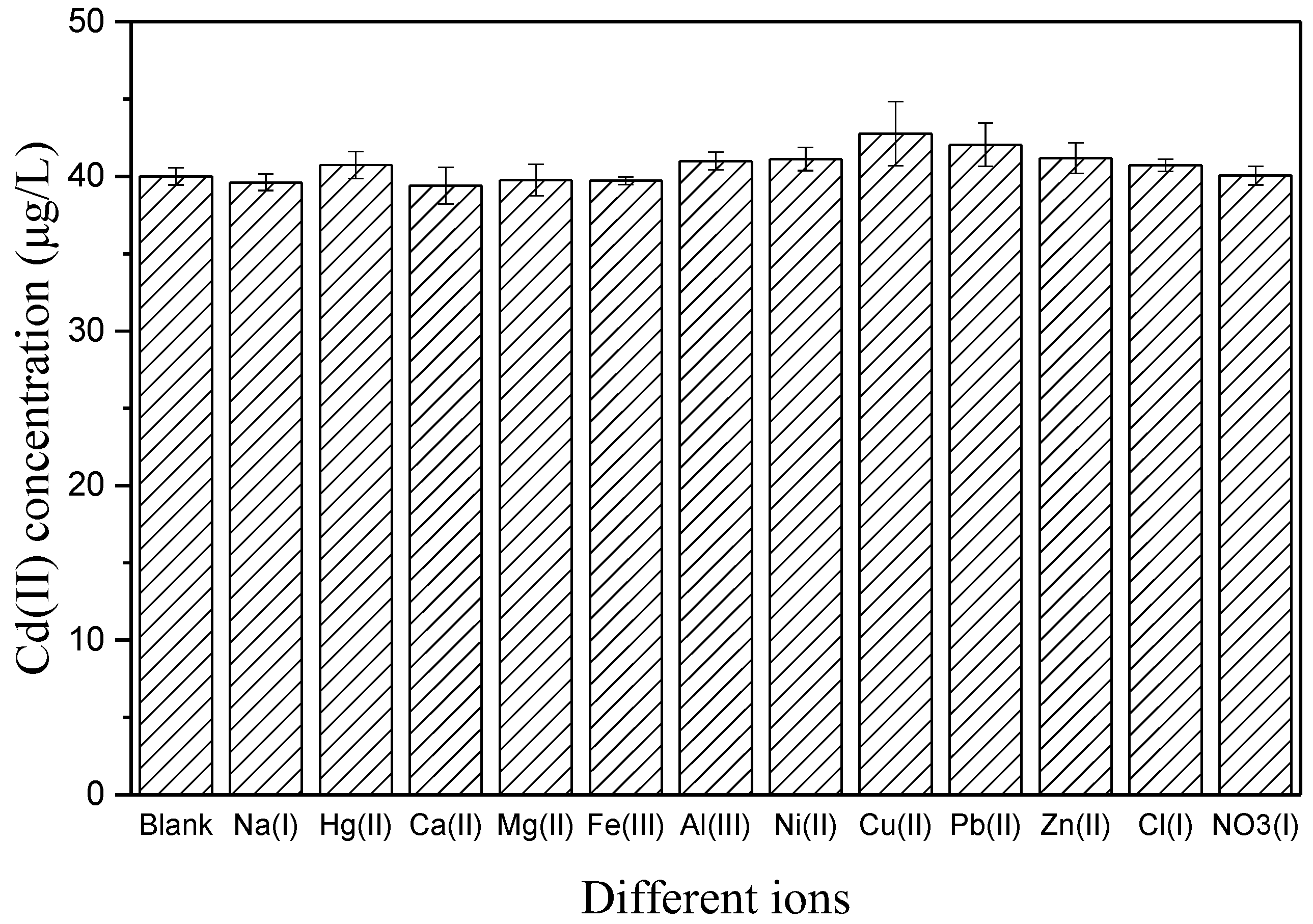
3.3. Interference Effects
3.4. The Reproducibility
3.5. Application to Real Sample Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wu, Y.; Zhang, H.; Liu, G.; Zhang, J.; Wang, J.; Yu, Y.; Lu, S. Concentrations and health risk assessment of trace elements in animal-derived food in southern China. Chemosphere 2016, 144, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, X.; Ding, C.; Wu, L. The dynamic simulation of rice growth parameters under cadmium stress with the assimilation of multi-period spectral indices and crop model. Field Crops Res. 2015, 183, 225–234. [Google Scholar] [CrossRef]
- Nharingo, T.; Ndumo, T.; Moyo, M. Human health risks due to heavy metals through consumption of wild mushrooms from Macheke forest, Rail Block forest and Muganyi communal lands in Zimbabwe. Environ. Monit. Assess. 2015, 187, 738. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, W.; Peng, C. Risk assessment of Cd polluted paddy soils in the industrial and township areas in Hunan, Southern China. Chemosphere 2016, 144, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Nakatsuka, H.; Watanabe, T.; Shimbo, S. Estimation of daily cadmium intake from cadmium in blood or cadmium in urine. Environ. Health Prev. Med. 2015, 20, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Jallad, K.N. Heavy metal exposure from ingesting rice and its related potential hazardous health risks to humans. Environ. Sci. Pollut. Res. Int. 2015, 22, 15449–15458. [Google Scholar] [CrossRef] [PubMed]
- Oteef, M.D.; Fawy, K.F.; Abd-Rabboh, H.S.; Idris, A.M. Levels of zinc, copper, cadmium, and lead in fruits and vegetables grown and consumed in Aseer Region, Saudi Arabia. Environ. Monit. Assess. 2015, 187, 676. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; McDonald, L.M. Metal Uptake in Plants and Health Risk Assessments in Metal-Contaminated Smelter Soils. Land Degrad. Dev. 2015, 26, 785–792. [Google Scholar] [CrossRef]
- Khan, M.U.; Muhammad, S.; Malik, R.N.; Khan, S.A.; Tariq, M. Heavy metals potential health risk assessment through consumption of wastewater irrigated wild plants: A case study. Hum. Ecol. Risk Assess. Int. J. 2015, 22, 141–152. [Google Scholar] [CrossRef]
- Panhwar, A.H.; Kazi, T.G.; Afridi, H.I.; Arain, S.A.; Arain, M.S.; Brahaman, K.D.; Naeemullah; Arain, S.S. Correlation of cadmium and aluminum in blood samples of kidney disorder patients with drinking water and tobacco smoking: Related health risk. Environ. Geochem. Health 2016, 38, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Liang, X.X.; Wang, P.; Wang, J.; Gao, Y.H.; Hu, S.G.; Huang, Q.; Huang, R.; Jiang, Q.; Wu, S.X.; et al. Assessment of dietary cadmium exposure: A cross-sectional study in rural areas of south China. Food Control 2016, 62, 284–290. [Google Scholar] [CrossRef]
- Sacristán, D.; Viscarra Rossel, R.A.; Recatalá, L. Proximal sensing of Cu in soil and lettuce using portable X-ray fluorescence spectrometry. Geoderma 2016, 265, 6–11. [Google Scholar] [CrossRef]
- Peng, Y.E.; Guo, W.; Zhang, P.; Jin, L.; Hu, S. Heated Slurry Sampling for the Determination of Cadmium in Food by Electrothermal Atomic Absorption Spectrometry. Anal. Lett. 2015, 48, 2894–2907. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Z.; Ross, A.; Huang, Z.; Chang, W.; Ou-Yang, K.; Chen, Y.; Wu, C. Determination of heavy metals in leather and fur by microwave plasma-atomic emission spectrometry. Spectrochim. Acta Part B At. Spectrosc. 2015, 112, 6–9. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, X.; Zhou, T.; Wang, J.; Li, H.; Zhang, P.; Ding, H.; Ding, L. Multi-throughput dynamic microwave-assisted leaching coupled with inductively coupled plasma atomic emission spectrometry for heavy metal analysis in soil. J. Anal. At. Spectrom. 2015, 30, 1920–1926. [Google Scholar] [CrossRef]
- Chen, L.; Yuan, D.; Zhang, X.; Lv, X.; Wang, L.; Li, J. Elemental analysis of Acori Tatarinowii Rhizomaby inductively coupled plasma emission and mass spectrometry with chemometrics. Anal. Lett. 2015, 48, 2882–2893. [Google Scholar] [CrossRef]
- Zhang, Y.; Mao, X.; Wang, M.; Gao, C.; Qi, Y.; Qian, Y.; Tang, X.; Zhou, J. Direct Determination of Cadmium in Grain by Solid Sampling Electrothermal Vaporization Atomic Fluorescence Spectrometry with a Tungsten Coil Trap. Anal. Lett. 2015, 48, 2908–2920. [Google Scholar] [CrossRef]
- Li, Y.; Chen, W.C.; Chen, S.M.; Lou, B.S.; Ali, M.A.; Al-Hemaid, F.M. Detection of real sample DNA at a cadmium sulfide-chitosan/gelatin modified electrode. Colloids Surf. B Biointerfaces 2014, 113, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Huerta-Aguilar, C.A.; Singh, H.; Pandiyan, T.; Singh, N. Voltammetric Simultaneous Determination of Cu2+, Cd2+ and Pb2+ in Full Aqueous Medium Using Organic Nanoparticles of Disulfide Based Receptor. Electroanalysis 2015, 27, 2544–2551. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zhang, L.; Wang, H. Electrochemical detection of trace cadmium in soil using a Nafion/stannum film-modified molecular wire carbon paste electrodes. Ionics 2013, 19, 1687–1693. [Google Scholar] [CrossRef]
- Bayrak, H.E.; Bulut, V.N.; TÜFekÇİ, M.; Bayrak, H.; Duran, C.; Soylak, M. Comparative study for the separation, preconcentration, and determination of copper and cadmium in real samples by using two different ligands. Turk. J. Chem. 2016, 40, 93–105. [Google Scholar] [CrossRef]
- Benvidi, A.; Jahanbani, S.; Akbari, A.; Zare, H.R. Simultaneous determination of hydrazine and hydroxylamine on a magnetic bar carbon paste electrode modified with reduced graphene oxide/Fe3O4 nanoparticles and a heterogeneous mediator. J. Electroanal. Chem. 2015, 758, 68–77. [Google Scholar] [CrossRef]
- Dehdashtian, S.; Gholivand, M.B.; Shamsipur, M.; Kariminia, S. Construction of a sensitive and selective sensor for morphine using chitosan coated Fe3O4 magnetic nanoparticle as a modifier. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.; Wang, J.; Xia, H.; Wang, S.; Yu, X. Direct Electrochemistry and Electrocatalysis of Horseradish Peroxidase Immobilized in a DNA/Chitosan-Fe3O4 Magnetic Nanoparticle Bio-Complex Film. Materials 2014, 7, 1069–1083. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yu, X.Y.; Xiong, S.Q.; Liu, J.H.; Huang, X.J. Electrochemical detection of arsenic(III) completely free from noble metal: Fe3O4 microspheres-room temperature ionic liquid composite showing better performance than gold. Anal. Chem. 2013, 85, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Srivastava, R. Ionic liquids coated Fe3O4 based inorganic–organic hybrid materials and their application in the simultaneous determination of DNA bases. Colloids Surf. B 2014, 118, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.L.; Lo, N.C.; Cheng, H.; Tsuda, T.; Sakamoto, T.; Chen, Y.H.; Kuwabata, S.; Chen, P.Y. An ionic liquid-Fe3O4 nanoparticles-graphite composite electrode used for nonenzymatic electrochemical determination of hydrogen peroxide. J. Electroanal. Chem. 2014, 729, 109–115. [Google Scholar] [CrossRef]
- Dimovasilis, P.A.; Prodromidis, M.I. Preparation of Screen-Printed Compatible Bismuth-Modified Sol-Gel Microspheres: Application to the Stripping Voltammetric Determination of Lead and Cadmium. Anal. Lett. 2015, 49, 979–989. [Google Scholar] [CrossRef]
- Promphet, N.; Rattanarat, P.; Rangkupan, R.; Chailapakul, O.; Rodthongkum, N. An electrochemical sensor based on graphene/polyaniline/polystyrene nanoporous fibers modified electrode for simultaneous determination of lead and cadmium. Sens. Actuators B Chem. 2015, 207, 526–534. [Google Scholar] [CrossRef]
- Sosa, V.; Barcelo, C.; Serrano, N.; Arino, C.; Diaz-Cruz, J.M.; Esteban, M. Antimony film screen-printed carbon electrode for stripping analysis of Cd(II), Pb(II), and Cu(II) in natural samples. Anal. Chim. Acta 2015, 855, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Y.; Ding, D.; Zhao, J.; Liu, J.; Yang, W.; Qu, K. On-site determination of Pb2+ and Cd2+ in seawater by double stripping voltammetry with bismuth-modified working electrodes. Microchem. J. 2016, 126, 280–286. [Google Scholar] [CrossRef]


| Cd(II) Concentration (µg/L) | Electrode Number | Current (µA) | Linear Method | ISN Method | ||
|---|---|---|---|---|---|---|
| Detection Concentration (µg/L) | Relative Error (%) | Detection Concentration (µg/L) | Relative Error (%) | |||
| 2 | E1 | 1.78 ± 0.05 | 1.93 ± 0.05 | 3.52 | 2.09 ± 0.06 | 4.48 |
| E2 | 1.63 ± 0.03 | 1.76 ± 0.03 | 11.89 | 2.03 ± 0.04 | 1.65 | |
| E3 | 1.73 ± 0.05 | 1.87 ± 0.05 | 6.49 | 1.92 ± 0.06 | 3.92 | |
| 5 | E1 | 4.33 ± 0.19 | 4.73 ± 0.22 | 5.32 | 5.13 ± 0.26 | 2.60 |
| E2 | 4.22 ± 0.09 | 4.61 ± 0.10 | 7.74 | 5.34 ± 0.12 | 6.80 | |
| E3 | 4.47 ± 0.12 | 4.89 ± 0.14 | 2.23 | 4.99 ± 0.16 | 0.24 | |
| 10 | E1 | 8.72 ± 0.29 | 9.57 ± 0.31 | 4.30 | 10.30 ± 0.37 | 3.00 |
| E2 | 8.53 ± 0.19 | 9.25 ± 0.21 | 7.49 | 10.64 ± 0.27 | 6.40 | |
| E3 | 9.73 ± 0.37 | 10.68±0.40 | 6.83 | 9.78 ± 0.46 | 2.20 | |
| Modifier | Accumulation Time (s) | Concentration Range (µg/L) | Detection Limit (µg/L) | Reference |
|---|---|---|---|---|
| Bi/SG/SPE | 240 | 0.95–16.9 | 1.4 | [28] |
| G/PANI/PS/SPE | 180 | 10–500 | 4.43 | [29] |
| SbSPCE | 120 | 11.5–72.4 | 3.4 | [30] |
| ILGPE | 300 | 0.1–3.2 | 0.01 | [31] |
| Bi/Fe3O4/ILSPE | 240 | 0.5–40 | 0.05 | The present |
| Sample | Added (μg/L) | Found (μg/L) | Found by FAAS (μg/L) | Recovery (%) |
|---|---|---|---|---|
| 1 | – | 1.45 ± 0.49 | 1.38 ± 0.11 | – |
| 5 | 6.88 ± 0.26 | 107.83 | ||
| 10 | 11.48 ± 0.45 | 100.87 | ||
| 2 | – | 2.85 ± 0.37 | 3.02 ± 0.08 | – |
| 5 | 7.36 ± 0.39 | 91.77 | ||
| 10 | 12.66 ± 0.19 | 97.23 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Zhao, G.; Yin, Y.; Wang, Z.; Liu, G. Screen-Printed Electrode Modified by Bismuth /Fe3O4 Nanoparticle/Ionic Liquid Composite Using Internal Standard Normalization for Accurate Determination of Cd(II) in Soil. Sensors 2018, 18, 6. https://doi.org/10.3390/s18010006
Wang H, Zhao G, Yin Y, Wang Z, Liu G. Screen-Printed Electrode Modified by Bismuth /Fe3O4 Nanoparticle/Ionic Liquid Composite Using Internal Standard Normalization for Accurate Determination of Cd(II) in Soil. Sensors. 2018; 18(1):6. https://doi.org/10.3390/s18010006
Chicago/Turabian StyleWang, Hui, Guo Zhao, Yuan Yin, Zhiqiang Wang, and Gang Liu. 2018. "Screen-Printed Electrode Modified by Bismuth /Fe3O4 Nanoparticle/Ionic Liquid Composite Using Internal Standard Normalization for Accurate Determination of Cd(II) in Soil" Sensors 18, no. 1: 6. https://doi.org/10.3390/s18010006




