Low Power Operation of Temperature-Modulated Metal Oxide Semiconductor Gas Sensors
Abstract
:1. Introduction
2. Materials and Methods
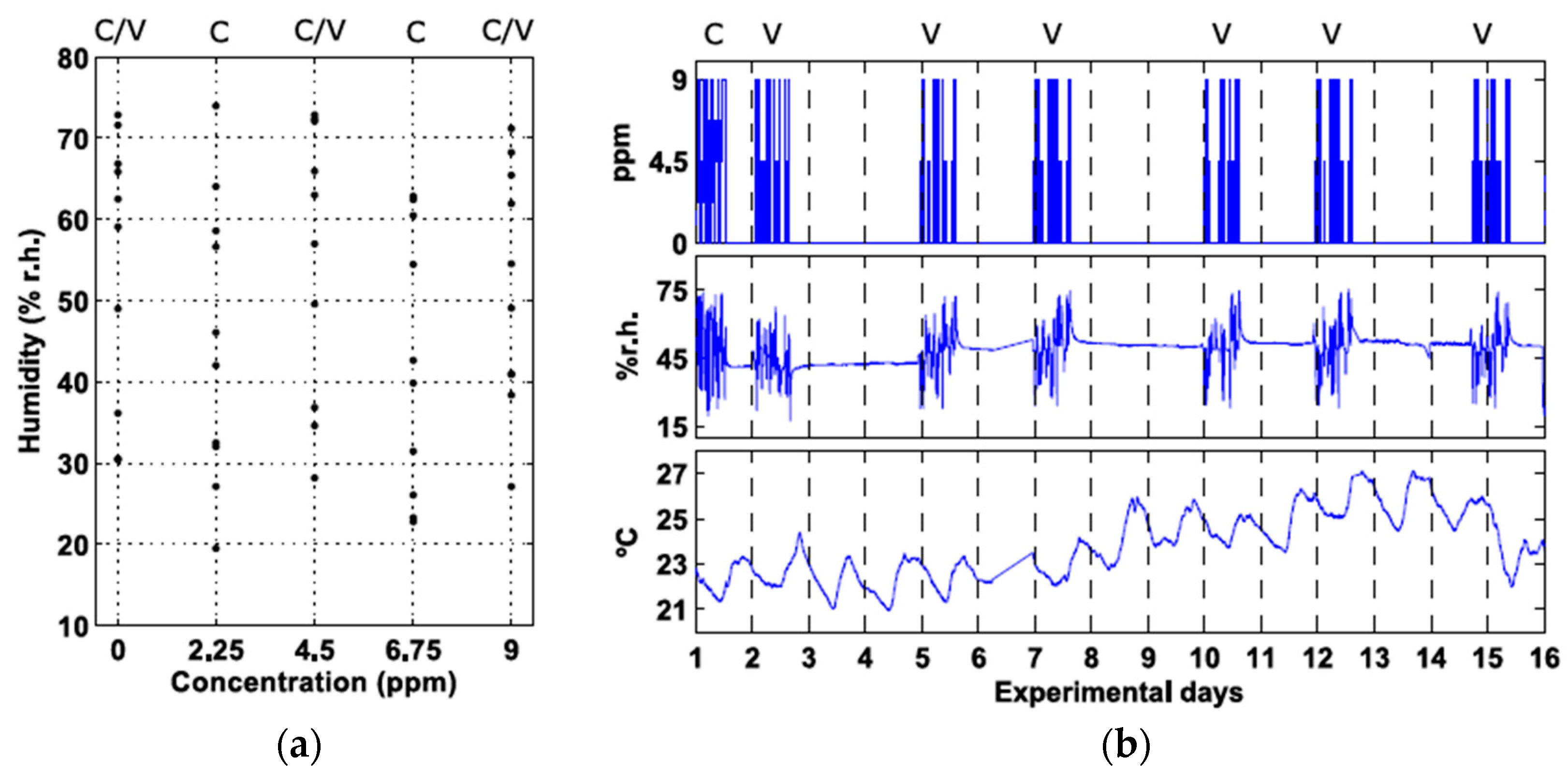
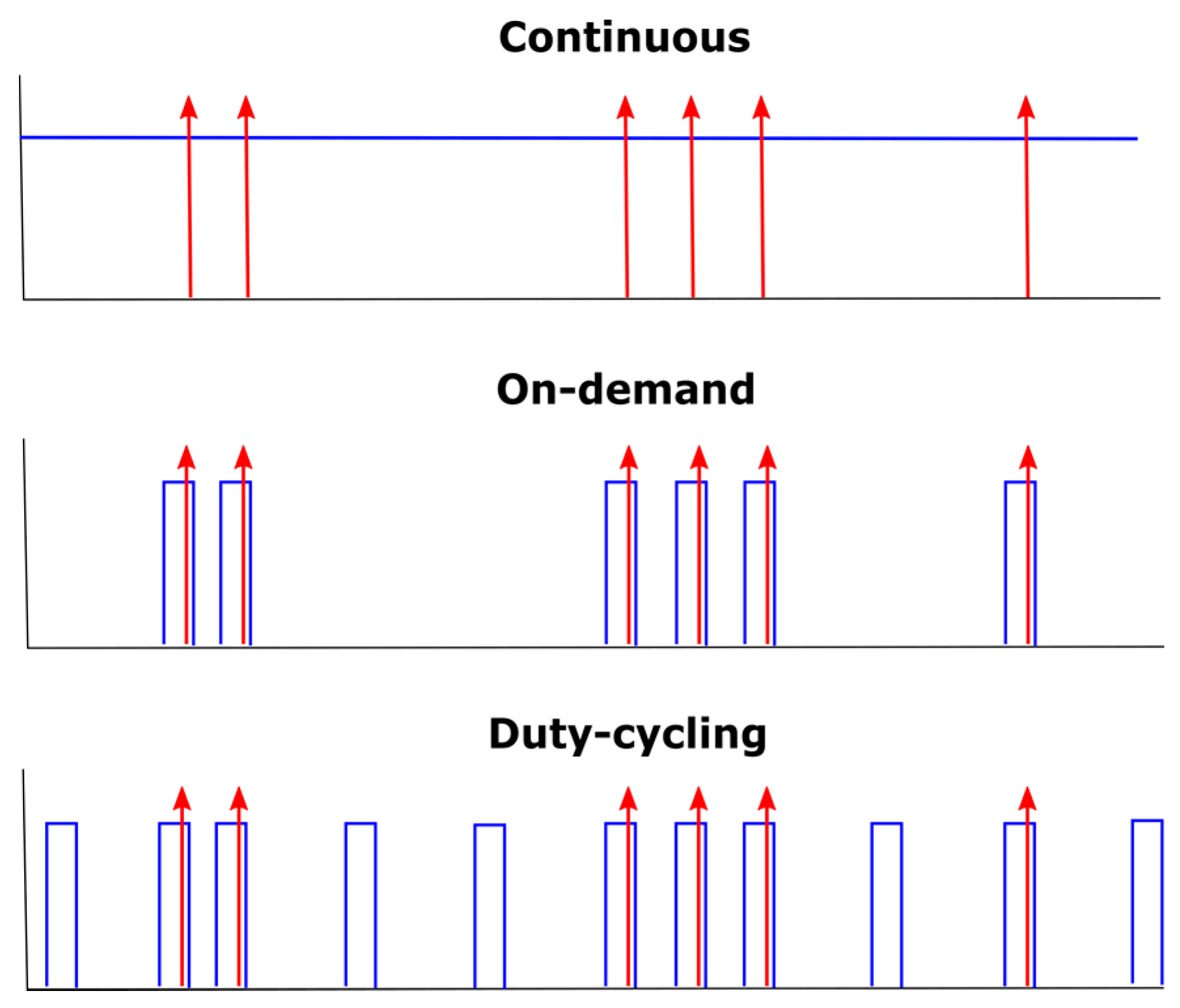
2.1. Experimental Design
2.2. Sensor Board
2.3. Calibration Models
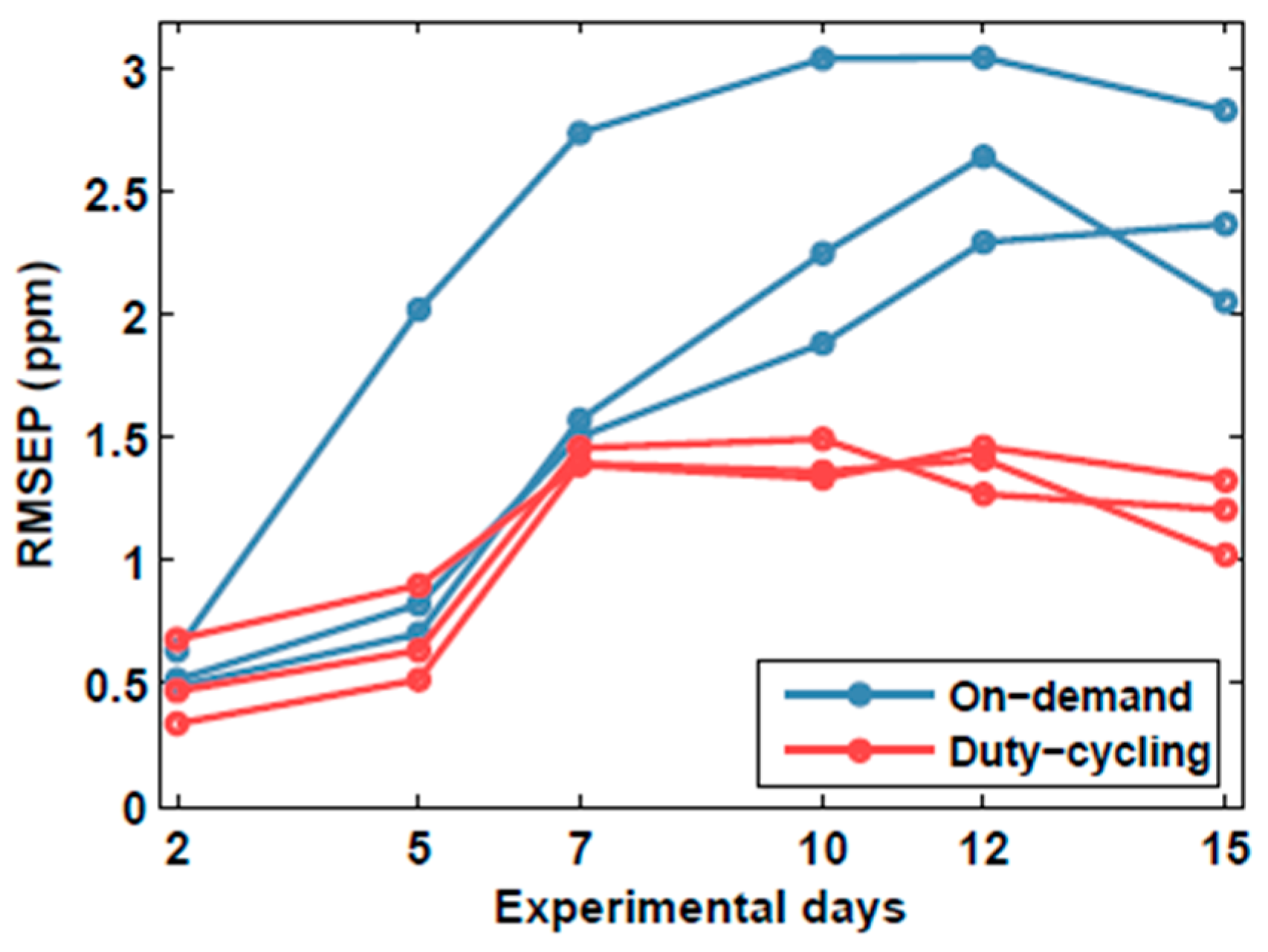
3. Results and Discussion
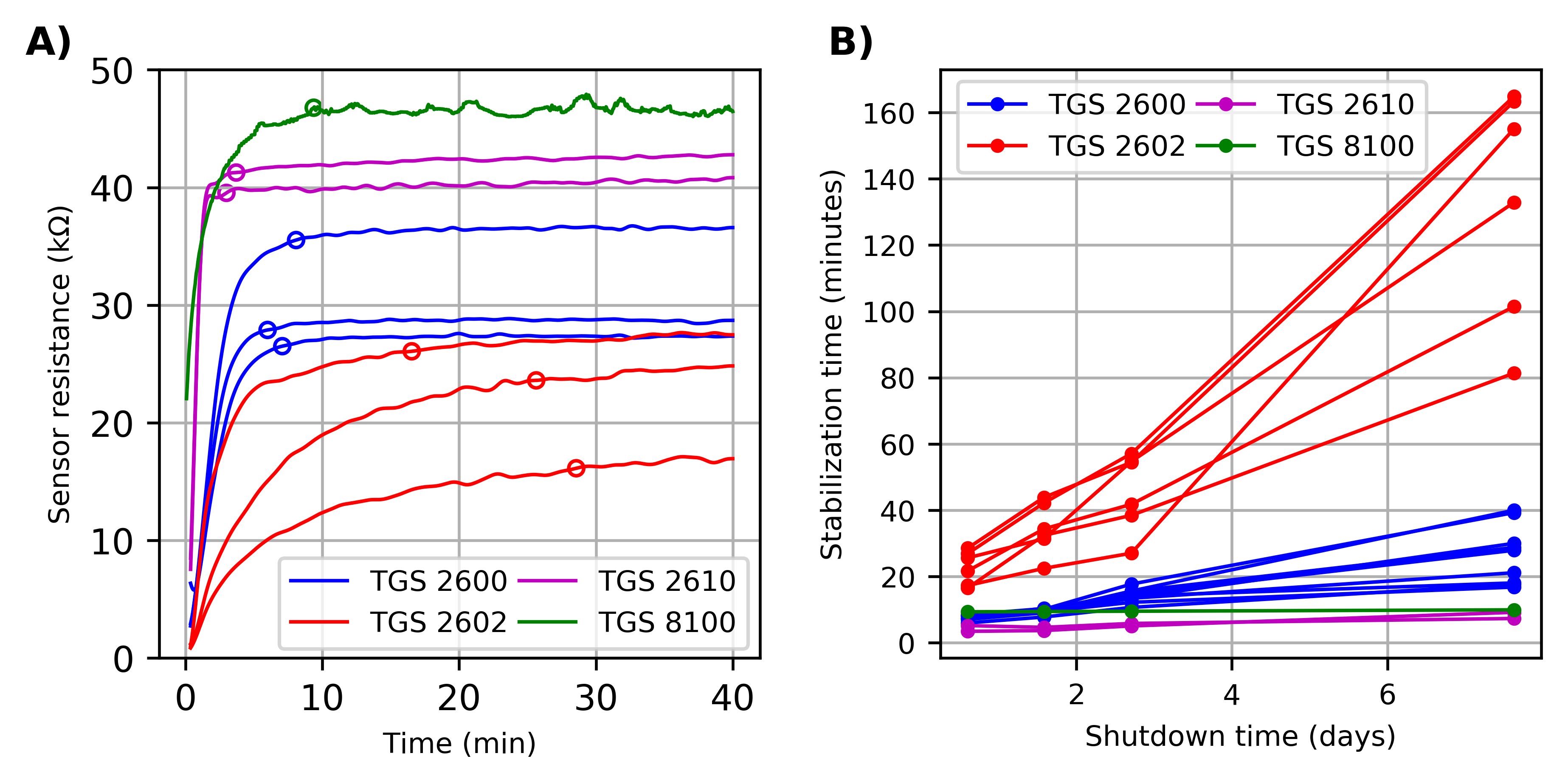
3.1. Drift in Sensor Conductance Patterns
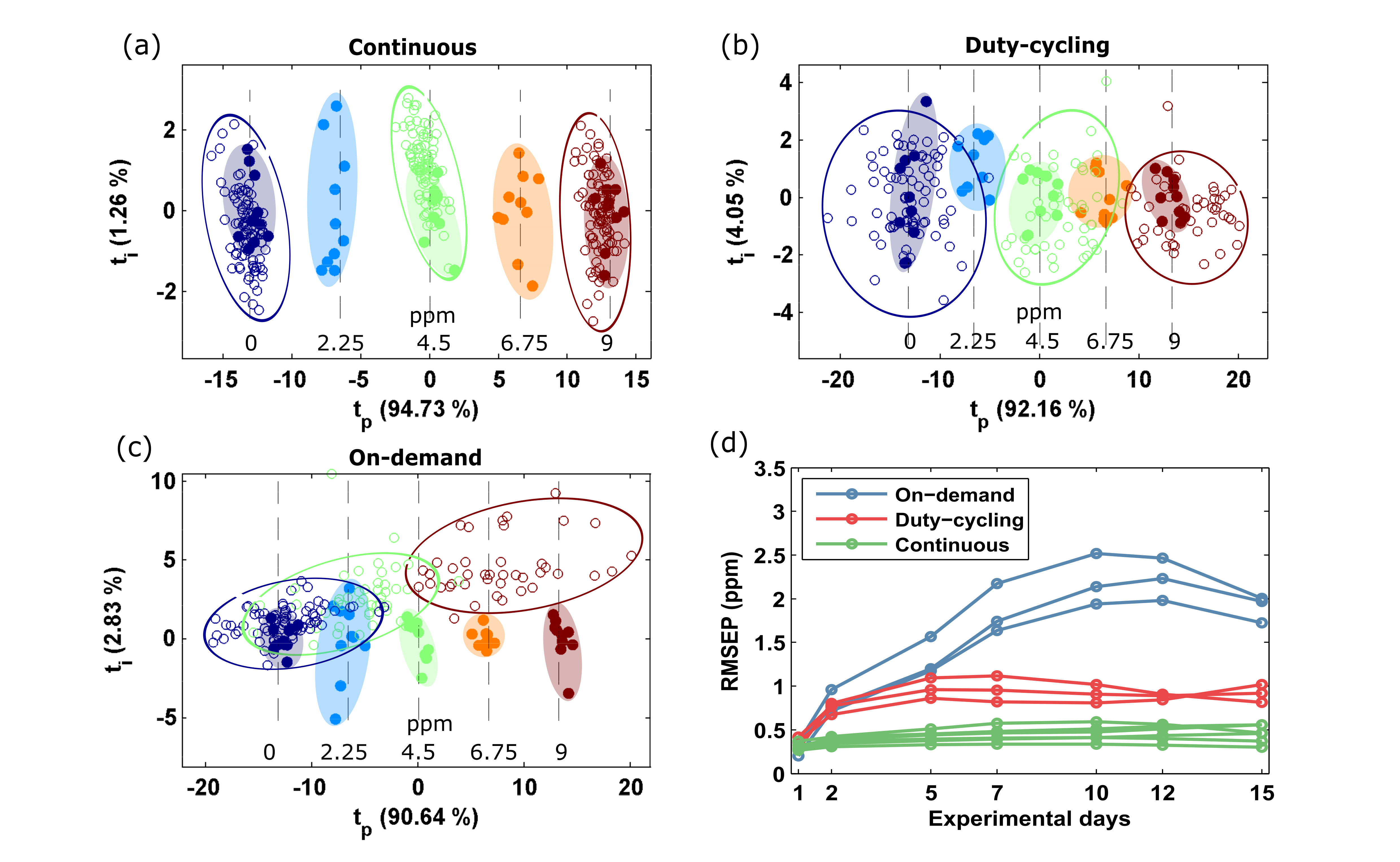
3.2. Prediction Error
3.3. Calibration in Discontinuous Mode
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
References
- Chansin, G.; Pugh, D. Environmental Gas Sensors 2017–2027; CISION: Cambridge, UK, 2017. [Google Scholar]
- Pouter, J. Smartphone Ownership and Internet Usage Continues to Climb in Emerging Economies; Pew Research Center: Washington, DC, USA, 2016. [Google Scholar]
- Joshi, S.M. The sick building syndrome. Indian J. Occup. Environ. Med. 2008, 12, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J. Indoor residential chemical emissions as risk factors for respiratory and allergic effects in children: A review. Indoor Air 2007, 17, 259–277. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Guidelines for Indoor Air Quality: Selected Pollutants; WHO: Geneva, Switzerland, 2010. [Google Scholar]
- EPA. Criteria Air Pollutants. In America’s Children and the Environment; EPA: Washington, DC, USA, 2015; pp. 1–22. [Google Scholar]
- Kay, M.; Santos, J.; Takane, M. mHealth: New horizons for health through mobile technologies. World Heal. Organ. 2011, 64, 66–71. [Google Scholar]
- Cavaliere, F.; Volpe, C.; Gargaruti, R.; Poscia, A.; Di Donato, M.; Grieco, G.; Moscato, U. Effects of acute hypoventilation and hyperventilation on exhaled carbon monoxide measurement in healthy volunteers. BMC Pulm. Med. 2009, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Beg, M.F.S.; Alzoghaibi, M.A.; Abba, A.A.; Habib, S.S. Exhaled nitric oxide in stable chronic obstructive pulmonary disease. Ann. Thorac. Med. 2009, 4, 65. [Google Scholar] [PubMed]
- Davies, S.; Spanel, P.; Smith, D. Quantitative analysis of ammonia on the breath of patients in end-stage renal failure. Kidney Int. 1997, 52, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.; Al-Moundhri, M.; Souberbielle, B.E.; Norton, A.; Priest, K.; Ryan, C.; Mendes, R.; Ashley, S.; Smith, I.E.; O’Brien, M.E.R. A randomised study of SRL172 (Mycobacterium vaccae) in patients with advanced lung cancer treated with chemotherapy. Br. J. Cancer 1998, 78, 4. [Google Scholar]
- Zhang, J.; Yao, X.; Yu, R.; Bai, J.; Sun, Y.; Huang, M.; Adcock, I.M.; Barnes, P.J. Exhaled carbon monoxide in asthmatics: a meta-analysis. Respir. Res. 2010, 11, 50. [Google Scholar] [CrossRef] [PubMed]
- King, J.; Kupferthaler, A.; Frauscher, B.; Hackner, H.; Unterkofler, K.; Teschl, G.; Hinterhuber, H.; Amann, A.; Högl, B. Measurement of endogenous acetone and isoprene in exhaled breath during sleep. Physiol. Meas. 2012, 33, 413. [Google Scholar] [CrossRef] [PubMed]
- Kundu, S.K.; Bruzek, J.A.; Nair, R.; Judilla, A.M. Breath acetone analyzer: diagnostic tool to monitor dietary fat loss. Clin. Chem. 1993, 39, 87–92. [Google Scholar] [PubMed]
- Beard, E.; West, R. Pilot study of the use of personal carbon monoxide monitoring to achieve radical smoking reduction. J. Smok. Cessat. 2012, 7, 12–17. [Google Scholar] [CrossRef]
- Delgado, M.K.; Huang, Y.; Wanner, K.; Goldberg, E.; Hemmons, J.; Spencer, E.; Wetherill, R. Test accuracy of smartphone-paired breathalysers: A validation study. Inj. Prev. 2017, 23, A15. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Hwang, I.-S.; Kim, S.-J.; Lee, C.-Y.; Lee, J.-H. CuO nanowire gas sensors for air quality control in automotive cabin. Sens. Actuators B Chem. 2008, 135, 298–303. [Google Scholar] [CrossRef]
- Romain, A.C.; Nicolas, J. Long term stability of metal oxide-based gas sensors for e-nose environmental applications: An overview. Sens. Actuators B Chem. 2010, 146, 502–506. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Borrego, C.; Costa, A.M.; Ginja, J.; Amorim, M.; Coutinho, M.; Karatzas, K.; Sioumis, T.; Katsifarakis, N.; Konstantinidis, K.; De Vito, S.; et al. Assessment of air quality microsensors versus reference methods: The EuNetAir joint exercise. Atmos. Environ. 2016, 147. [Google Scholar] [CrossRef]
- Adam, G.; Lemaigre, S.; Goux, X.; Delfosse, P.; Romain, A.-C. Upscaling of an electronic nose for completely stirred tank reactor stability monitoring from pilot-scale to real-scale agricultural co-digestion biogas plant. Bioresour. Technol. 2015, 178, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic noses for food quality: A review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Perera, A.; Pardo, A.; Barrettino, D.; Hierlermann, A.; Marco, S. Evaluation of fish spoilage by means of a single metal oxide sensor under temperature modulation. Sens. Actuators B Chem. 2010, 146, 477–482. [Google Scholar] [CrossRef]
- Sekhar, P.K.; Brosha, E.L.; Mukundan, R.; Li, W.; Nelson, M.A.; Palanisamy, P.; Garzon, F.H. Application of commercial automotive sensor manufacturing methods for NOx/NH3 mixed potential sensors for on-board emissions control. Sens. Actuators, B Chem. 2010, 144, 112–119. [Google Scholar] [CrossRef]
- Boon-Brett, L.; Bousek, J.; Moretto, P. Reliability of commercially available hydrogen sensors for detection of hydrogen at critical concentrations: Part II—Selected sensor test results. Int. J. Hydrogen Energy 2009, 34, 562–571. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in gas sensor technology for hydrogen safety. Int. J. Hydrogen Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Kwor, R. Carbon Monoxide Detectors, Carbon Monoxide Toxicity; CRC: Boca Raton, FL, USA, 2000; pp. 61–82. [Google Scholar]
- Tomchenko, A.A.; Harmer, G.P.; Marquis, B.T. Detection of chemical warfare agents using nanostructured metal oxide sensors. Sens. Actuators B Chem. 2005, 108, 41–55. [Google Scholar] [CrossRef]
- Šundić, T.; Marco, S.; Perera, A.; Pardo, A.; Hahn, S.; Bârsan, N.; Weimar, U. Fuzzy inference system for sensor array calibration: prediction of CO and CH4 levels in variable humidity conditions. Chemom. Intell. Lab. Syst. 2002, 64, 103–122. [Google Scholar] [CrossRef]
- Kuske, M.; Padilla, M.; Romain, A.C.; Nicolas, J.; Rubio, R.; Marco, S. Detection of diverse mould species growing on building materials by gas sensor arrays and pattern recognition. Sens. Actuators B Chem. 2006, 119, 33–40. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Advances in electronic-nose technologies developed for biomedical applications. Sensors 2011, 11, 1105–1176. [Google Scholar] [CrossRef] [PubMed]
- Padilla, M.; Perera, A.; Montoliu, I.; Chaudry, A.; Persaud, K.; Marco, S. Drift compensation of gas sensor array data by Orthogonal Signal Correction. Chemom. Intell. Lab. Syst. 2010, 100, 28–35. [Google Scholar] [CrossRef]
- Padilla, M.; Fonollosa, J.; Marco, S. Improving the Robustness of Odour Sensing Systems by Multivariate Signal Processing; IGI-Global: Hershey, PA, USA, 2013. [Google Scholar]
- Ruhland, B.; Becker, T.; Müller, G. Gas-kinetic interactions of nitrous oxides with SnO2 surfaces. Sens. Actuators B Chem. 1998, 50, 85–94. [Google Scholar] [CrossRef]
- Zampolli, S.; Elmi, I.; Cozzani, E.; Cardinali, G.C.; Scorzoni, A.; Cicioni, M.; Marco, S.; Palacio, F.; Gómez-Cama, J.M.; Sayhan, I.; et al. Ultra-low-power components for an RFID Tag with physical and chemical sensors. Microsyst. Technol. 2008, 14, 581–588. [Google Scholar] [CrossRef]
- AMG AG. AMS AS-MLV-P2 Air Quality Sensor; AMS AG: Unterpremstätten, Austria, 2015. [Google Scholar]
- Figaro Engineering. Inc. Technical Information for Tgs2610; Figaro Engineering. Inc.: Arlington Heights, IL, USA, 2012. [Google Scholar]
- Clifford, P.K.; Tuma, D.T. Characteristics of semiconductor gas sensors II. Transient response to temperature change. Sens. Actuators 1982, 3, 255–281. [Google Scholar] [CrossRef]
- Sayhan, I.; Helwig, A.; Becker, T.; Muller, G.; Elmi, I.; Zampolli, S.; Padilla, M.; Marco, S. Discontinuously Operated Metal Oxide Gas Sensors for Flexible Tag Microlab Applications. IEEE Sens. J. 2008, 8, 176–181. [Google Scholar] [CrossRef]
- Wu, H.; Siegel, M. Odor-based incontinence sensor. In Proceedings of the 17th IEEE Instrumentation and Measurement Technology Conference, Baltimore, MD, USA, 1–4 May 2000; Volume 1, pp. 63–68. [Google Scholar]
- Macias, M.M.; Orellana, C.J.G.; Velasco, H.M.G.; Manso, A.G.; Garzon, J.E.A.; Santamaria, H.S. Gas sensor measurements during the initial action period of duty-cycling for power saving. Sens. Actuators B Chem. 2017, 239, 1003–1009. [Google Scholar] [CrossRef]
- Jelicic, V.; Oletic, D.; Sever, T.; Bilas, V. Evaluation of mox gas sensor transient response for low-power operation. In Proceedings of the 2015 IEEE Sensors Applications Symposium (SAS), Zadar, Croatia, 13–15 April 2015; pp. 1–5. [Google Scholar]
- Rossi, M.; Brunelli, D. Ultra low power CH4 monitoring with wireless sensors. In Proceedings of the 2015 IEEE Sensors Applications Symposium (SAS), Baltimore, MD, USA, 3–6 November 2013; pp. 1–4. [Google Scholar]
- Choi, S.; Kim, N.; Cha, H.; Ha, R. Micro sensor node for air pollutant monitoring: Hardware and software issues. Sensors 2009, 9, 7970–7987. [Google Scholar] [CrossRef] [PubMed]
- Oletic, D.; Jelicic, V.; Antolovic, D.; Bilas, V. Energy-efficient atmospheric CO concentration sensing with on-demand operating MOX gas sensor. In Proceedings of the 2014 IEEE Sensors, Valencia, Spain, 2–5 November 2014; pp. 795–798. [Google Scholar]
- Bicelli, S.; Depari, A.; Faglia, G.; Flammini, A.; Fort, A.; Mugnaini, M.; Ponzoni, A.; Vignoli, V.; Rocchi, S. Model and experimental characterization of the dynamic behavior of low-power carbon monoxide MOX sensors operated with pulsed temperature profiles. IEEE Trans. Instrum. Meas. 2009, 58, 1324–1332. [Google Scholar] [CrossRef]
- Barsan, N.; Koziej, D.; Weimar, U. Metal oxide-based gas sensor research: How to? Sens. Actuators B Chem. 2007, 121, 18–35. [Google Scholar] [CrossRef]
- Lee, A.P.; Reedy, B.J. Temperature modulation in semiconductor gas sensing. Sens. Actuators B Chem. 1999, 60, 35–42. [Google Scholar] [CrossRef]
- Vergara, A.; Ramírez, J.L.; Llobet, E. Reducing power consumption via a discontinuous operation of temperature-modulated micro-hotplate gas sensors: Application to the logistics chain of fruit. Sens. Actuators B Chem. 2008, 129, 311–318. [Google Scholar] [CrossRef]
- F.I.S. Inc. Fis Gas Sensor SB-500-12; F.I.S. Inc.: Jacksonville, FL, USA, 2017. [Google Scholar]
- Conrad, T.; Hiry, P.; Schutze, A. PuMaH-a temperature control and resistance read-out system for microstructured gas sensors based on PWM signals. In Proceedings of the 2005 IEEE Sensors, Irvine, CA, USA, 31 October–3 November 2005. [Google Scholar]
- Sensirion, A.G. Data Sheet SHT7x (SHT71, SHT75)-Humidity and Temperature Sensor IC. Available online: http//www. Sensirion. Com/en/products/humidity-Temperature/humidity-Sensor-sht75 (accessed on 12 November 2017).
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-regression: A basic tool of chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Wold, S.; Trygg, J.; Berglund, A.; Antti, H. Some recent developments in PLS modeling. Chemom. Intell. Lab. Syst. 2001, 58, 131–150. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (O-PLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Burgues, J.; Marco, S. Multivariate estimation of the limit of detection by orthogonal partial least squares in temperature-modulated MOX sensors. Anal. Chim. Acta. 2018, accepted. [Google Scholar]
- Di Carlo, S.; Falasconi, M. Drift correction methods for gas chemical sensors in artificial olfaction systems: techniques and challenges. In Advances in Chemical Sensors; InTech: London, UK, 2012. [Google Scholar]
- Korotcenkov, G.; Cho, B.K. Instability of metal oxide-based conductometric gas sensors and approaches to stability improvement (short survey). Sens. Actuators B Chem. 2011, 156, 527–538. [Google Scholar] [CrossRef]
- Everitt, B.; Skrondal, A. The Cambridge Dictionary of Statistics; Cambridge University Press: Cambridge, UK, 2002; Volume 106. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burgués, J.; Marco, S. Low Power Operation of Temperature-Modulated Metal Oxide Semiconductor Gas Sensors. Sensors 2018, 18, 339. https://doi.org/10.3390/s18020339
Burgués J, Marco S. Low Power Operation of Temperature-Modulated Metal Oxide Semiconductor Gas Sensors. Sensors. 2018; 18(2):339. https://doi.org/10.3390/s18020339
Chicago/Turabian StyleBurgués, Javier, and Santiago Marco. 2018. "Low Power Operation of Temperature-Modulated Metal Oxide Semiconductor Gas Sensors" Sensors 18, no. 2: 339. https://doi.org/10.3390/s18020339





