The Trace Detection of Nitrite Ions Using Neutral Red Functionalized SH-β-Cyclodextrin @Au Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Apparatus
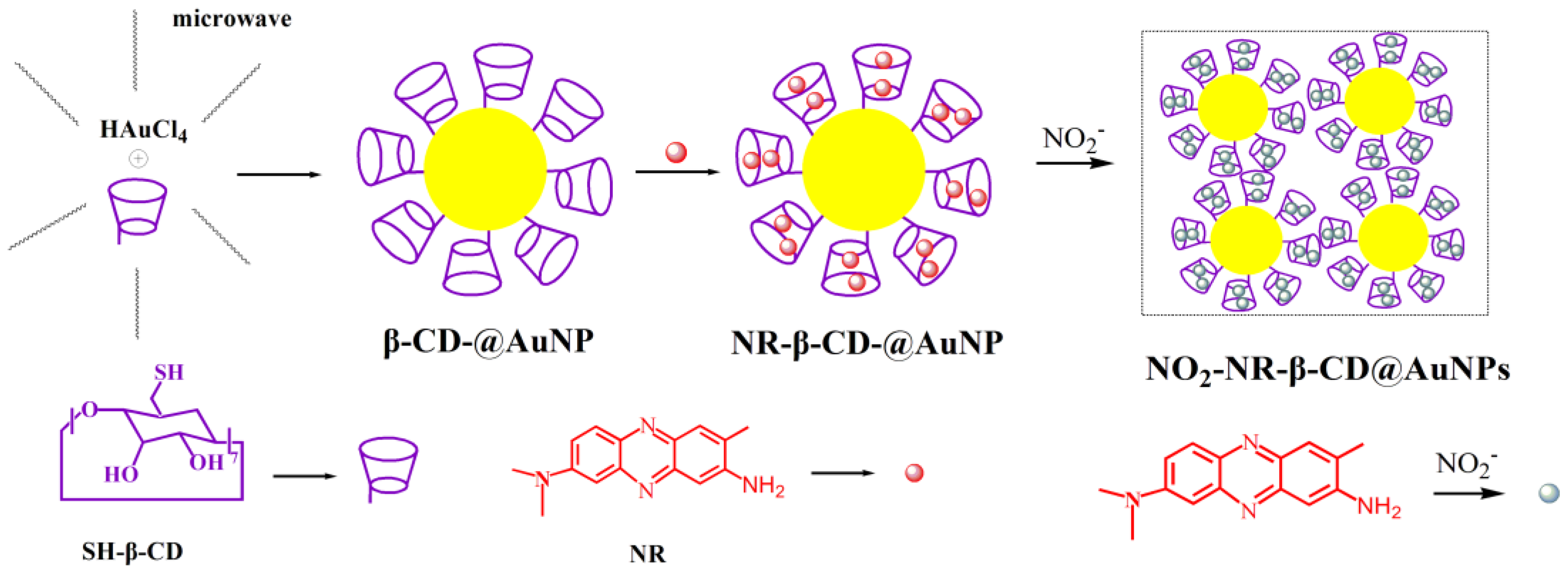
2.3. Preparation of the SH-β-CD Functionalized AuNPs (β-CD@AuNPs)
2.4. Preparation of Fluorescence Dye-Incorporated SH-β-CD Functionalized Gold Nanoparticles (NR-β-CD@AuNPs)
2.5. Detection of Nitrite Ions
2.6 Detection of Nitrite Ions in Real Samples
3. Results
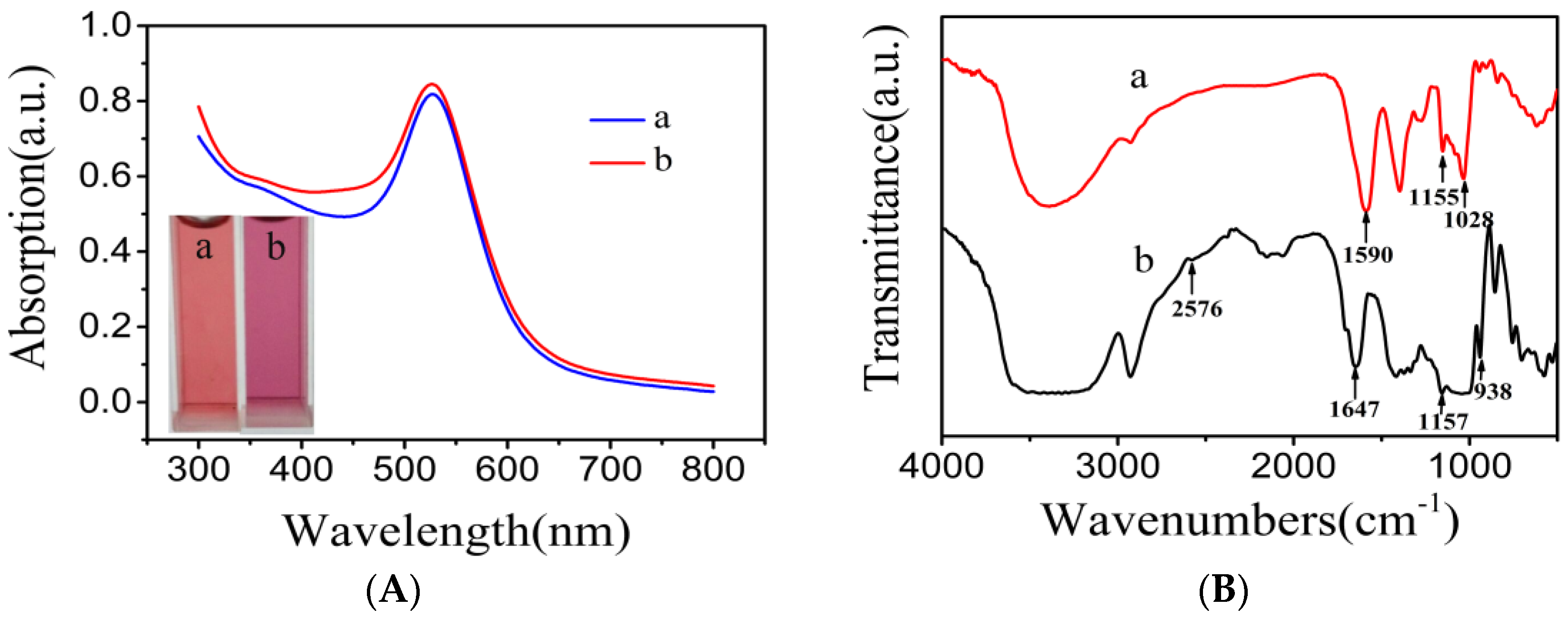
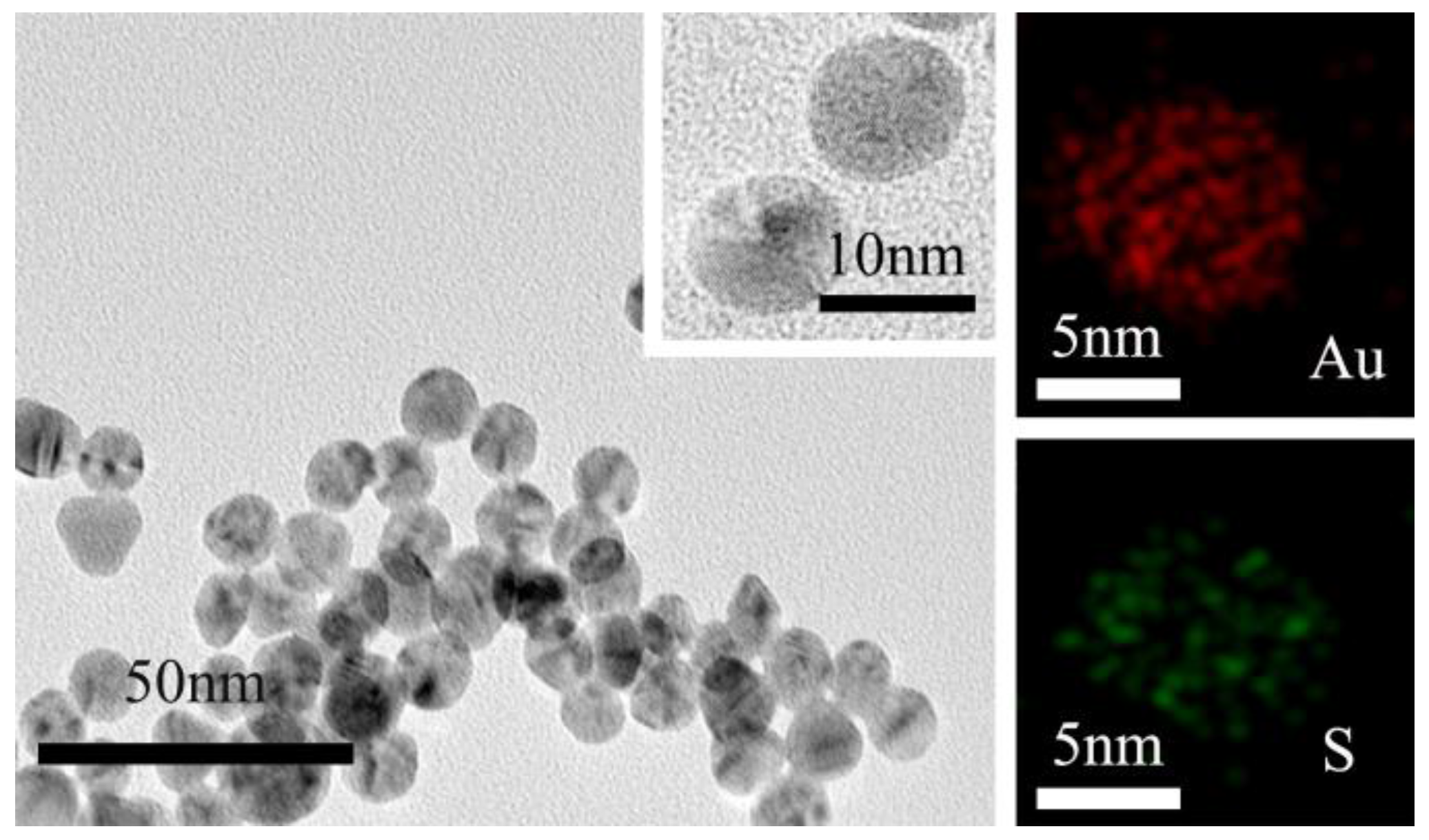
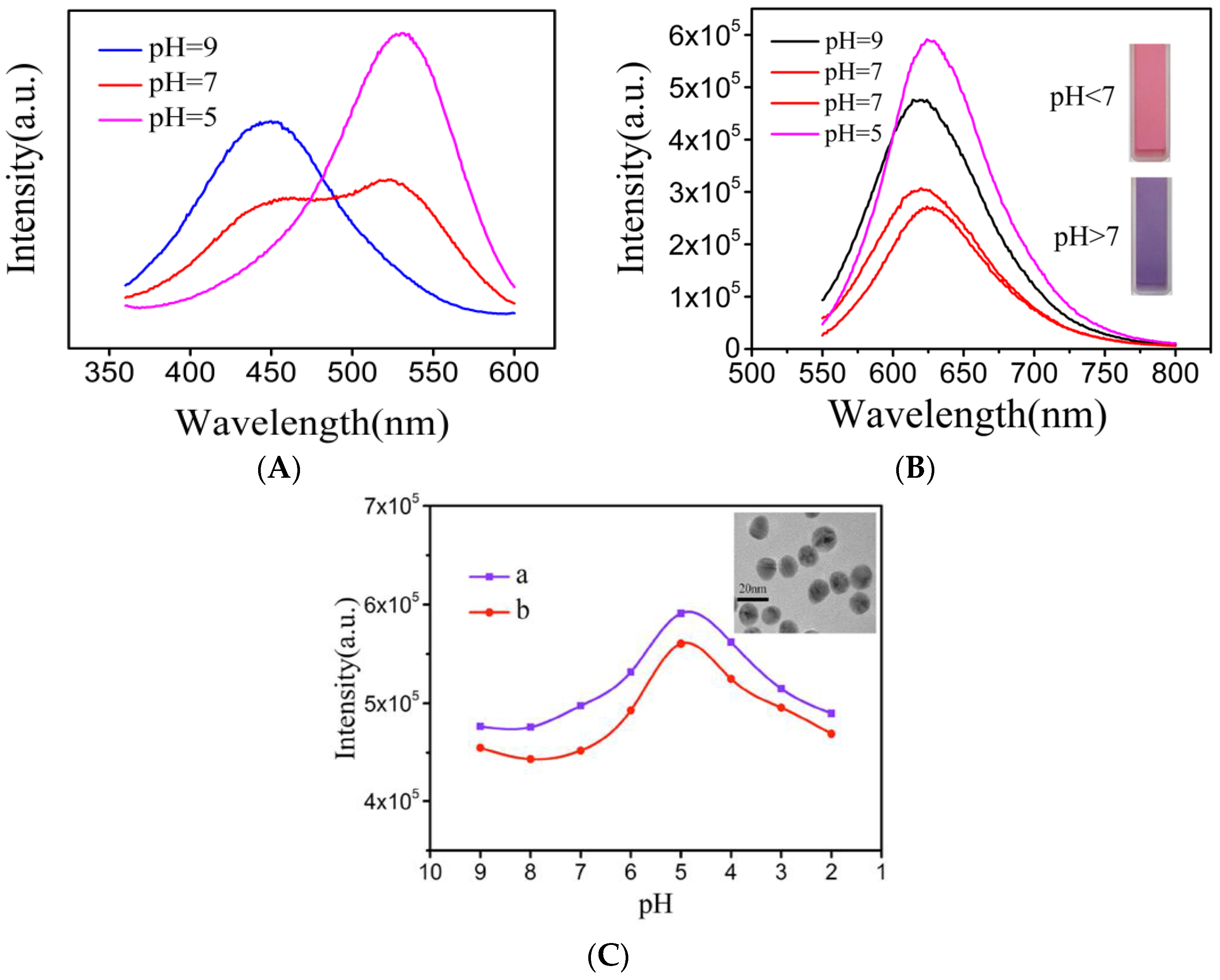
3.1. Characterization of the β-CD@AuNPs
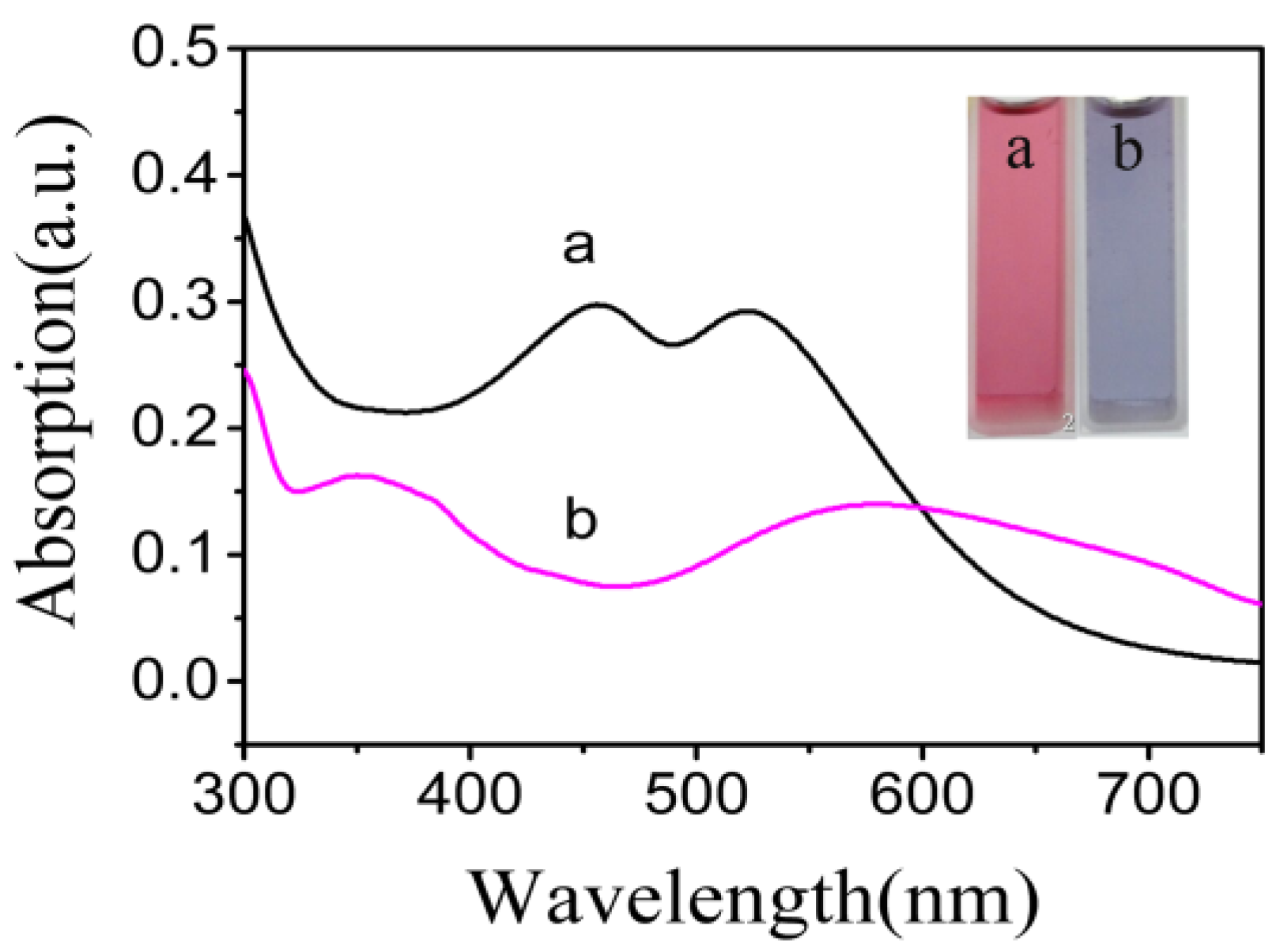
3.2. Characterization of the NR-β-CD@AuNPs
3.3. Effect of pH on the Fluorescence Property of the NR-β-CD@AuNPs
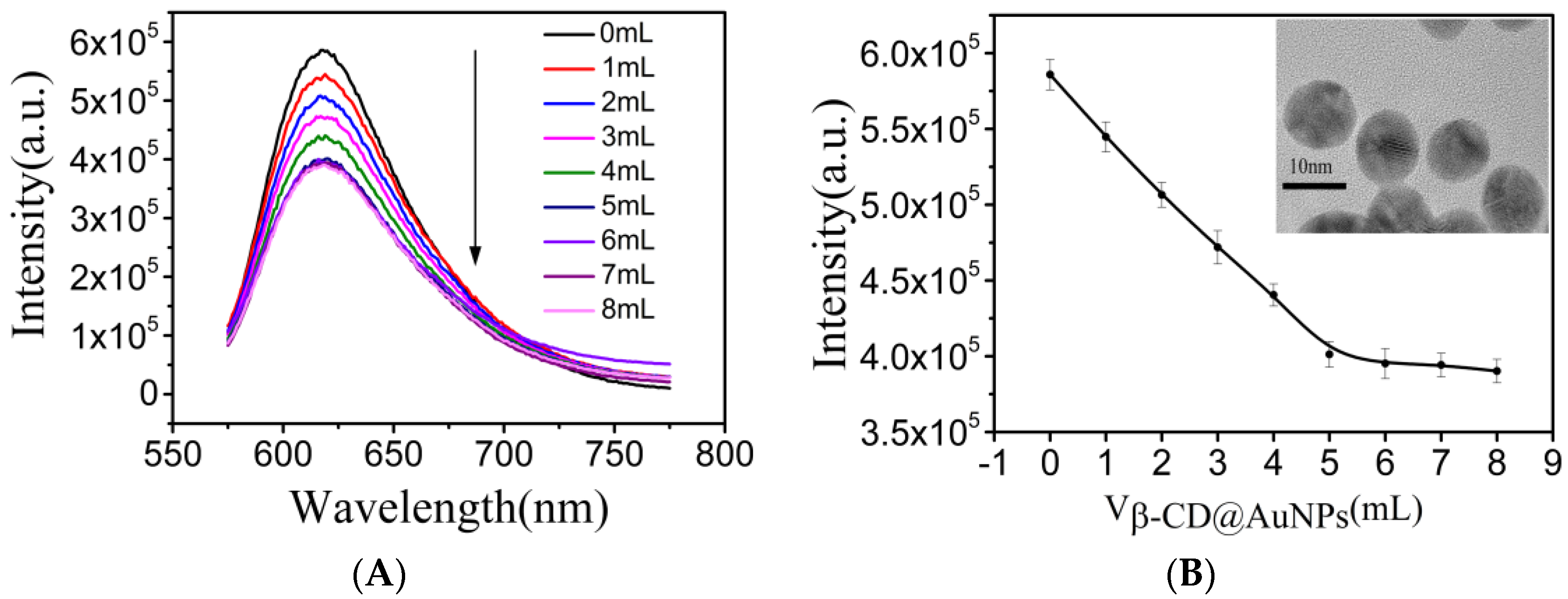
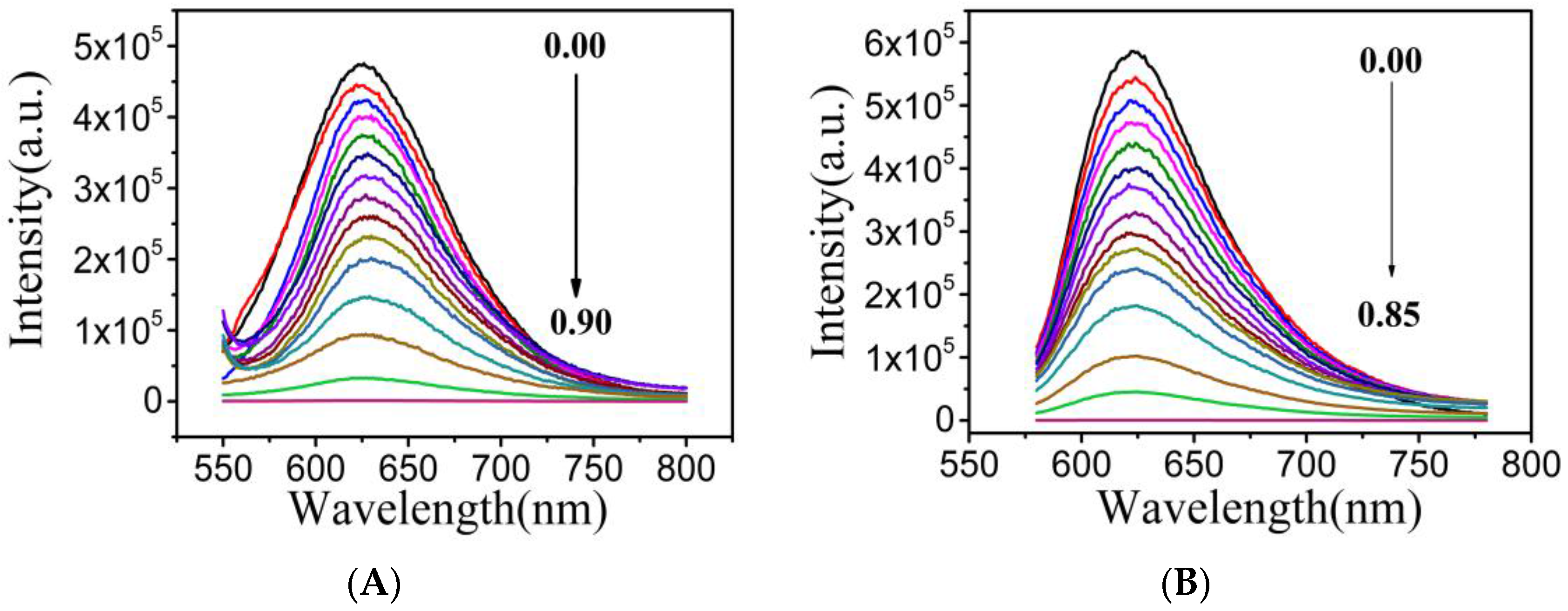
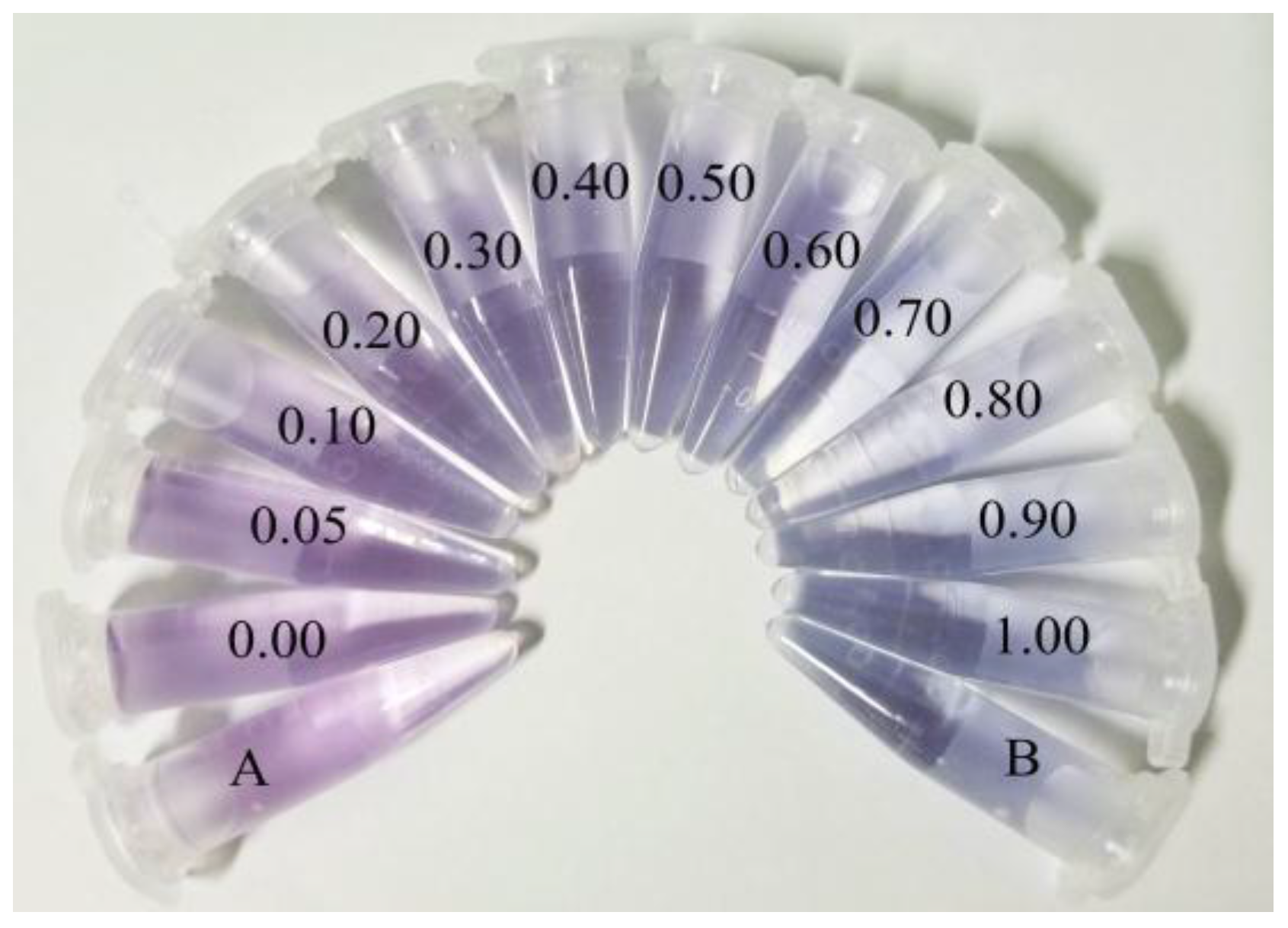
3.4. The Detection of NO2− in an Aqueous Solution
3.5. Selectivity
3.6. Application of NO2− Detection in Real Samples
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Daniel, M.C.; Astruc, D. Gold nanoparticles: assembly, supramolecular chemistry, quantum−size−related properties, and applications toward biology, catalysis, and nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B. 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Rana, S.; Bajaj, A.; Mout, R.; Rotello, V.M. Monolayer coated gold nanoparticles for delivery applications. Adv. Drug Delivery Rev. 2012, 64, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, M.; Pang, B.; Vara, M.; Xia, Y. Gold nanomaterials at work in biomedicine. Chem. Rev. 2015, 115, 10410–10488. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Algar, W.R.; Berti, L.; Gemmill, K.B.; Casey, B.J.; Oh, E.; Stewart, M.H.; Medintz, I.L. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem. Rev. 2013, 113, 1904–2074. [Google Scholar] [CrossRef] [PubMed]
- Ray, P.C.; Fan, Z.; Crouch, R.A.; Sinha, S.S.; Pramanik, A. Nanoscopic optical rulers beyond the FRET distance limit: fundamentals and applications. Chem. Soc. Rev. 2014, 43, 6370–6404. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.W.; Lian, J.Y.; Liu, Q.; Xu, C.L.; Li, B.X. β−Cyclodextrin−modified silver nanoparticles as colorimetric probes for the direct visual enantioselective recognition of aromatic α−amino acids. Anal. Methods 2016, 8, 5794–5800. [Google Scholar] [CrossRef]
- Liu, J.; Lu, Y. Preparation of aptamer−linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat. Protoc. 2006, 1, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Deeb, C.; Zhou, X.A.; Gerard, D.; Bouhelier, A.; Jain, P.K.; Plain, J.; Soppera, O.; Royer, P.; Bachelott, R. Off−resonant optical excitation of gold nanorods: nanoscale imprint of polarization surface charge distribution. J. Phys. Chem. Lett. 2011, 2, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Stender, A.S.; Wang, G.F.; Sun, W.; Fang, N. Influence of gold nanorod geometry on optical response. ACS Nano 2010, 4, 7667–7675. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.J.; Wang, E.K. Synthesis and electrochemical applications of gold nanoparticles. Anal. Chim. Acta 2007, 598, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Serafin, V.; Eguilaz, M.; Agui, L.; Yanez−Sedeno, P.; Pingarron, J.M. An electrochemical immunosensor for testosterone using gold nanoparticles–carbon nanotubes composite electrodes. Electroanalysis 2011, 23, 169–176. [Google Scholar] [CrossRef]
- Pingarron, J.M.; Yanez−Sedeno, P.; Gonzalez−Cortes, A. Gold nanoparticle−based electrochemical biosensors. Electrochim. Acta 2008, 53, 5848–5866. [Google Scholar] [CrossRef]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent dyes and their supramolecular host/guest complexes with macrocycles in aqueous solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.W. Towards biocompatible nanovalves based on mesoporous silica nanoparticles. Med. Chem. Commun. 2011, 2, 1033–1049. [Google Scholar] [CrossRef]
- Sun, Y.L.; Yang, B.J.; Zhang, X.A.; Yang, Y.W. Cucurbit [7] uril pseudorotaxane−based photo responsive supramolecular nanovalve. Chem. Eur. J. 2012, 18, 9212–9216. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, R.K.; Thennarasu, S.; Mandal, A.B. Resveratrol stabilized gold nanoparticles enable surface loading of doxorubicin and anticancer activity. Colloids Surf. B 2014, 114, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, S.; Sakthinathan, S.; Chen, S.M.; Thirumalraj, B.; Wua, T.H.; Loub, B.S.; Liuc, X.H. Preparation of β−cyclodextrin entrapped graphite composite for sensitive detection of dopamine. Carbohydr. Polym. 2016, 135, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Finder, T.; Bahshi, L.; Willner, I. β−cyclodextrin−modified CdSe/ZnS quantum dots for sensing and chiroselective analysis. Nano Lett. 2009, 9, 2073–2076. [Google Scholar] [CrossRef] [PubMed]
- Wayu, M.B.; Schwarzmann, M.A.; Gillespie, S.D.; Leopold, M.C. Enzyme−free uric acid electrochemical sensors using β−cyclodextrin−modified carboxylic acid−functionalized carbon nanotubes. J. Mater. Sci. 2017, 52, 6050–6062. [Google Scholar] [CrossRef]
- Du, D.; Wang, M.H.; Cai, J.; Zhang, A.D. Sensitive acetylcholinesterase biosensor based on assembly of β−cyclodextrins onto multiwall carbon nanotubes for detection of organophosphates pesticide. Sens. Actuators B Chem. 2010, 146, 337–341. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Eskandari, M.; Hosseini, S.H.; Nazari, M. Synthesis of water dispersible reduced graphene oxide via supramolecularcomplexation with modified β−cyclodextrin. Int. J. Polym. Mater. Polym. Biomater. 2017, 66, 235–242. [Google Scholar] [CrossRef]
- Abbaspour, A.; Noori, A. A cyclodextrin host–guest recognition approach to an electrochemical sensor for simultaneous quantification of serotonin and dopamine. Biosens. Bioelectron. 2011, 26, 4674–4680. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, Y. Construction and function of cyclodextrin−based 1D supramolecular strands and their secondary assemblies. Adv. Mater. 2015, 27, 5403–5409. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Q.; Zhao, Y.M.; Lu, D.T.; Wu, H.J.; Fan, M.; Wei, Y.L.; Shuang, S.M.; Dong, C. β−Cyclodextrin functionalized gold nanoparticles: Characterization and its analytical application for L−tyrosine. J. Inclusion Phenom. Macrocyclic Chem. 2014, 78, 275–286. [Google Scholar] [CrossRef]
- Luo, C.H.; Zheng, Z.H.; Ding, X.B.; Peng, Y.X. Supramolecular assembly of β−cyclodextrin−capped gold nanoparticles on ferrocene−functionalized ITO surface for enhanced voltammetric analysis of ascorbic acid. Electroanalysis 2008, 20, 894–899. [Google Scholar] [CrossRef]
- Kodamatania, H.; Yamazakib, S.; Saitoc, K.; Tomiyasua, T.; Komatsud, Y. Selective determination method for measurement of nitrite and nitrate in water samples using high−performance liquid chromatography with post−column photochemical reaction and chemiluminescence detection. J. Chromatogr. A 2009, 1216, 3163–3167. [Google Scholar] [CrossRef] [PubMed]
- Beamonte, E.; Bermudez, J.D.; Casino, A. A statistical study of the quality of surface water intended for human consumption near Valencia (Spain). J. Environ. Manag. 2007, 83, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Takayama, Y.; Makabe, N.; Mitsui, R.; Hirokawa, T. Ion chromatography for determination of nitrite and nitrate in seawater using monolithic columns. J. Choromatogr. A 2005, 1083, 63–67. [Google Scholar] [CrossRef]
- Zuo, Y.G.; Wang, C.J.; Van, T. Simultaneous determination of nitrite and nitrate in dew, rain, snow and lake water samples by ion−pair high−performance liquid chromatography. Talanta 2006, 70, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qi, S.D.; Dong, Y.L. A sensitive colorimetric method for the determination of nitrite in water supplies, meat and dairy products using ionic liquid−modified methyl red as a colour reagent. Food Chem. 2014, 151, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.; Ercan, Ö.; Taşcıoğlu, S. A novel method for the spectrophotometric determination of nitrite in water. Talanta 2005, 66, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Li, B.; Zhang, L.M.; Zhang, L.G.; Zhao, H.F. Fabrication and characterization of a fluorescent sensor based on Rh 6G−functionlized silica nanoparticles for nitrite ion detection. Sens. Actuators B Chem. 2012, 171–172, 946–953. [Google Scholar] [CrossRef]
- Ojani, R.; Raoof, J.B.; Zarei, E. Electrocatalytic reduction of nitrite using ferricyanide; Application for its simple and selective determination. Electrochim. Acta 2006, 52, 753–759. [Google Scholar] [CrossRef]
- Paixão, T.R.L.C.; Cardoso, J.L.; Bertotti, M. Determination of nitrate in mineral water and sausage samples by using a renewable in situ copper modified electrode. Talanta 2007, 71, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Lin, J.M.; Huie, C.W.; Yamada, M. Chemiluminescence study of carbonate and peroxynitrous acid and its application to the direct determination of nitrite based on solid surface enhancement. Anal. Chim. Acta 2004, 510, 29–34. [Google Scholar] [CrossRef]
- Pelletier, M.M.; Kleinbongard, P.; Ringwood, L.; Hito, R.; Hunter, C.J.; Schechter, A.N.; Gladwin, M.T.; Dejam, A. The measurement of blood and plasma nitrite by chemiluminescence: Pitfalls and solutions. Free Radic. Biol. Med. 2006, 41, 541–548. [Google Scholar] [CrossRef] [PubMed]
- He, D.Y.; Zhang, Z.J.; Huang, Y.; Hu, Y.F. Chemiluminescence microflow injection analysis system on a chip for the determination of nitrite in food. Food Chem. 2007, 101, 667–672. [Google Scholar] [CrossRef]
- Mikuška, P.; Večeřa, Z. Chemiluminescent flow−injection analysis of nitrates in water using on−line ultraviolet photolysis. Anal. Chim. Acta 2002, 474, 99–105. [Google Scholar] [CrossRef]
- Mikuška, P.; Večeřa, Z. Simultaneous determination of nitrite and nitrate in water by chemiluminescent flow−injection analysis. Anal. Chim. Acta 2003, 495, 225–232. [Google Scholar] [CrossRef]
- Zhang, T.; Fan, H.L.; Jin, Q.H. Sensitive and selective detection of nitrite ion based on fluorescence super quenching of conjugated polyelectrolyte. Talanta 2010, 81, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.; Pal, H.; Koti, A.S.R.; Sapre, A.V. Photophysical properties and rotational relaxation dynamics of neutral red bound to β−cyclodextrin. J. Phys. Chem. A 2004, 108, 1465–1474. [Google Scholar] [CrossRef]
- Mohanty, J.; Bhasikuttan, A.C.; Nau, W.M.; Pal, H. Host−guest complexation of neutral red with macrocyclic host molecules: Contrasting pKa shifts and binding affinities for cucurbit [7] uril and β−cyclodextrin. J. Phys. Chem. B 2006, 110, 5132–5138. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, D.X.; Sun, Y.L.; Zheng, Y.B.; Tan, L.L.; Weiss, P.S.; Wang, Y.Y. Viologen−mediated assembly of and sensing with carboxylatopillar [5] arene−modified gold nanoparticles. J. Am. Chem. Soc. 2013, 135, 1570–1576. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Meng, F.; Qi, L. Facile synthesis and one−dimensional assembly of cyclodextrin−capped gold nanoparticles and their applications in catalysis and surface−enhanced Raman scattering. J. Phys. Chem. C 2009, 113, 13636–13642. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.C.; Zhu, H.; Zhu, Q.Q.; Xia, Y.S. Three−in−One: Sensing, Self−Assembly, and Cascade Catalysis of Cyclodextrin Modified Gold Nanoparticles. J. Am. Chem. Soc. 2016, 138, 16645–16654. [Google Scholar] [CrossRef] [PubMed]
- Eftink, M.R.; Ghiron, C.A. Anal Biochem, Fluorescence quenching studies with proteins. Anal. Biochem. 1981, 114, 199–227. [Google Scholar] [CrossRef]
- Liu, Y.L.; Kang, N.; Ke, X.B.; Wang, D.; Ren, L.; Wang, H.J. A fluorescent nanoprobe based on metal−enhanced fluorescence combined with Förster resonance energy transfer for the trace detection of nitrite ions. RSC Adv. 2016, 6, 27395–27403. [Google Scholar] [CrossRef]
- Adarsh, N.; Shanmugasundaram, M.; Ramaiah, D. Efficient reaction based colorimetric probe for sensitive detection, quantification, and on−site analysis of nitrite ions in natural water resources. Anal. Chem. 2013, 85, 10008–10012. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Pang, S.; He, L.L.; Nugen, S.R. Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface−enhanced Raman spectroscopy. Biosens. Bioelectron. 2016, 85, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Li, Y.X.; Chen, Y.L.; Wang, L. Electrochemical determination of nitrite and iodate by use of gold nanoparticles/poly(3−methylthiophene) composites coated glassy carbon electrode. Sens. Actuators B Chem. 2008, 134, 780–786. [Google Scholar] [CrossRef]

| Samples | Content (NO2−, μg·mL−1) | Added (NO2−, μg·mL−1) | Found (NO2−, μg·mL−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 0.23±0.01 | 0.10 | 0.33±0.01 | 101.00±1.5 | 1.20±0.5 |
| 2 | 0.32±0.02 | 0.10 | 0.42±0.01 | 99.80±1.2 | 2.10±0.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Zhang, X.; Jiang, C.; Zhang, W.; Yang, L. The Trace Detection of Nitrite Ions Using Neutral Red Functionalized SH-β-Cyclodextrin @Au Nanoparticles. Sensors 2018, 18, 681. https://doi.org/10.3390/s18030681
Du X, Zhang X, Jiang C, Zhang W, Yang L. The Trace Detection of Nitrite Ions Using Neutral Red Functionalized SH-β-Cyclodextrin @Au Nanoparticles. Sensors. 2018; 18(3):681. https://doi.org/10.3390/s18030681
Chicago/Turabian StyleDu, Xiaoyang, Xiaoxia Zhang, Chunlai Jiang, Weilu Zhang, and Lizhu Yang. 2018. "The Trace Detection of Nitrite Ions Using Neutral Red Functionalized SH-β-Cyclodextrin @Au Nanoparticles" Sensors 18, no. 3: 681. https://doi.org/10.3390/s18030681




