1. Introduction
Bacterial sepsis can be described as a condition in which patients experience systemic inflammatory response syndrome associated with infection and one of the leading causes of death in Malaysia [
1]. Currently available routine screening for bacterial sepsis include the white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), although these have poor sensitivity and specificity [
2]. There is a need to distinguish biomarkers that are specific and sensitive for bacterial sepsis detection. Pierrakos and Vincent [
3] reported that the Secretory Group IIA Phospholipase A2 (sPLA2-IIA) have high sensitivity and specificity in determining sepsis (~95%). This biomarker was also tested positive in humans for the diagnosis of septicemia [
4,
5,
6].
sPLA2-IIA is an acute phase protein and participates in the host response to inflammation and generates inflammatory arachidonic acid metabolites [
7]. The levels of sPLA2-IIA is effective in measuring systemic of inflammation in various bacteremic and non-bacteremic infections [
8,
9,
10] and in differentiating between bacterial and viral infections [
11]. Interestingly, the level of sPLA2-IIA correlate well with the severity of septic shock and its outcome [
12,
13]. High or markedly increase levels of sPLA2-IIA have also been shown to associate with adverse outcome in sepsis [
14,
15]. Rintala et al. [
11] have proved that sPLA2-IIA reached maximal levels even at the beginning of the follow-up and suggested the cut-off value of 150 mg/L for sPLA2-IIA to identifying bacteremia within the first 24 h.
Current diagnosis of bacterial sepsis involves cumbersome and time-consuming methods of bacterial cultures or ELISA (enzyme-linked immunosorbent assay) in which antibodies produced towards the pathogen are measured. Thus, a rapid and simple technology to aid bacteria sepsis diagnosis will be useful. Enzyme based electrochemical biosensors have a potential to fulfill the requirements, through the development of portable instruments which is specific, fast and cost effective with sufficient sensitivity and selectivity to diagnose sepsis directly.
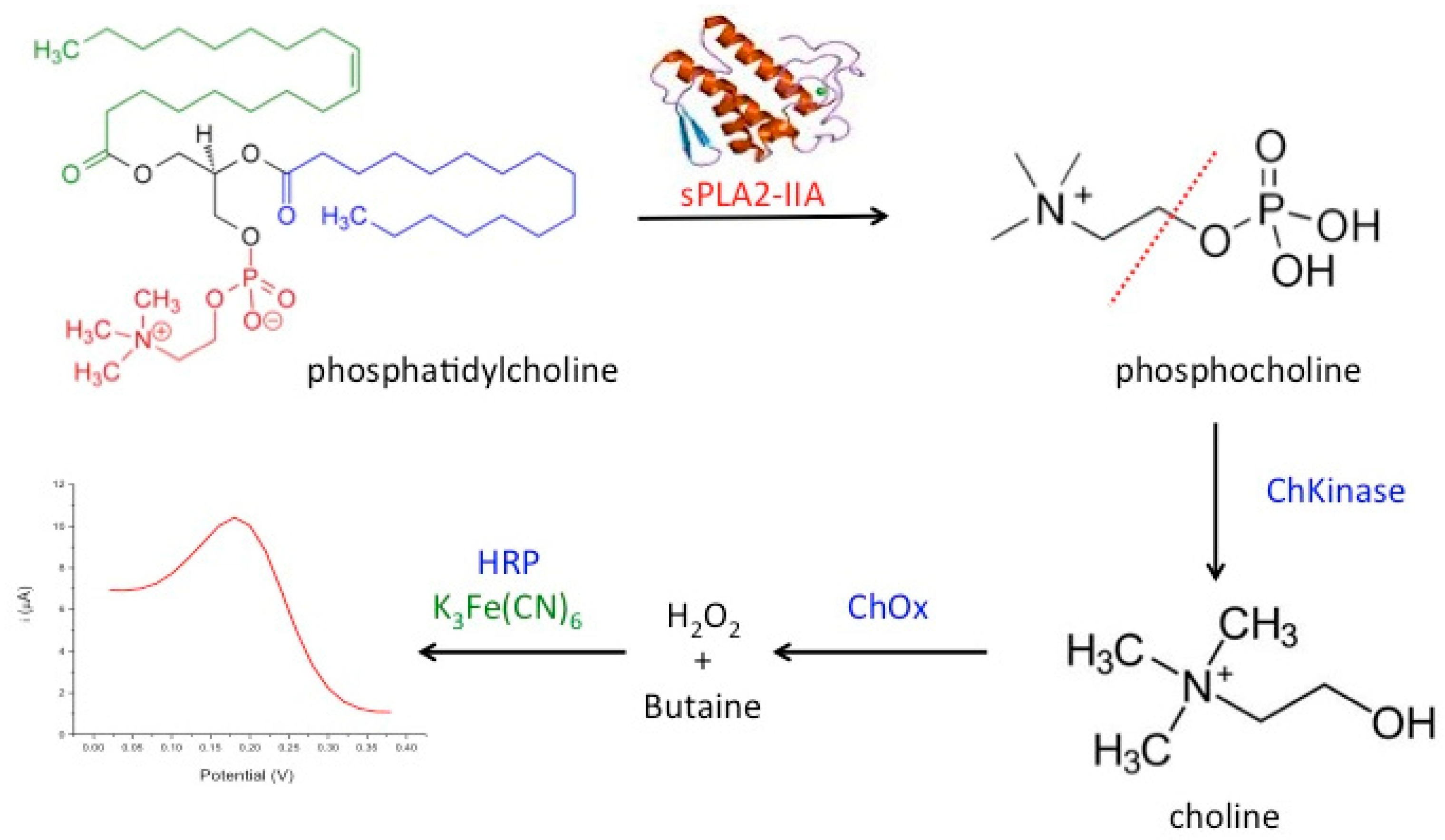
In the present work, we developed a novel amperometric biosensor for direct determination of the bacterial sepsis biomarker, sPLA2-IIA based on tri-enzyme system consisted of choline kinase (ChKinase), choline oxidase (ChOx) and horseradish peroxidase (HRP), which were covalently immobilized onto modified acrylic microspheres surface. The production of the enzyme sPLA2-IIA during sepsis conditions can be reacted with the substrate, phosphatidylcholine (PC) to form phosphocholine. Choline kinase (also known as choline phosphokinase) is an enzyme which catalyses the conversion of phosphocholine to choline. This is then acted upon by the second enzyme choline oxidase (ChOx) to produce hydrogen peroxide. Further, the third enzyme horseradish peroxidase (HRP) was used to react with hydrogen peroxide and this reaction is indicated by the redox mediator, potassium ferricyanide K
3Fe(CN)
6 operating at lower potential. The tri-enzyme system for sPLA2-IIA determination is summarized in
Figure 1.
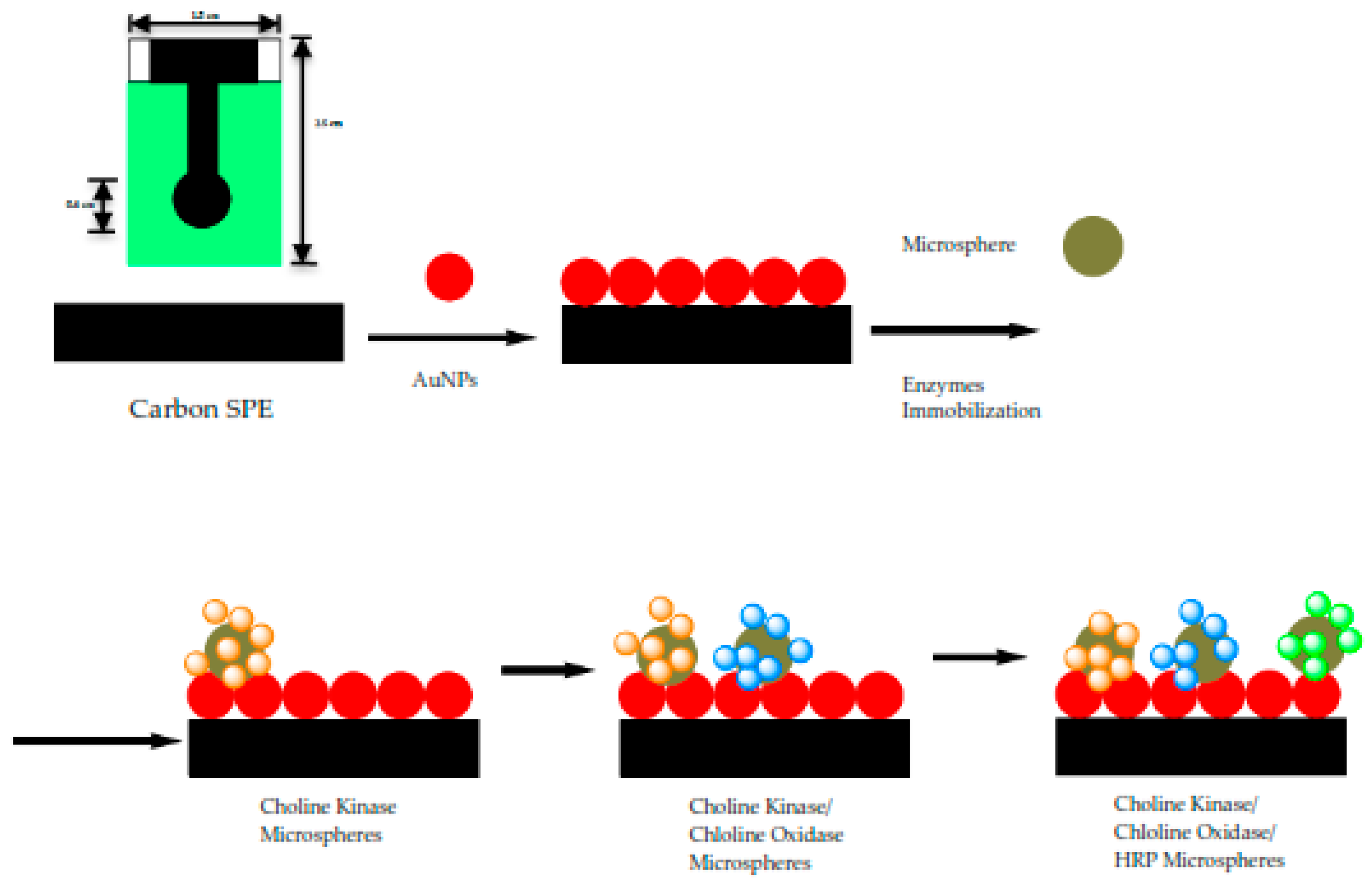
To fabricate an electrochemical biosensor, these enzymes were immobilized on acrylic microspheres functionalized with succinimide groups (NBA-NAS). The quantities of immobilized enzyme were optimized before immobilization onto the microspheres, independently. Thus, the amount of enzyme needed for an optimum detection system can be controlled by mixing the right weight of enzyme-conjugated microspheres before they were coated onto the electrode. Enzyme loaded microspheres were subsequently mixed with gold nanoparticles (AuNPs) to form a composite that was coated onto a carbon paste screen-printed electrode (SPE). The role of AuNPs is to improve the electrode conductivity as acrylic microspheres do not possess electrical conductivity. The use of acrylic microspheres modified with
N-acryloxysuccinimide (NAS) for enzyme immobilization had been reported for amperometric capsaicin biosensor [
16], potentiometric formaldehyde biosensor [
17], and optical urea biosensor [
18]. Enzymes deposited onto microspheres through covalent bonding have benefited from leaching and instability issue, hence enhance the overall performance.
2. Materials and Methods
2.1. Reagents and Solutions
All chemicals from commercial sources were of analytical grade and used as obtained. Choline oxidase from Alcaligenes sp., peroxidase from horseradish in lyophilized powder, approximately 150 U/mg and potassium ferricyanide, K3Fe(CN)6 were purchased from Sigma-Aldrich (Saint Louis, MO, USA). The source of sPLA2-IIA is honey bee venom (Apis mellifera), salt-free in lyophilized powder, 600–2400 U/mg protein and the substrate, L-a-phosphatidylcholine was obtained from egg yolk, type XVI-E (≥99%), 100 mg in lyophilized powder, ultrapure grade and kept in phosphate buffered saline (Sigma). Choline chloride 99%, Choline Kinase-a Inhibitor (CK37), 5 mg, was obtained from Merck. Potassium chloride (KCl), sodium hydroxide (NaOH), sodium acetate, sodium phosphate, n-butyl acrylate (NBA), 2-2-dimethoxy-2-phenylacetophenone (DMPP), 1,6-hexanediol diacrylate (HDDA), gold nanoparticles (AuNPs) and tris-hydroxymethyl aminomethane (Tris-HCl) were also supplied by Sigma-Aldrich). N-acryloxysuccinimide (NAS), sodium dodecyl sulfate (SDS) and ethanol 95% were from Systerm while hydrochloride acid 37% (HCl) from Riedel-de Haen. Phosphate buffer solution (PBS) was prepared using potassium dihydrogen phosphate and dipotassium hydrogen phosphate (Systerm) to achieve concentration of 0.1 M at pH 7. Preparation of standard solutions and cleaning laboratory glassware were performed using deionized water.
2.2. Apparatus and Electrode Deionized Water Was Used
Differential pulse voltammetry (DPV) experiments were operated using potentiostat, Autolab PGSTAT 12 (Metrohm, Herisau, Switzerland) at 0.02 mV step potential in the scanning range of −0.4–0.4 mV. SPE (Scrint Technology (M) Sdn. Bhd., Penang, Malaysia) modified with acrylic microspheres and gold nanoparticles (AuNPs) was used as working electrode. Meanwhile, a glassy carbon electrode and Ag/AgCl electrode were used as auxiliary and reference electrodes, independently. KCl solution of 3.0 M was utilized as the internal solution of the Ag/AgCl whereas all potential measured in this study were later referred to Ag/AgCl electrode. Homogeneous mixture of acrylic spheres was produced using sonicator bath, model Elma S30H and followed by centrifugation at 4000 rpm for 30 min (Centrifuge, HERMLE Z230A, 5500 rpm). These microspheres were washed three times using 5 × 10−2 M K-phosphate buffer (pH 7.0) followed by air-drying at ambient temperature. The morphology of poly(NBA) microspheres were analyzed using scanning electron microscope (SEM, LEO 1450VP, 20 kV acceleration voltage).
2.3. Immobilization Matrix—Acrylic Microspheres
NBA monomer (7 mL, SDS (0.01 g), HDDA (450 μL), DMPP (0.1 g), NAS (6 mg) and deionized water (15 mL) were mixed in a volumetric flask. At the end of sonication (10 min), the resulting mixtures turned milky white and was subsequently photocured for 600 s under continuous nitrogen gas purging in an ultraviolet exposure unit (R.S. Components Ltd., Northants, UK) of 15 Watt light intensity at 350 nm wavelength. Poly(NBA-NAS) microspheres were later collected by centrifugation (4000 rpm) for 30 min and washed repeatedly with potassium phosphate buffer solution of 5 × 10−2 M (pH 7) and dried at room temperature. The clean microspheres were stored at 4 °C when not in use.
2.4. Preparation of Modified SPEs
Screen-printed electrodes (SPEs) were cut into strips of 2.0 cm × 0.6 cm. The SPEs were modified with gold nanoparticles (1 mg AuNPs was dissolved in 300 µL EtOH) and this was accomplished by drop coating them on the SPEs. The three enzymes i.e., Horseradish peroxidase (4 µL), choline oxidase (8 µL) and choline kinase (4 µL) (All the enzymes = 0.5 mg enzymes in 100 µL potassium phosphate buffer solution of 0.01 M at pH 7) were immobilized separately on acrylic microspheres (1 mg microsphere was mixed in 100 µL potassium buffer solution of 0.01 M at pH 7) for 24 h. After overnight immobilization, all the mixture of acrylic microsphere (ACMS)-enzymes were centrifuged at speed: 10,000 rpm; duration: 10 min; temperature: 4 °C and washed with PBS solution 0.01 M pH 7 for three times. Each enzyme-conjugated microsphere was suspended in buffer solution again before carefully pipetted and dropped onto the confined area of the SPEs. The solution was left to dry on the working electrode up to 2 h before proceeding with the analysis. The modified SPEs were stored at chill temperature of 4 °C. The fabrication procedure of the biosensor is as shown in
Figure 2.
2.5. Optimization of Bacterial Sepsis Biosensor
The response of bacterial sepsis biosensor was optimized to achieve the optimum working conditions for sepsis determination. The AuNps deposition was optimized from 0.03 to 0.1 mg, whilst acrylic microspheres (ACMS) were loaded between 0.02 and 0.2 mg onto the AuNPs-modified SPE surface. Effect of buffer type to the enzyme biosensor was investigated by using K-phosphate, tris-HCl, Na-phosphate and Na-acetate buffer. pH effect on the bacterial sepsis biosensor were carried out by varying the electrolyte pH from 5.0 to 9.0. Ratio of enzyme (HRP:Chox:ChKinase) was loaded between 0:0:0 and 2:1:1 mg on the ACMS-AuNPs-SPE electrode. For the optimization of the response time, the designed enzyme biosensors were dipped into a solution that contained the sPLA2-IIA enzyme at a fixed concentration from 0 to 30 min.
2.6. Selectivity Response
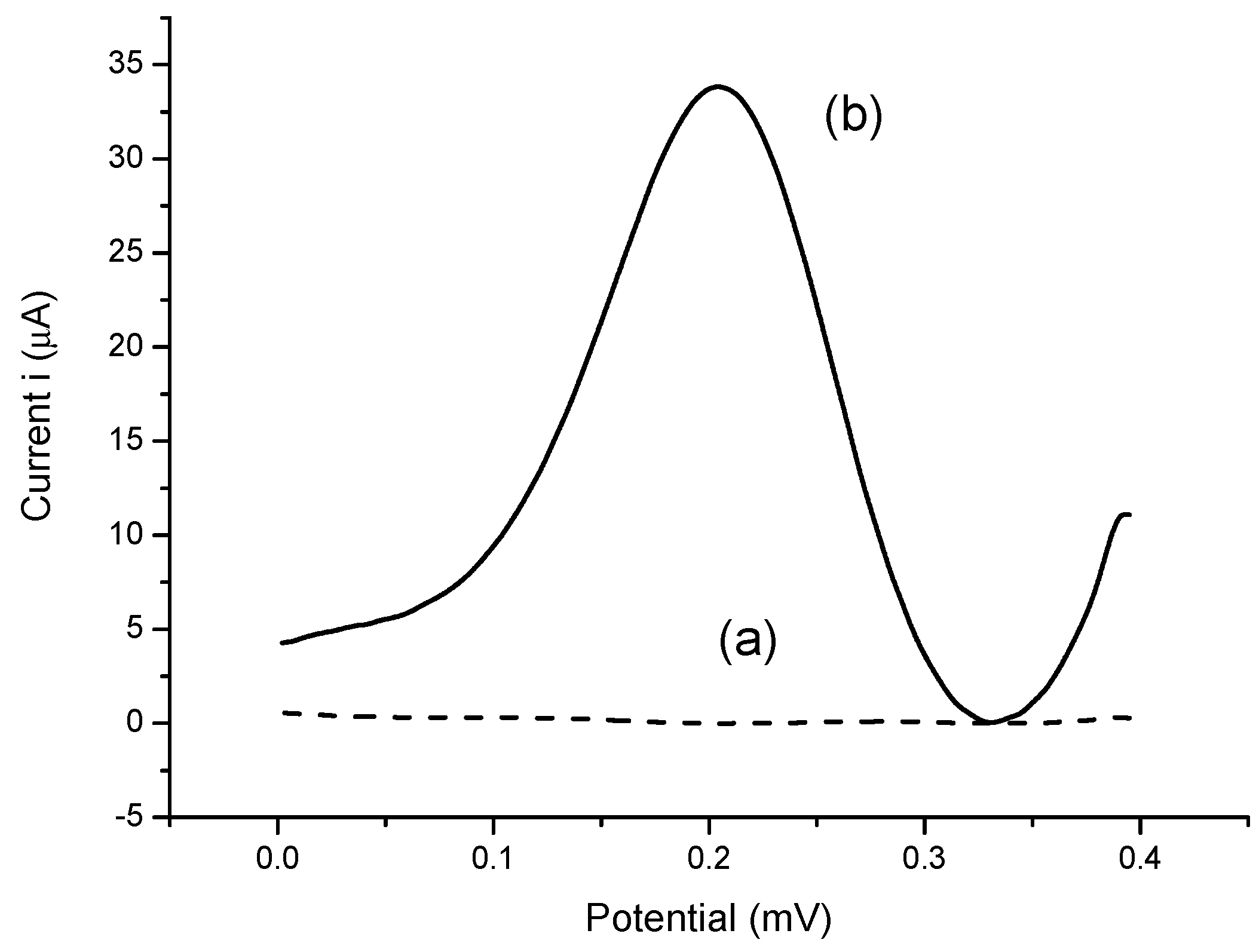
The selectivity response towards the biosensor was carried out with five metabolite compounds that can be found in human body fluids (e.g., serum, whole blood, etc.) at concentrations of 40 ng/mL and 80 ng/mL to match their concentrations in the diluted serum samples used for the tests later. The chosen metabolite compounds were urea, ascorbic acid, glucose, sucrose and citric acid. The selectivity study was carefully conducted via dipping the enzyme biosensor into the pure metabolite solutions alone and the DPV peak current response was recorded immediately. The biosensor responses obtained from these metabolite compounds were then compared to the response of the analyte sPLA2-IIA alone.
2.7. Amperometric Measurement
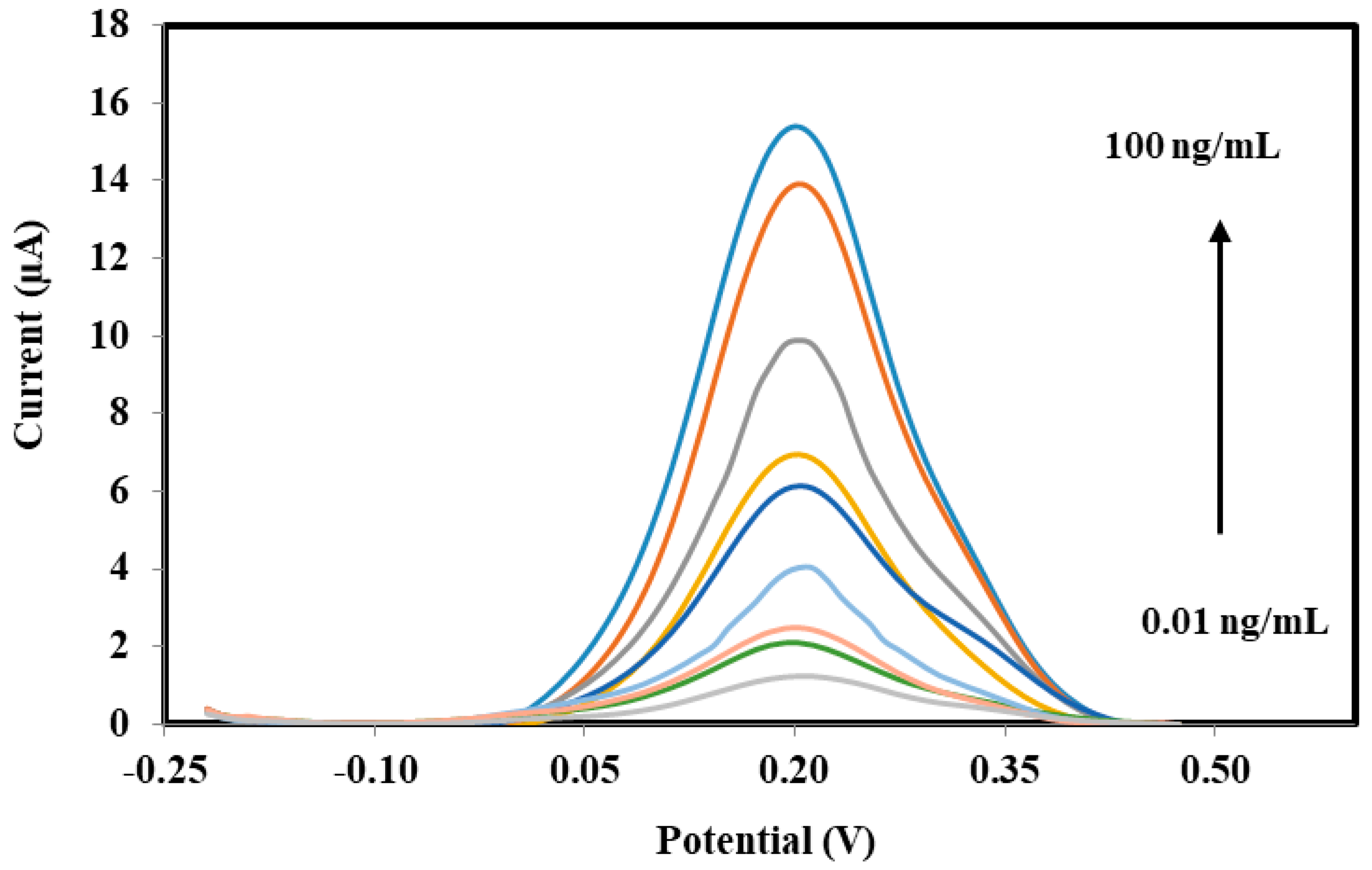
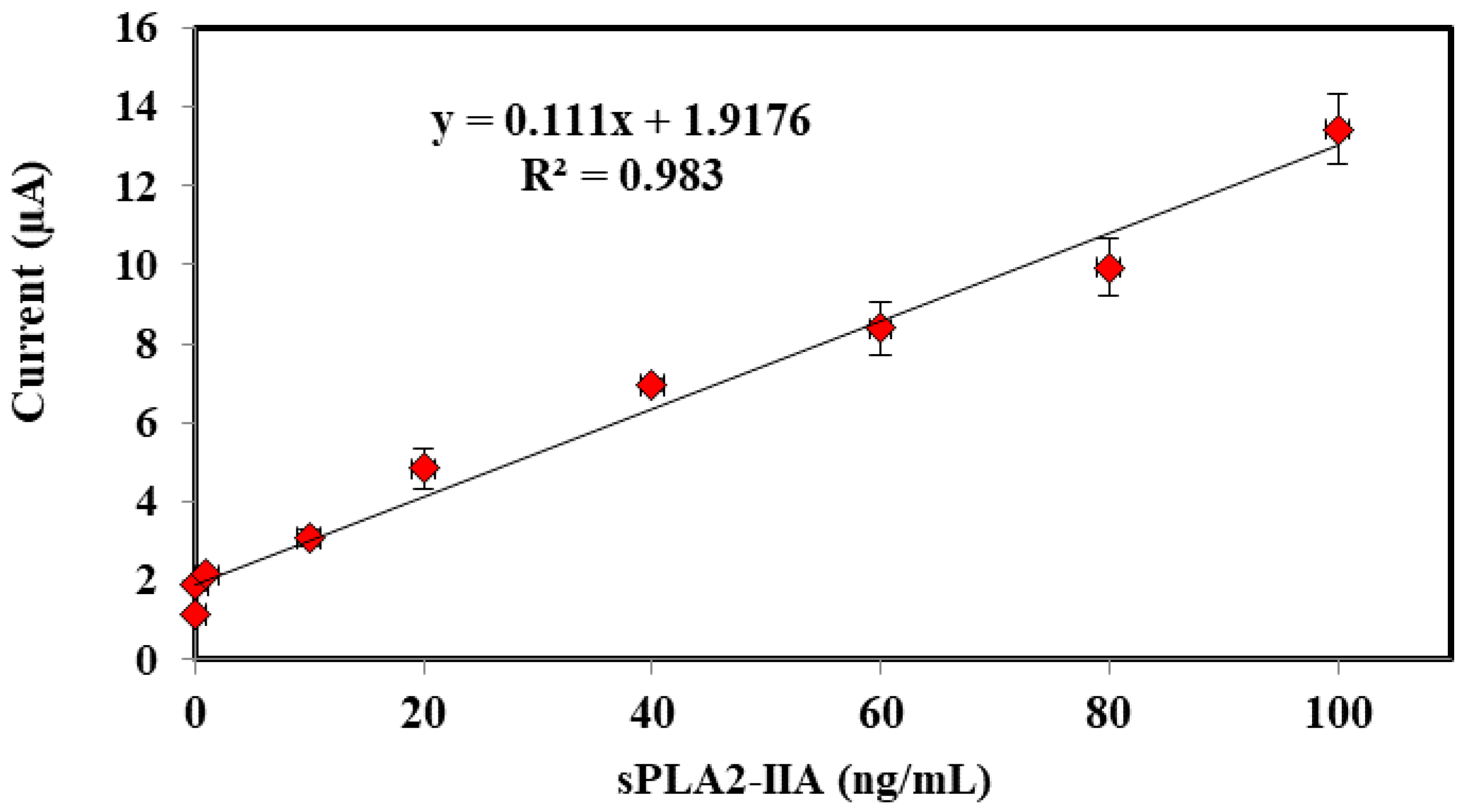
In order to detect sPLA2-IIA found in bacterial infection and sepsis, a potentiostat is used to record the electrochemical measurements through three electrode system developed consists of SPE as working electrode, a platinum electrode as counter electrode and a Ag/AgCl as reference electrode, respectively. Amperometric measurements are performed using the enzyme modified-SPEs as electrochemical transducer connected to a potentiostat (Metrohm Autolab B.V.) to generate electric current, which correlated to the enzymatic reaction progress and concentration of hydrogen peroxide, specifically. The response of the enzyme biosensor was obtained in a series of sPLA2-IIA enzyme solution from 0.01 ng/mL to 100 ng/mL where a linear calibration curve and the lowest detection limit were determined. The modified-SPEs were incubated for 10 min in 4 mL of solution containing PBS buffer, sPLA2-IIA enzyme and its substrate phosphatidylcholine. After incubation, 1 mL K3Fe(CN)6 (1 mM) was added and further incubated of another 5 min before the current response parallel to the enzymatic reaction was measured between the potential from −0.4 V to 0.4 V, where peak current was registered at 0.2 V.
2.8. Human Blood Serum Analysis
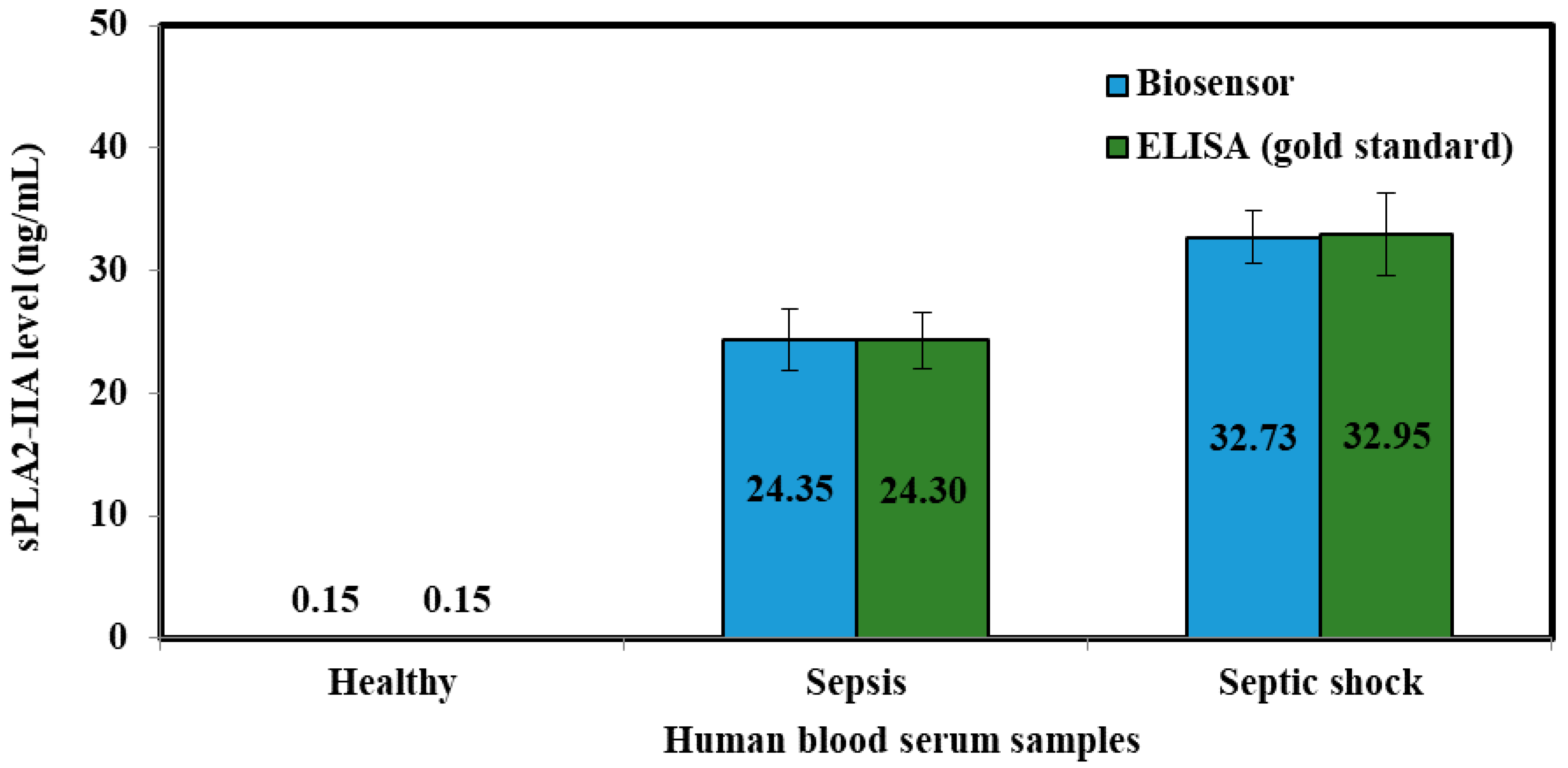
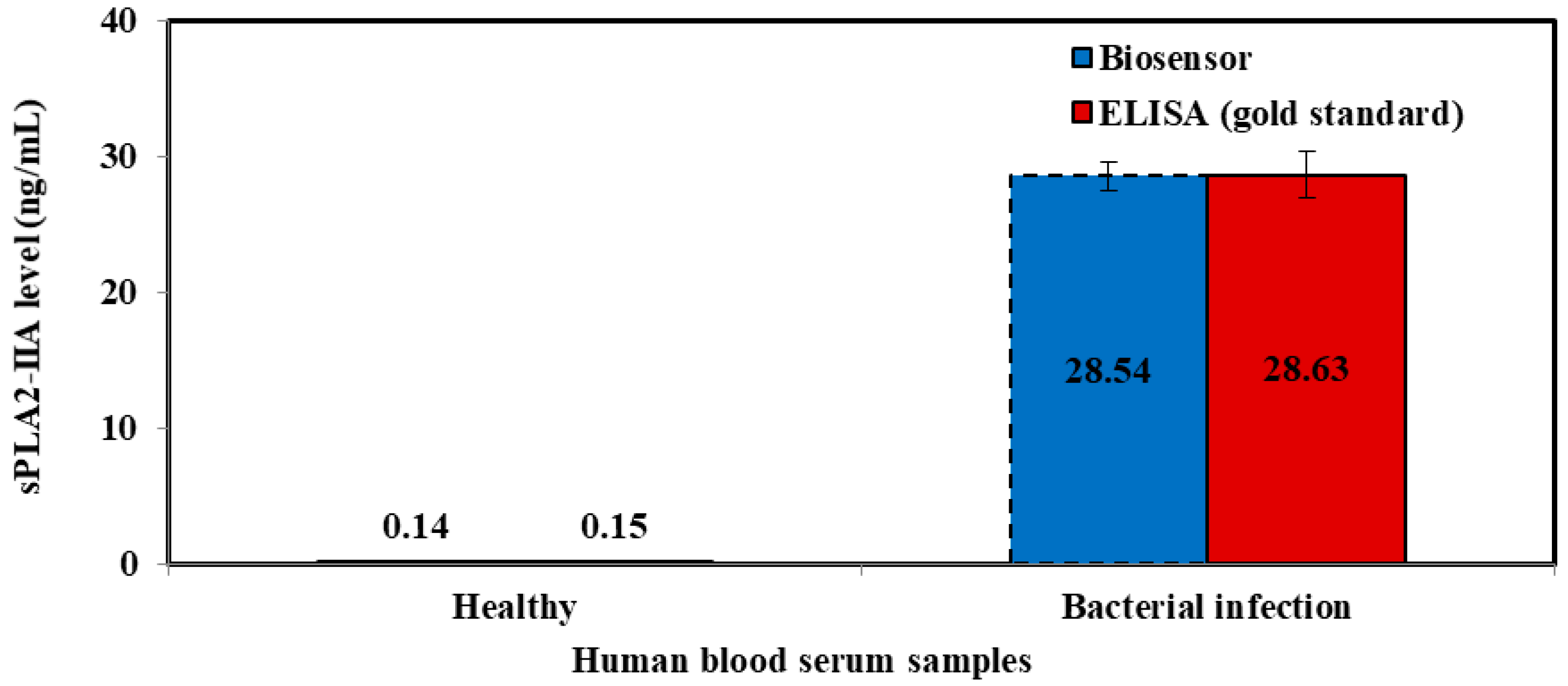
Human blood serum was analyzed to verify the accuracy and validity of sepsis biosensor based on developed amperometric method. These samples were collected using sterilized plastic bottles and kept frozen at all times. Serum collection was fully handled by Universiti Kebangsaan Malaysia Medical Centre (UKMMC). PBS buffer solution was added into serum samples to obtain 300 times dilution before measurement and kept the similar matrix conditions as KCl standard solutions. The samples were categorized into three categories, which were healthy samples, sepsis samples and septic shock samples. These diluted samples were analyzed according to the procedure mentioned in 2.7 using the biosensor.
The serum samples collected were evaluated using an ELISA test kit (Enzyme Immunometric Assay Kit; 585000, Cayman Chemical, Ann Arbor, MI, USA), concurrently. Each well of the plate has been coated with a monoclonal antibody specific for sPLA2, known as sPLA2 capture antibody which bind to any sPLA2-IIa introduced into the well. An Acetylcholinesterase:Fab’ Conjugate (AChE:Fab’) binds selectively to a different epitope on the sPLA2 molecule and form a ‘sandwich’ by linking on the opposite sides of the sPLA2 molecule.
These “sandwiches” were later deposited on the plate so the excess reagents would be washed away. Ellman’s Reagent was added to each well and the concentration of the analyte was determined by measuring the enzymatic activity of the AChE. The AChE:Fab’ was specific for sPLA2, and will not cross-react with other types of sPLAs, or other inflammatory mediators such as interleukin-1, platelet activating or tumor necrosis factor. The product of the AChE-catalyzed reaction has a distinct yellow color, which absorbs strongly at 412 nm. The intensity of this color was determined spectrophotometrically and proportional to the amount of bound conjugate, thus was also correspond to the concentration of sPLA2. The concentration of sPLA2 in the serum was tested as triplicates, indicated as pg/mL and determined against the standard curve of each ELISA assay. The ELISA kit has a minimum detectable concentration of 15.6 pg/mL.