Optical Fibre NO2 Sensor Based on Lutetium Bisphthalocyanine in a Mesoporous Silica Matrix
Abstract
:1. Introduction
2. Materials and Methods
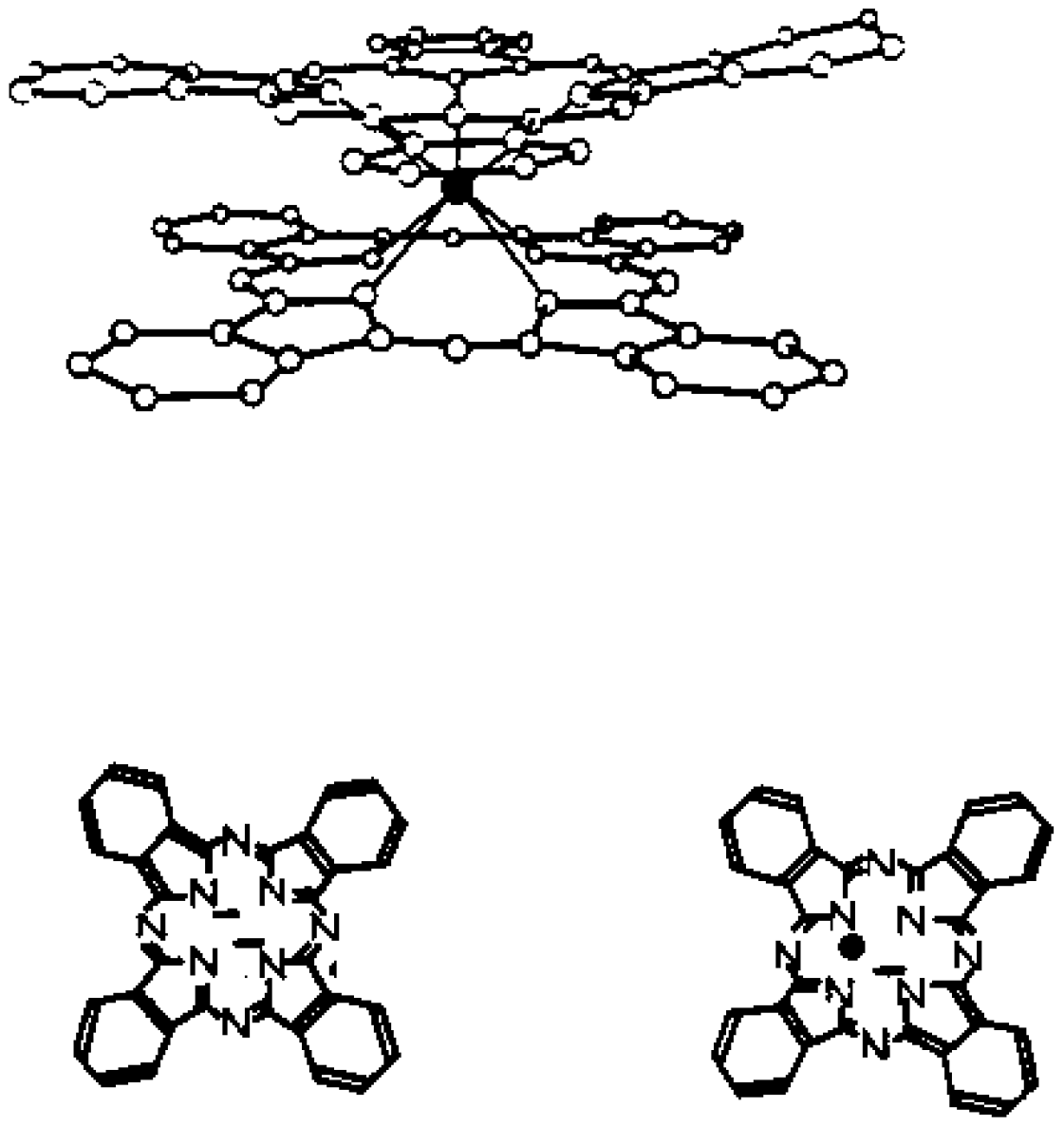
2.1. Sensitive Material
2.2. Mesoporous Matrix
2.3. Impregnation with LuPc2
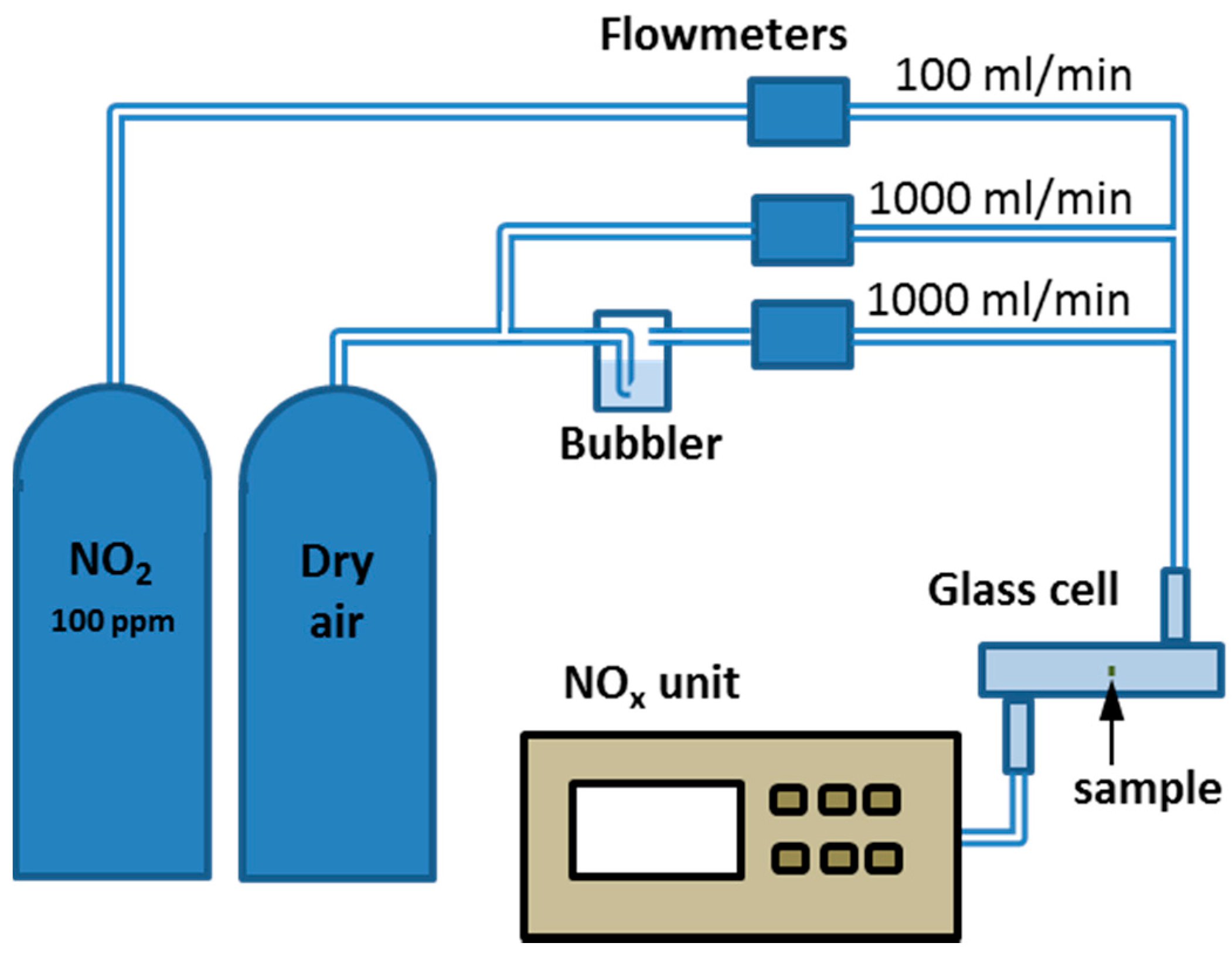
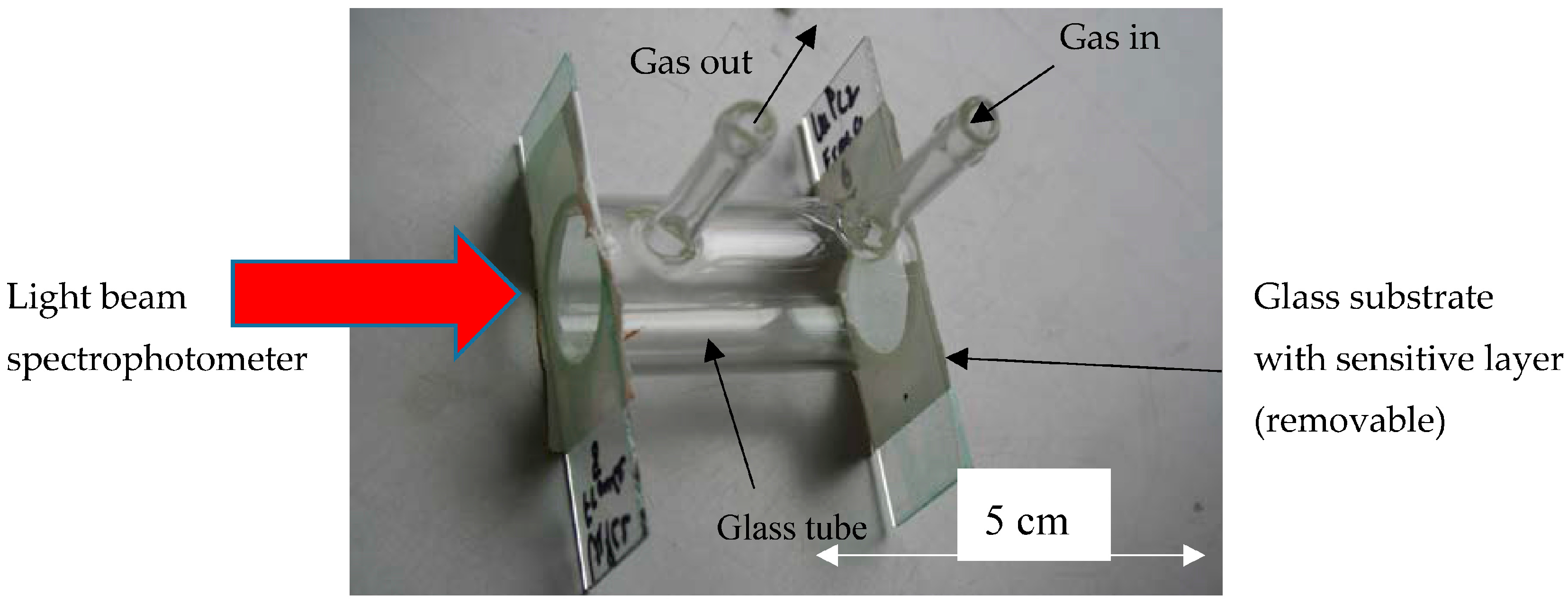
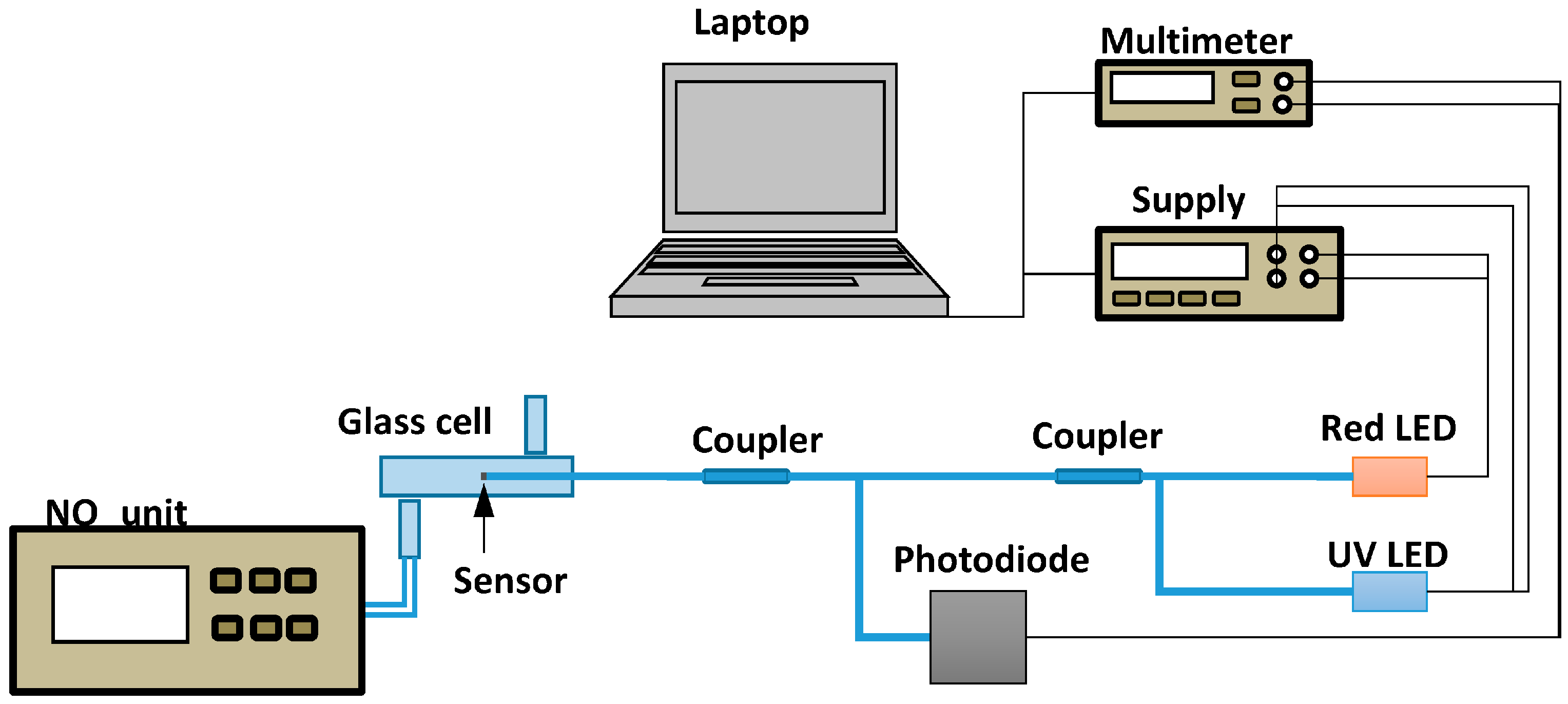
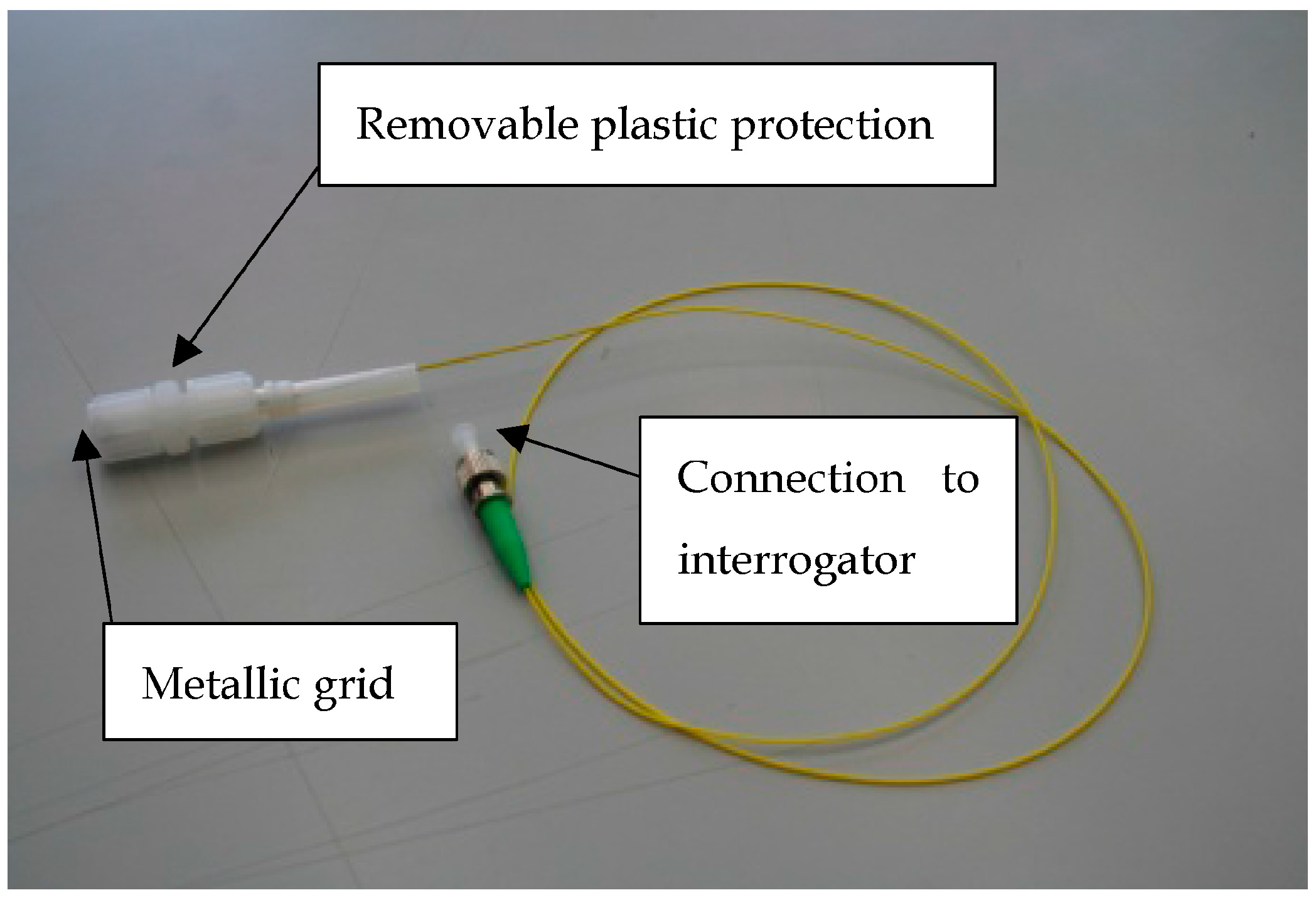
2.4. Measurement Setup for Gas Sensing Characterization
3. Results and Discussion
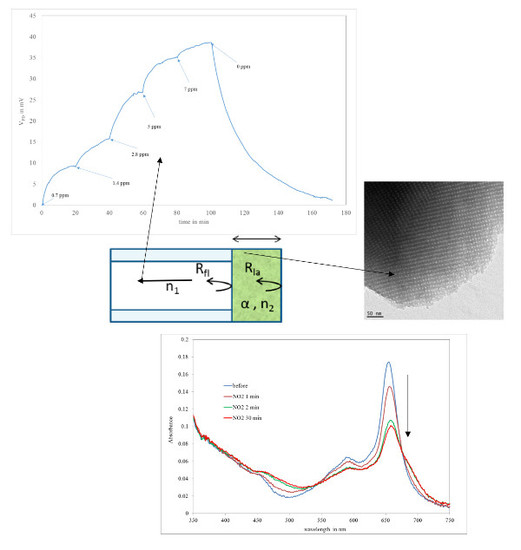
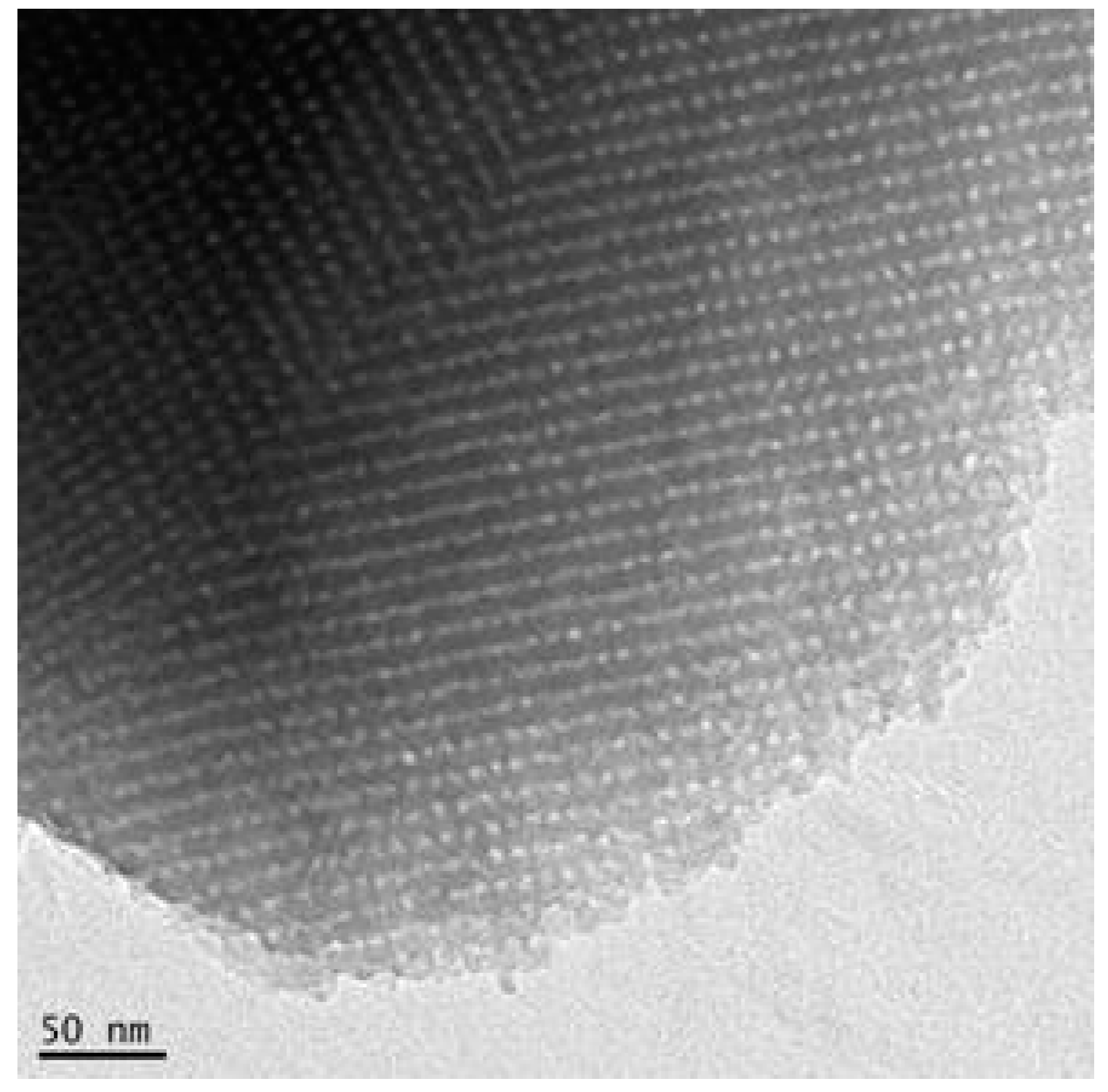
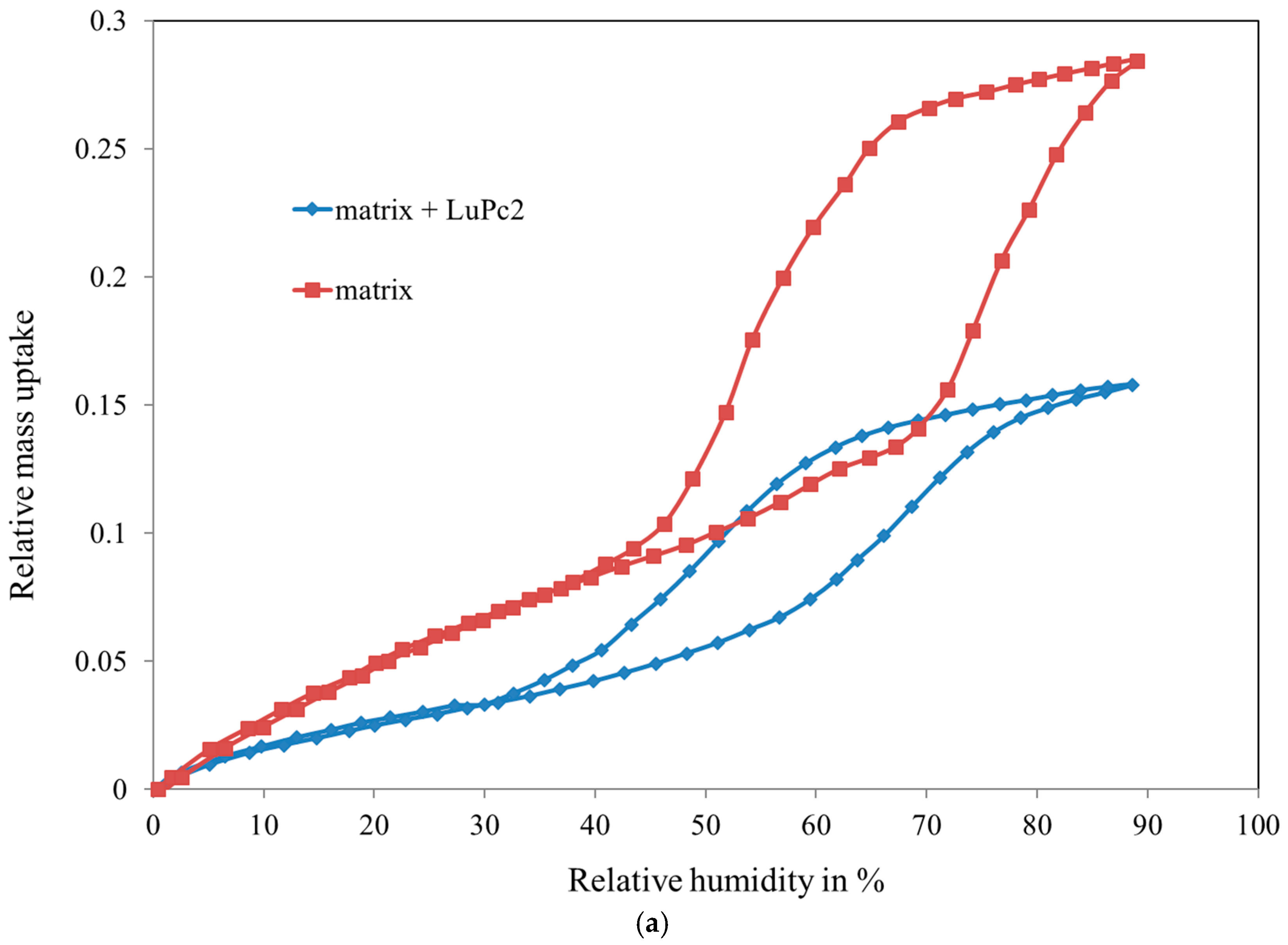
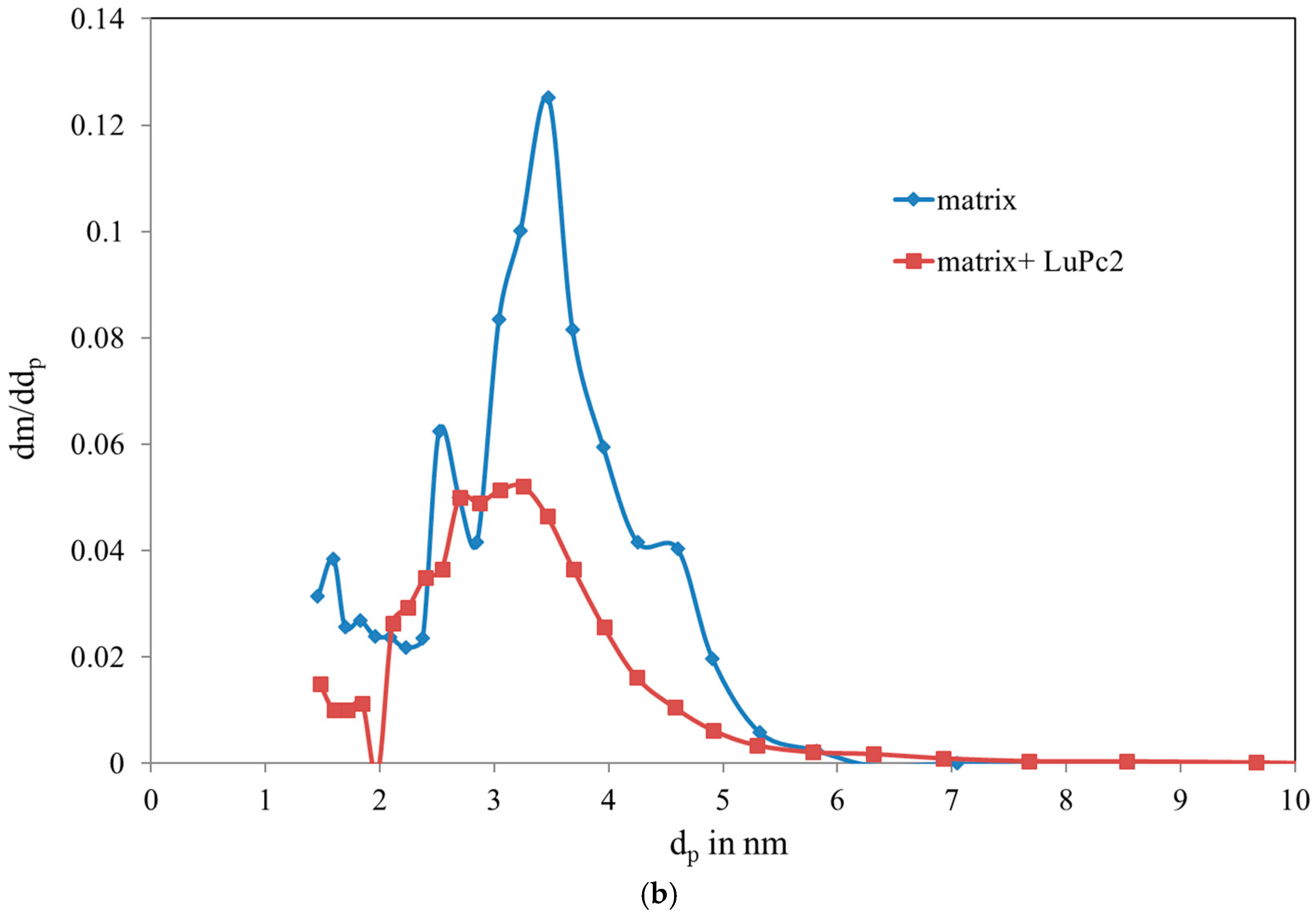
3.1. Characterization of the Mesoporous Matrix
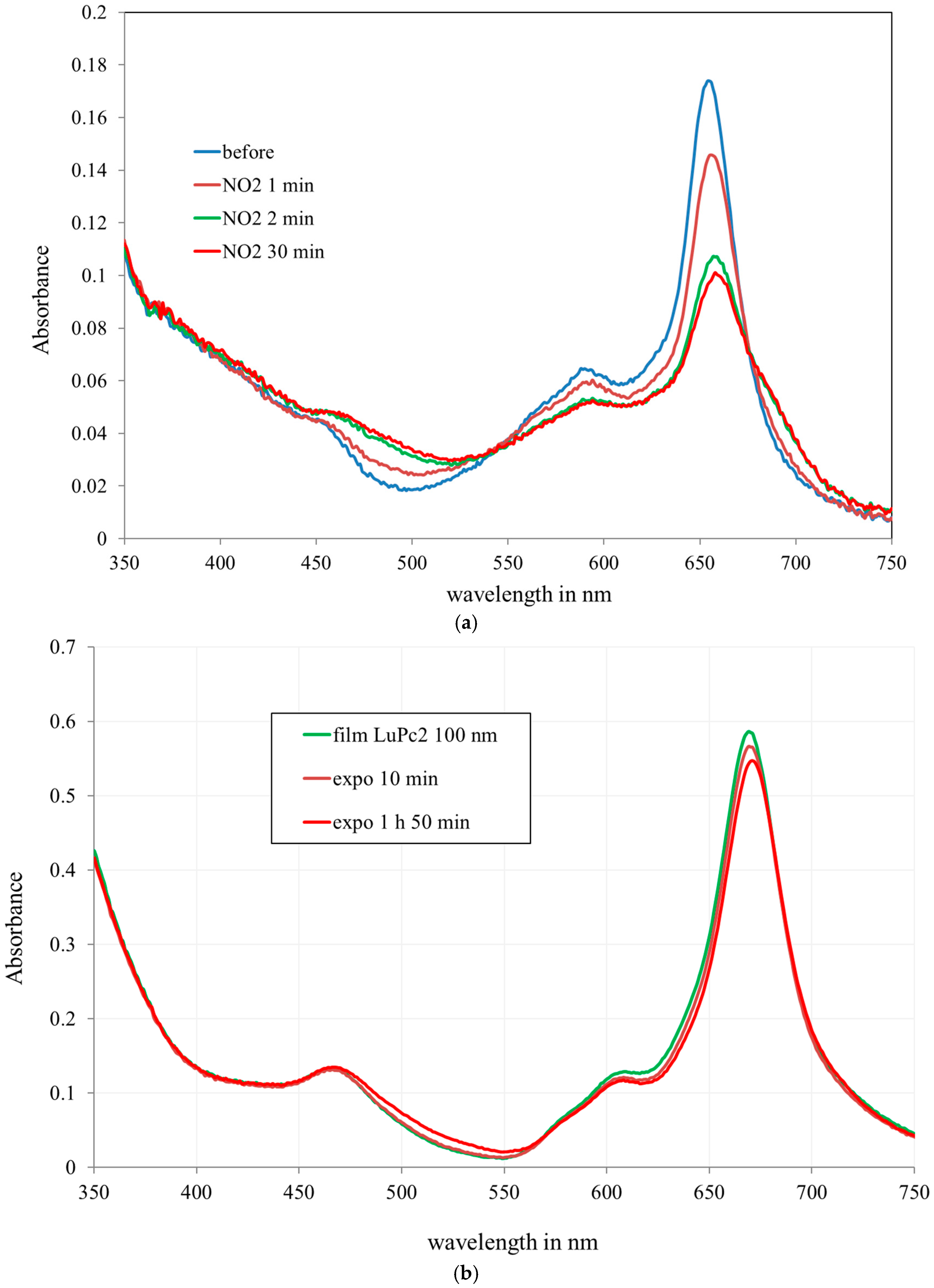
3.2. Optical Characterization on Flat Substrates
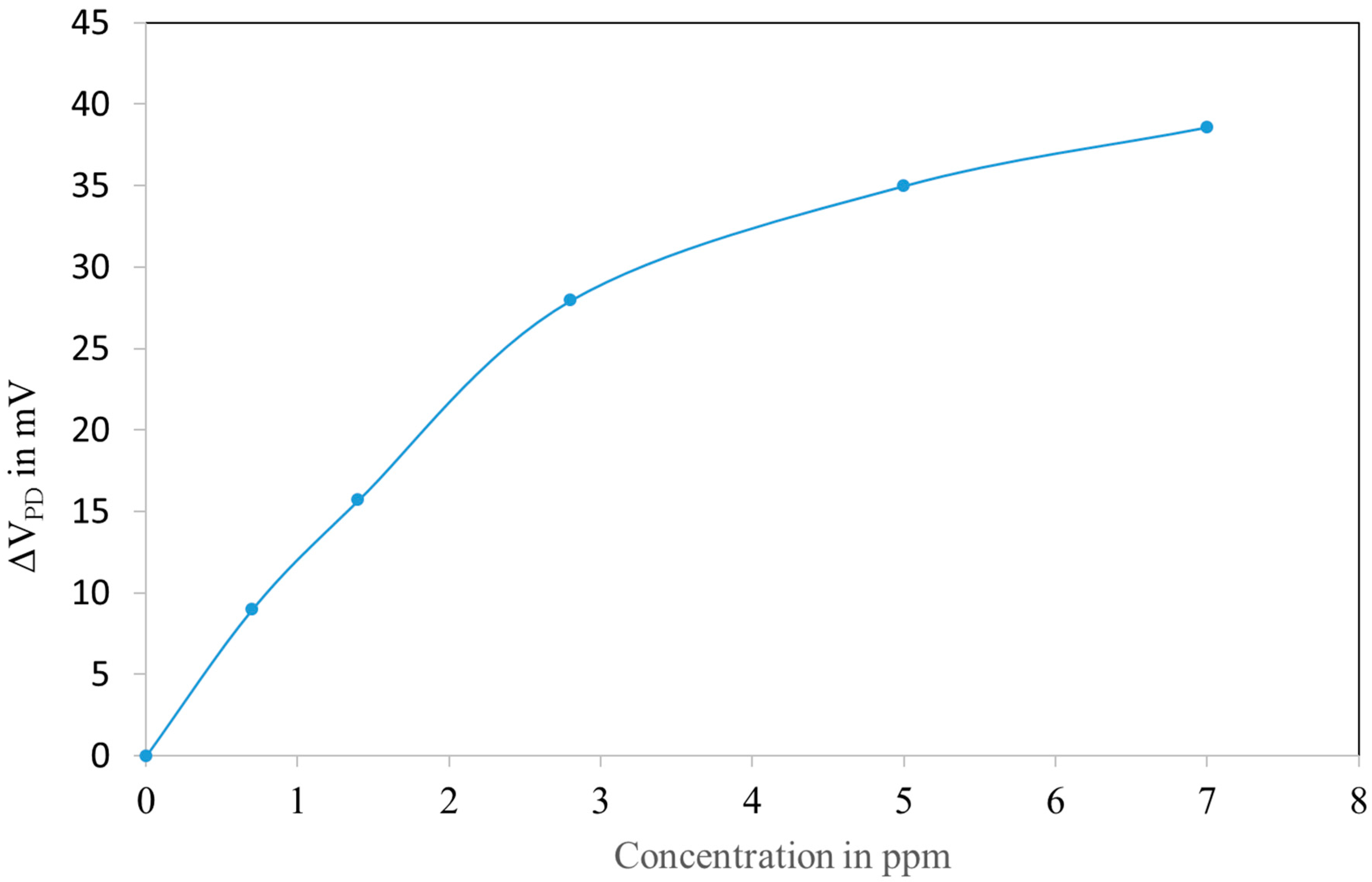
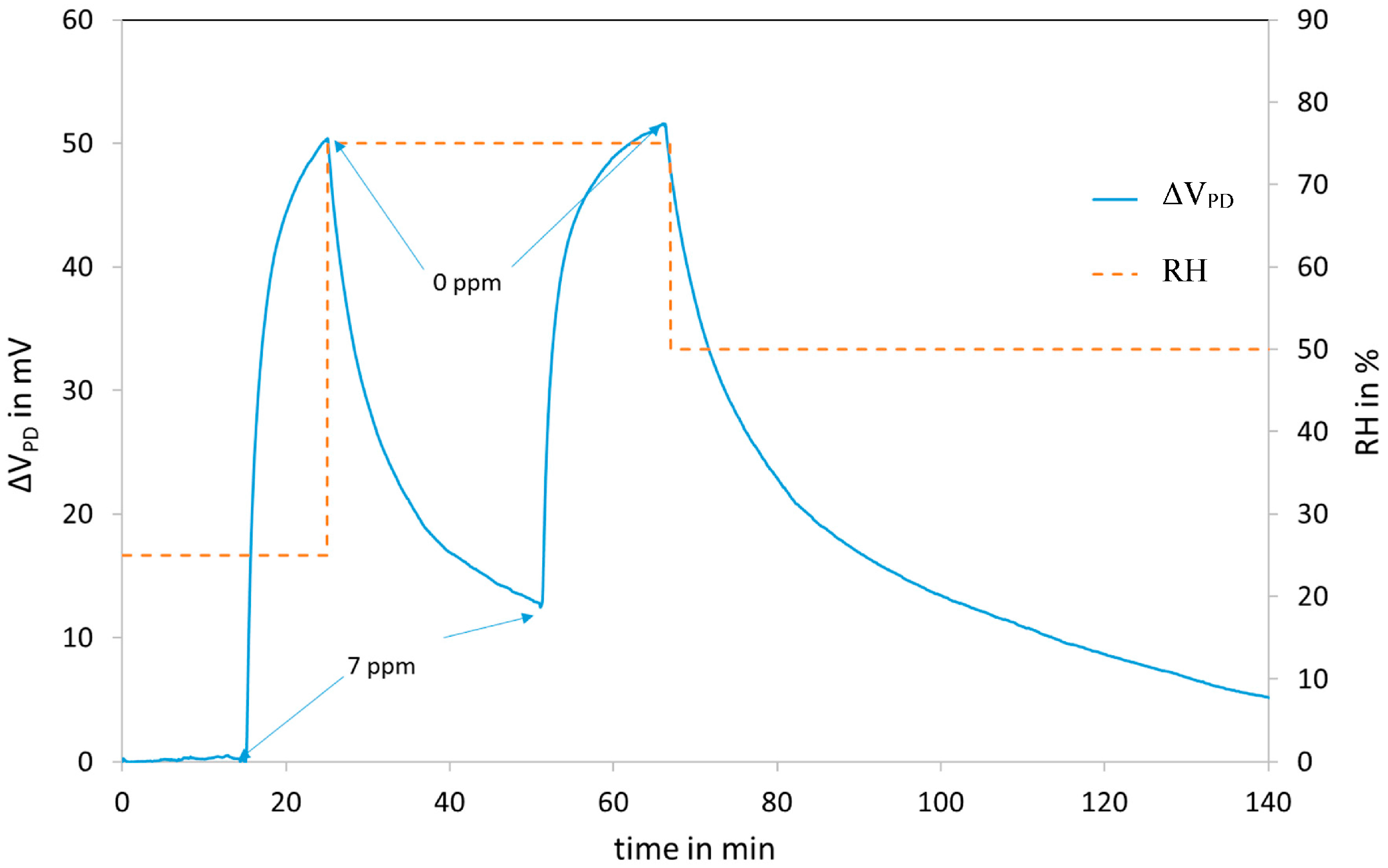
3.3. NO2 Sensing on Fibre
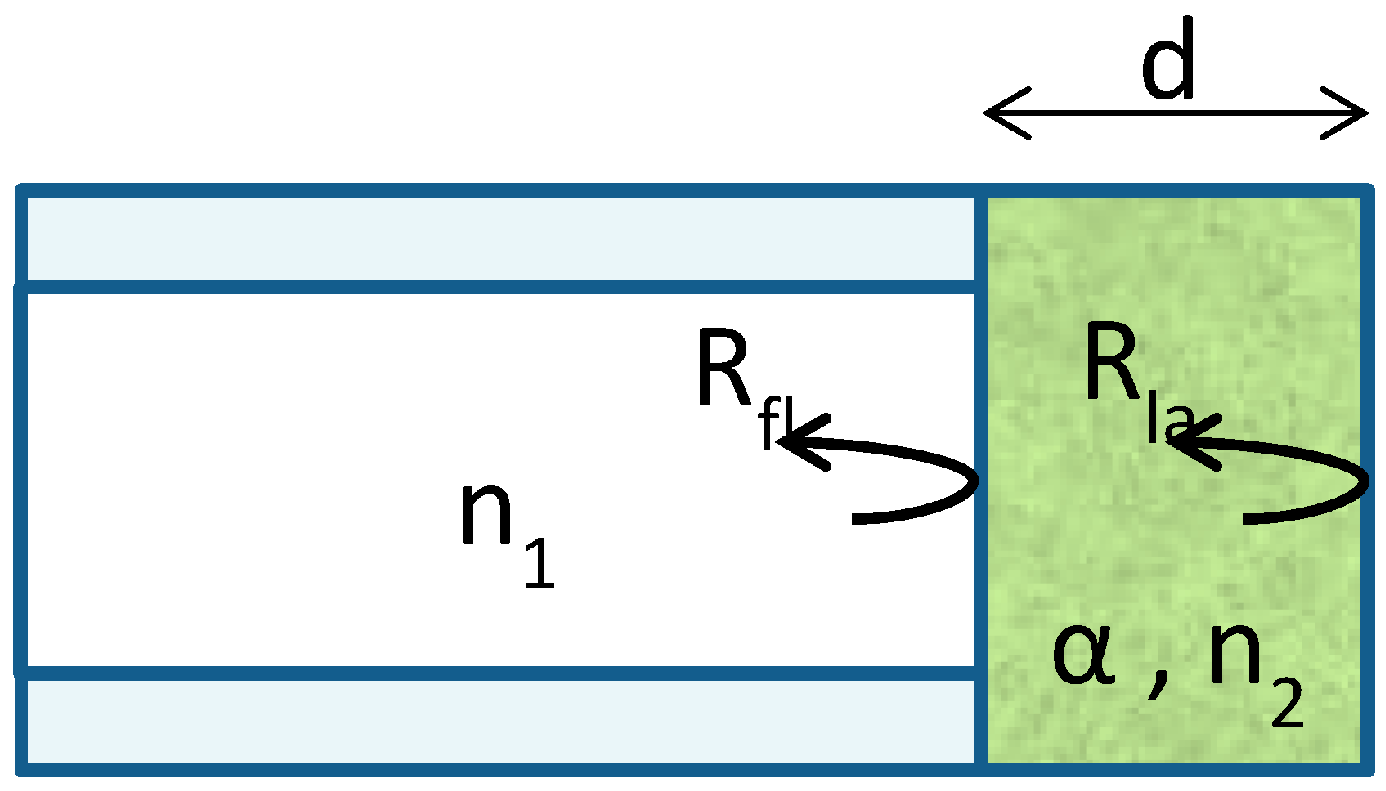
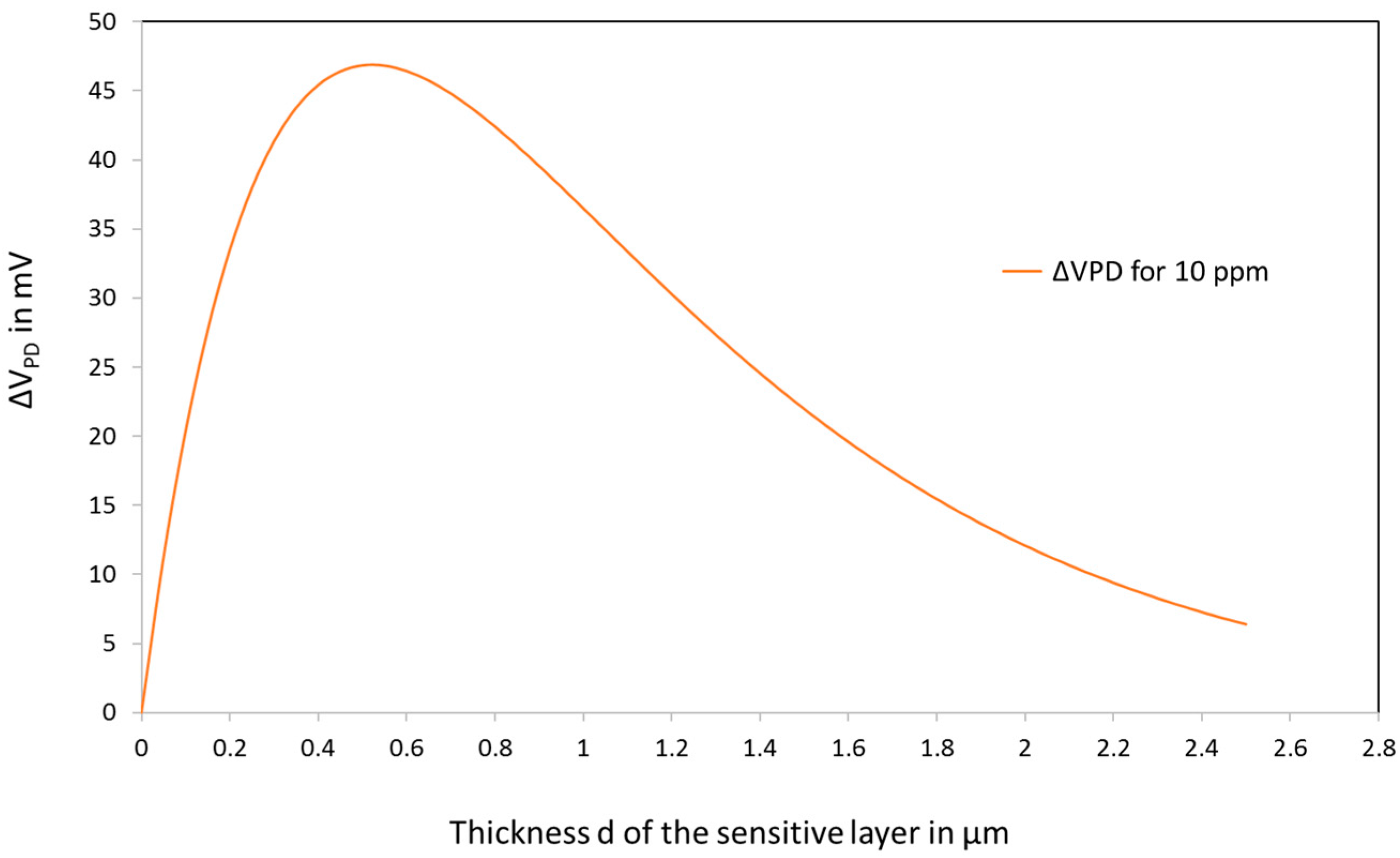
3.4. Simple Model for the Sensor
- PLED is the output power of the red LED (emitting at 660 nm) in W;
- is the responsivity of the photodiode in A/W;
- GPD is the transimpedance gain of the photodiode in V/A;
- GT represents the optical losses and is lower than 1.
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Olivier, J.J.M.; Bouwman, J.G.J.; Van Der Hoek, A.F.; Berdowski, K.W. Global air emission inventories for anthropogenic sources of NOx, NH3 and N2O in 1990. Environ. Pollut. 1998, 102, 135–148. [Google Scholar] [CrossRef]
- Maître, A.; Bonneterre, V.; Huillard, L.; Sabatier, P.; Gaudemaris, R. Impact of urban atmospheric pollution on coronary disease. Eur. Heart J. 2006, 27, 2275–2284. [Google Scholar] [CrossRef] [PubMed]
- Technical Committee on Road Tunnels. Pollution by Nitrogen Dioxide in Road Tunnels; World Road Association: La defense, France, 2000. [Google Scholar]
- Forsberg, B. Bedömning av Exponering för Vägtunnelluft, Yrkes-och Miljömedicin; Umeǻ Universitet: Umeǻ, Sweden, 2009. [Google Scholar]
- Langris, H.B.M.; Lundbäck, M.; Barath, S.; Söderberg, S.; Mills, N.; Newby, D.E.; Sandström, T.; Blomberg, A. Exposure to nitrogen dioxide is not associated with vascular dysfunction in man. Inhal. Toxicol. 2010, 22, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Statens Vegvesen Vegdirektoratet. Available online: http://www.vegvesen.no/_attachment/61913 (accessed on 28 February 2018).
- Mac Craith, B.D.; O’Keefe, G.; McDonagh, C.; McEvoy, A.K. LED-based fibre optic oxygen sensor using sol-gel coating. Electron. Lett. 1994, 30, 888–889. [Google Scholar] [CrossRef]
- Bariáin, C.; Matías, I.R.; Arregui, F.J.; López-Amo, M. Experimental results towards development of humidity sensors by using hygroscopic material on biconically tapered optical fibre. Proc. SPIE 1998, 3555, 95–105. [Google Scholar]
- Caucheteur, C.; Debliquy, M.; Lahem, D.; Mégret, P. Catalytic fibre Bragg grating sensor for hydrogen leak detection in air. IEEE Photonics Technol. Lett. 2008, 20, 96–98. [Google Scholar] [CrossRef]
- Caucheteur, C.; Debliquy, M.; Lahem, D.; Mégret, P. Hybrid fibre gratings coated with a catalytic sensitive layer for hydrogen sensing in air. Opt. Express 2008, 16, 16854–16859. [Google Scholar] [CrossRef] [PubMed]
- Mechery, S.J.; Singh, J.P. Fibre optic based gas sensor with nanoporous structure for the selective detection of NO2 in air samples. Anal. Chim. Acta 2006, 557, 123–129. [Google Scholar] [CrossRef]
- Bezunartea, M.; Estella, J.; Echeverría, J.C.; Elosúa, C.; Bariáin, C.; Laguna, M.; Luquin, A.; Garrido, J.J. Optical fibre sensing element based on xerogel-supported [Au2Ag2(C6F5)4(C14H10)]n for the detection of methanol and ethanol in the vapour phase. Sens. Actuators B Chem. 2008, 134, 966–973. [Google Scholar] [CrossRef]
- Schmidlin, E.M.; Mendoza, E.A.; Ferrell, D.J.; Syracuse, S.J.; Khalil, A.N.; Lieberman, R.A. A fibre optic NO2 sensor for combustion monitoring. Proc. SPIE 1994, 2068, 41–48. [Google Scholar]
- John, M.S.; Unnikrishnan, K.P.; Thomas, J.; Radhakrishnan, P.; Nampori, V.P.N.; Vallabhan, C.P.G. Characterization of an optical fibre sensor in detecting NO2 gas. In Proceedings of the International Conference on Fibre Optics & Photonics PHOTONICS-2000, Kolkata, India, 18–20 December 2000; pp. 612–616. [Google Scholar]
- Mechery, S.J.; Singh, J.P. Self-calibrated fibre optic transflection probe for NO2 detection. Proc. SPIE 2004, 5272, 110–115. [Google Scholar]
- Baldini, F.; Capobianchi, A.; Falai, A.; Mencaglia, A.A.; Pennesi, G. Reversible and selective detection of NO2 by means of optical fibre. Sens. Actuators B Chem. 2001, 74, 12–17. [Google Scholar] [CrossRef]
- Ohira, S.-I.; Wanigasekara, E.; Rudkevich, D.M.; Dasgupta, P.K. Sensing parts per million levels of gaseous NO2 by an optical fibre transducer based on calix[4]arenes. Talanta 2009, 77, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Bariáin, C.; Matías, I.R.; Fernández-Valdivielso, C.; Arregui, F.J.; Rodríguez-Méndez, M.L.; de Saja, J.A. Optical fibre sensor based on lutetium bisphthalocyanine for the detection of gases using standard telecommunication wavelengths. Sens. Actuators B Chem. 2003, 93, 153–158. [Google Scholar] [CrossRef]
- Bueno Martinez, A.; Lahem, D.; Caucheteur, C.; Debliquy, M. Reversible NO2 Optical Fibre Chemical Sensor Based on LuPc2 Using Simultaneous Transmission of UV and Visible Light. Sensors 2015, 15, 9870–9881. [Google Scholar] [CrossRef] [PubMed]
- Leznoff, C.C.; Lever, A.B.P. Phthalocyanines: Properties and Applications; VCH Publishers: New York, NY, USA, 1989; Volume 1–3, ISBN 0895737531. [Google Scholar]
- McKeown, N.B. Phthalocyanine Materials: Synthesis, Structure and Function. Chemistry of Solid State Materials; Cambridge University Press: Cambridge, UK, 1998; Volume 6, ISBN 0521496233. [Google Scholar]
- Simon, J.; André, J.-J. Molecular Semiconductors; Springer: Berlin/Heidelberg, Germany, 1985; ISBN 978-3-642-70012-5. [Google Scholar]
- Wright, J.D. Gas adsorption on phthalocyanines and its effects on electrical properties. Prog. Surf. Sci. 1991, 31, 1–60. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Hogarth, C.A. Gas sensing properties of phthalocyanine Langmuir–Blodgett films. Adv. Mater. 1994, 6, 162–164. [Google Scholar] [CrossRef]
- Capone, S.; Mongelli, S.; Rella, R.; Siciliano, P.; Valli, L. Gas sensitivity measurements on NO2 sensors based on Coper(II) tetrakis(n-butylaminocarbonyl) phthalocyanine LB films. Langmuir 1999, 15, 1748–1753. [Google Scholar] [CrossRef]
- Simon, J.; Bouvet, M.; Bassoul, P. The Encyclopedia of Advanced Materials; Pergamon: Oxford, UK, 1994; pp. 1680–1692. [Google Scholar]
- Rodriguez-Mendez, M.L.; Aroca, R.; De Saja, J.A. Electrochromic and gas adsorption properties of Langmuir-Blodgett films of lutetium bisphthalocyanine complexes. Chem. Mater. 1993, 5, 933–937. [Google Scholar] [CrossRef]
- Weiss, R.; Fischer, J. Lanthanide phthalocyanine complexes. In The Porphyrin Handbook, 1st ed.; Kadish, K., Smith, K.M., Guilard, R., Eds.; Academic Press Inc.: New York, NY, USA, 2006; Volume 16, pp. 171–246. [Google Scholar]
- Rodriguez-Mendez, M.L.; Aroca, R.; DeSaja, J.A. Electrochromic properties of Langmuir-Blodgett films of bisphthalocyanine complexes of rare earth elements. Chem. Mater. 1992, 4, 1017–1020. [Google Scholar] [CrossRef]
- Maitrot, M.; Guillaud, G.; Boudjema, B.; André, J.-J.; Strzelecka, H.; Simon, J.; Even, R. Lutetium bisphthalocyanine: The first molecular semiconductor. Conduction properties of thin films of p- and n-doped materials. Chem. Phys. Lett. 1987, 133, 59–62. [Google Scholar] [CrossRef]
- Bouvet, M. Radical phthalocyanines and intrinsic semiconduction. In Porphyrin Handbook; Kadish, K.M., Guilard, R., Smith, K., Eds.; Academic Press: New York, NY, USA, 2003; Volume 19, pp. 37–104. ISBN 9780123932211. [Google Scholar]
- Gutierrez, N.; Rodríguez-Méndez, M.L.; de Saja, J.A. Array of sensors based on lanthanide bisphtahlocyanine Langmuir–Blodgett films for the detection of olive oil aroma. Sens. Actuators B Chem. 2001, 77, 437–442. [Google Scholar] [CrossRef]
- De Saja, J.A.; Rodríguez-Méndez, M.L. Sensors based on double-decker rare earth phthalocyanines. Adv. Colloid Interface Sci. 2005, 116, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Méndez, M.L.; Gorbunova, Y.; de Saja, J.A. Spectroscopic Properties of Langmuir–Blodgett Films of Lanthanide Bis(phthalocyanine)s Exposed to Volatile Organic Compounds. Sensing Applications. Langmuir 2002, 18, 9560–9565. [Google Scholar] [CrossRef]
- Clarisse, C.; Riou, M.-T. Synthesis and characterization of some lanthanide phthalocyanines. Inorg. Chim. Acta 1987, 130, 139–144. [Google Scholar] [CrossRef]
- Zhao, D.; Feng, J.; Huo, Q.; Melosh, N.; Fredrickson, G.H.; Chmelka, B.F.; Stucky, G.D. Triblock Copolymer Syntheses of Mesoporous Silica with Periodic 50 to 300 Angstrom Pores. Science 1998, 279, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Al Othman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Dewalque, J.; Cloots, R.; Mathis, F.; Dubreuil, O.; Krins, N.; Henrist, C. TiO2 multilayer thick films (up to 4 µm) with ordered mesoporosity: Influence of template on the film mesostructure and use as high efficiency photoelectrode in DSSCs. J. Mater. Chem. 2011, 21, 7356–7363. [Google Scholar] [CrossRef]
- Taguchi, A.; Schüth, F. Ordered mesoporous materials in catalysis. Microporous Mesoporous Mater. 2005, 7, 1–45. [Google Scholar] [CrossRef]
- Soler-Illia, G.J.A.; Angelome, P.C.; Fuertes, M.C.; Calvo, A.; Wolosiuk, A.; Zelcer, A.M.; Bellino, G.; Martınez, E.D. Mesoporous hybrid and nanocomposite thin films. A sol–gel toolbox to create nanoconfined systems with localized chemical properties. J. Sol-Gel Sci. Technol. 2011, 57, 299–312. [Google Scholar] [CrossRef]
- Hua, Z.-L.; Shi, J.-L.; Wang, L.; Zhang, W.-H. Preparation of mesoporous silica films on a glass slide: Surfactant template removal by solvent extraction. J. Non-Cryst. Solids 2001, 292, 177–183. [Google Scholar] [CrossRef]
- Recloux, I.; Debliquy, M.; Baroni, A.; Paint, Y.; Lanzutti, A.; Fedrizzi, L.; Olivier, M.-G. Optimization of synthesis parameters of mesoporous silica sol-gel thin films for application on 2024 aluminum alloy substrates. Appl. Surf. Sci. 2013, 277, 201–210. [Google Scholar] [CrossRef]
- Cagnol, F.; Grosso, D.; Soler-Illia, G.J.A. Humidity-controlled mesostructuration in CTAB-templated silica thin film processing. The existence of a modulable steady state. J. Mat. Chem. 2003, 13, 61–66. [Google Scholar] [CrossRef]
- Parra, V.; Brunet, J.; Pauly, A.; Bouvet, M. Molecular semiconductor–doped insulator (MSDI) heterojunctions: An alternative transducer for gas chemosensing. Analyst 2009, 134, 1776–1778. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Bouvet, M.; Sizun, T.; Gao, Y.; Plassard, C.; Lesniewska, E.; Jiang, J. Facile Approaches to build Ordered Amphiphilic Tris(phthalocyaninato) Europium Triple-Decker Complex Thin Films and their comparative performances in ozone sensing. Phys. Chem. Chem. Phys. 2010, 12, 12851–12861. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Dubois, M.; Brunet, J.; Guérin, K.; Parra, V.; Varenne, C.; Lauron, B.; Bouvet, M.; Hamwi, A.; Pauly, A. The use of nanocarbons as chemical filters for selective detection of nitrogen dioxide and ozone. J. Nanosci. Nanotechnol. 2010, 10, 5653–5661. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Spinelle, L.; Pauly, A.; Dubois, M.; Guerin, K.; Bouvet, M.; Varenne, C.; Lauron, B.; Hamwicd, A. All-organic device with integrated chemical filter for the selective measurement of NO2 in air. Org. Electron. 2010, 11, 1223–1229. [Google Scholar] [CrossRef]
- Castell, N.; Dauge, F.R.; Schneider, P.; Vogt, M.; Lerner, U.; Fishbain, B.; Broday, D.; Bartonova, A. Can commercial low-cost sensor platforms contribute to air quality monitoring and exposure estimates? Environ. Int. 2017, 99, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Saffell, J.; Baron, R. Differentiating NO2 and O3 at Low Cost Air Quality Amperometric Gas Sensors. ACS Sens. 2016, 1, 1291–1294. [Google Scholar] [CrossRef]
- Suriano, D.; Prato, M.; Pfister, V.; Cassano, G.; Camporeale, G.; Dipinto, S.; Penza, M. Stationary and Mobile Low Cost Gas Sensor Systems for Air Quality Monitoring Applications. In Proceedings of the Fourth Scientific Meeting EuNetAir, Linkoping, Sweden, 3–5 June 2015; pp. 55–58. [Google Scholar] [CrossRef]
- Williams, R.; Long, R.; Beaver, M.; Kaufman, A.; Zeiger, F.; Heimbinder, M.; Hang, I.; Yap, R.; Acharya, B.; Ginwald, B.; et al. Sensor Evaluation Report; EPA 600/R-14/143; US Environmental Protection Agency: Research Triangle Park, NC, USA, 2014; pp. 21–28.



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Debliquy, M.; Lahem, D.; Bueno-Martinez, A.; Caucheteur, C.; Bouvet, M.; Recloux, I.; Raskin, J.-P.; Olivier, M.-G. Optical Fibre NO2 Sensor Based on Lutetium Bisphthalocyanine in a Mesoporous Silica Matrix. Sensors 2018, 18, 740. https://doi.org/10.3390/s18030740
Debliquy M, Lahem D, Bueno-Martinez A, Caucheteur C, Bouvet M, Recloux I, Raskin J-P, Olivier M-G. Optical Fibre NO2 Sensor Based on Lutetium Bisphthalocyanine in a Mesoporous Silica Matrix. Sensors. 2018; 18(3):740. https://doi.org/10.3390/s18030740
Chicago/Turabian StyleDebliquy, Marc, Driss Lahem, Antonio Bueno-Martinez, Christophe Caucheteur, Marcel Bouvet, Isaline Recloux, Jean-Pierre Raskin, and Marie-Georges Olivier. 2018. "Optical Fibre NO2 Sensor Based on Lutetium Bisphthalocyanine in a Mesoporous Silica Matrix" Sensors 18, no. 3: 740. https://doi.org/10.3390/s18030740