A Study on the Impact of Poly(3-hexylthiophene) Chain Length and Other Applied Side-Chains on the NO2 Sensing Properties of Conducting Graft Copolymers
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Materials Characterization
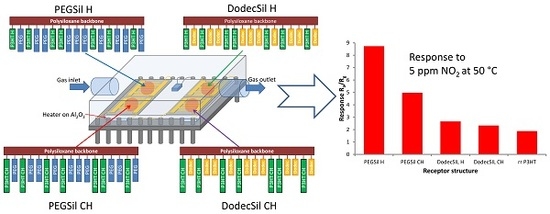
3.2. Gas Sensing Mesurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A




References
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.-M.; Jeong, M.-J.; Kim, D.; Stockwell, W.R.; Yang, J.-H.; Shin, H.-W.; Lee, M.-I.; Song, C.-K.; Lee, S.-D. Spatiotemporal variations of air pollutants (O3, NO2, SO2, CO, PM10 and VOCs) with land-use types. Atmos. Chem. Phys. Discuss. 2015, 15, 16985–17050. [Google Scholar] [CrossRef]
- Halfaya, Y.; Bishop, C.; Soltani, A.; Sundaram, S.; Aubry, V.; Voss, P.L.; Salvestrini, J.P.; Ougazzaden, A. Investigation of the performance of HEMT-based NO, NO2 and NH3 exhaust gas sensors for automotive antipollution systems. Sensors 2016, 16, 273. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-J.; Song, I.-G.; Kim, J.-S. In2O3-based micro gas sensor for detecting NOx gases. Electron. Mater. Lett. 2014, 10, 509–513. [Google Scholar] [CrossRef]
- Procek, M.; Stolarczyk, A.; Pustelny, T.; Maciak, E. A Study of a QCM Sensor Based on TiO2 Nanostructures for the Detection of NO2 and Explosives Vapours in Air. Sensors 2015, 15, 9563–9581. [Google Scholar] [CrossRef] [PubMed]
- Bielecki, Z.; Janucki, J.; Kawalec, A.; Mikolajczyk, J.; Palka, N.; Pasternak, M.; Pustelny, T.; Stacewicz, T.; Wojtas, J. Sensors and systems for the detection of explosive devices—An overview. Metrol. Meas. Syst. 2012, 19, 3–28. [Google Scholar] [CrossRef]
- Blue, R.; Uttamchandani, D.; Thomson, N.; Skabara, P. Novel polymer materials for low-cost nitro vapor detection sensors. In Proceedings of the 2015 IEEE Sensors, Busan, Korea, 1–4 November 2015; pp. 1–4. [Google Scholar]
- Yunusa, Z.; Hamidon, M.N.; Kaiser, A.; Awang, Z. Gas sensors: A review. Sens. Transducers 2014, 168, 61–75. [Google Scholar]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Procek, M.; Stolarczyk, A.; Pustelny, T. Impact of Temperature and UV Irradiation on Dynamics of NO2 Sensors Based on ZnO Nanostructures. Nanomaterials 2017, 7, 312. [Google Scholar] [CrossRef] [PubMed]
- Ponzoni, A.; Baratto, C.; Cattabiani, N.; Falasconi, M.; Galstyan, V.; Nunez-Carmona, E.; Rigoni, F.; Sberveglieri, V.; Zambotti, G.; Zappa, D. Metal Oxide Gas Sensors, a Survey of Selectivity Issues Addressed at the SENSOR Lab, Brescia (Italy). Sensors 2017, 17, 714. [Google Scholar] [CrossRef] [PubMed]
- Gardon, M.; Guilemany, J.M. A review on fabrication, sensing mechanisms and performance of metal oxide gas sensors. J. Mater. Sci. Mater. Electron. 2013, 24, 1410–1421. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Miasik, J.J.; Hooper, A.; Tofield, B.C. Conducting polymer gas sensors. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1986, 82, 1117. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Kumar, V.; Kim, K.-H.; Kumar, P.; Jeon, B.-H.; Kim, J.-C. Functional hybrid nanostructure materials: Advanced strategies for sensing applications toward volatile organic compounds. Coord. Chem. Rev. 2017, 342, 80–105. [Google Scholar] [CrossRef]
- Maciak, E.; Procek, M.; Kepska, K.; Stolarczyk, A. Study of optical and electrical properties of thin films of the conducting comb-like graft copolymer of polymethylsiloxane with poly(3-hexyltiophene) and poly(ethylene) glycol side chains for low temperature NO2 sensing. Thin Solid Films 2016, 618, 277–285. [Google Scholar] [CrossRef]
- Öztürk, S.; Kösemen, A.; Şen, Z.; Kılınç, N.; Harbeck, M. Poly(3-Methylthiophene) Thin Films Deposited Electrochemically on QCMs for the Sensing of Volatile Organic Compounds. Sensors 2016, 16, 423. [Google Scholar] [CrossRef] [PubMed]
- Stahl, U.; Voigt, A.; Dirschka, M.; Barié, N.; Richter, C.; Waldbaur, A.; Gruhl, F.; Rapp, B.; Rapp, M.; Länge, K. Long-Term Stability of Polymer-Coated Surface Transverse Wave Sensors for the Detection of Organic Solvent Vapors. Sensors 2017, 17, 2529. [Google Scholar] [CrossRef] [PubMed]
- Inzelt, G.; Pineri, M.; Schultze, J.; Vorotyntsev, M. Electron and proton conducting polymers: Recent developments and prospects. Electrochim. Acta 2000, 45, 2403–2421. [Google Scholar] [CrossRef]
- Lange, U.; Roznyatovskaya, N.V.; Mirsky, V.M. Conducting polymers in chemical sensors and arrays. Anal. Chim. Acta 2008, 614, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Park, C.; Yoon, H. Chemo-Electrical Gas Sensors Based on Conducting Polymer Hybrids. Polymers 2017, 9, 155. [Google Scholar] [CrossRef]
- Kepska, K.; Jarosz, T.; Januszkiewicz-Kaleniak, A.; Domagala, W.; Lapkowski, M.; Stolarczyk, A. Spectroelectrochemistry of poly(3-hexylthiophenes) in solution. Chem. Pap. 2018, 72, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Zhuang, X.; Shi, W.; Yang, X.; Li, L.; Yu, J. Poly(3-hexylthiophene)/polystyrene (P3HT/PS) blends based organic field-effect transistor ammonia gas sensor. Sens. Actuators B Chem. 2016, 225, 10–15. [Google Scholar] [CrossRef]
- Li, H.; Dailey, J.; Kale, T.; Besar, K.; Koehler, K.; Katz, H.E. Sensitive and Selective NO2 Sensing Based on Alkyl- and Alkylthio-Thiophene Polymer Conductance and Conductance Ratio Changes from Differential Chemical Doping. ACS Appl. Mater. Interfaces 2017, 9, 20501–20507. [Google Scholar] [CrossRef] [PubMed]
- Maciak, E.; Stolarczyk, A.; Procek, M. Surface plasmon resonance study of comb copolymers containing regioregular poly-3-hexylothiophene. In Optical Fibers and Their Applications 2015; Romaniuk, R.S., Wojcik, W., Smolarz, A., Eds.; SPIE—International Society for Optical Engineering: Bellingham, WA, USA, 2015. [Google Scholar]
- Procek, M.; Stolarczyk, A.; Maciak, E. Study of the impact of UV radiation on NO2 sensing properties of graft comb copolymers of poly(3-hexylthiophene) at room temperature. Proc. SPIE Int. Soc. Opt. Eng. 2017, 10455, 104550N. [Google Scholar]
- Jeffries-El, M.; Geneviève, S.; McCullough, R.D. Facile Synthesis of End-Functionalized Regioregular Poly(3-alkylthiophene)s via Modified Grignard Metathesis Reaction. Macromolecules 2005, 38, 10346–10352. [Google Scholar] [CrossRef]
- Stolarczyk, A.; Turczyn, R.; Januszkiewicz-Kaleniak, A.; Kępska, K. The macromolecule comb-graft polymer of polydimethylsiloxane graft-conjugated polyether and a method for its preparation. Pol. Pat. Appl. 2014, 407784, RR10. [Google Scholar]
- Li, D.; Shi, P.; Wang, J.; Li, J.; Su, R. High-efficiency absorption of high NOx concentration in water or PEG using capillary pneumatic nebulizer packed with an expanded graphite filter. Chem. Eng. J. 2014, 237, 8–15. [Google Scholar] [CrossRef]
- Cerıon Solis, J.; De la Rosa, E.; Pena Cabrera, E. Absorption and refractive index changes of poly (3-octylthiophene) under NO2 gas exposure. Opt. Mater. 2006, 29, 167–172. [Google Scholar] [CrossRef]
- Xie, T.; Xie, G.; Du, H.; Zhou, Y.; Xie, F.; Jiang, Y.; Tai, H. The Fabrication and Optimization of Thin-Film Transistors Based on Poly(3-Hexylthiophene) Films for Nitrogen Dioxide Detection. IEEE Sens. J. 2016, 16, 1865–1871. [Google Scholar] [CrossRef]
- Potje-Kamloth, K. Semiconductor junction gas sensors. Chem. Rev. 2008, 108, 367–399. [Google Scholar] [CrossRef] [PubMed]
- Kroon, R.; Mengistie, D.A.; Kiefer, D.; Hynynen, J.; Ryan, J.D.; Yu, L.; Müller, C. Thermoelectric plastics: From design to synthesis, processing and structure–property relationships. Chem. Soc. Rev. 2016, 45, 6147–6164. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, F.F.; Ribeiro Junior, L.A.; de Almeida Fonseca, A.L.; e Silva, G.M.; da Cunha, W.F. Electron–phonon coupling effects on intrachain polaron recombination in conjugated polymers. J. Mol. Model. 2017, 23, 1299–1308. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-C.; Liao, H.-C.; Lo, H.-H.; Chen, S.; Lin, Y.-Y.; Yen, W.-C.; Zeng, T.-W.; Chen, C.-W.; Chen, Y.-F.; Su, W.-F. Nanostructured polymer blends (P3HT/PMMA): Inorganic titania hybrid photovoltaic devices. Sol. Energy Mater. Sol. Cells 2009, 93, 961–965. [Google Scholar] [CrossRef]
- Lu, F.; Cai, W.; Zhang, Y. ZnO Hierarchical Micro/Nanoarchitectures: Solvothermal Synthesis and Structurally Enhanced Photocatalytic Performance. Adv. Funct. Mater. 2008, 18, 1047–1056. [Google Scholar] [CrossRef]
- Xie, T.; Xie, G.; Zhou, Y.; Huang, J.; Wu, M.; Jiang, Y.; Tai, H. Thin film transistors gas sensors based on reduced graphene oxide poly(3-hexylthiophene) bilayer film for nitrogen dioxide detection. Chem. Phys. Lett. 2014, 614, 275–281. [Google Scholar] [CrossRef]
- Xie, T.; Xie, G.; Du, H.; Su, Y.; Ye, Z.; Chen, Y.; Jiang, Y. Two novel methods for evaluating the performance of OTFT gas sensors. Sens. Actuators B Chem. 2016, 230, 176–183. [Google Scholar] [CrossRef]
- Zhao, T.; Fu, X.; Cui, X.; Lian, G.; Liu, Y.; Song, S.; Wang, Q.; Wang, K.; Cui, D. An in-situ surface modification route for realizing the synergetic effect in P3HT-SnO2 composite sensor and strikingly improving its sensing performance. Sens. Actuators B Chem. 2017, 241, 1210–1217. [Google Scholar] [CrossRef]
- Saxena, V.; Aswal, D.K.; Kaur, M.; Koiry, S.P.; Gupta, S.K.; Yakhmi, J.V.; Kshirsagar, R.J.; Deshpande, S.K. Enhanced NO2 selectivity of hybrid poly(3-hexylthiophene): ZnO-nanowire thin films. Appl. Phys. Lett. 2007, 90, 2005–2008. [Google Scholar] [CrossRef]





| Sample | 1H-NMR δ (ppm) |
|---|---|
| DodecSil CH | h: 0,09 (CH3-Si-O-); g, i: 0,50 (−CH2-Si-O-) a, k: 0,91 (−CH2-CH3); b, j: 1,34 (−(CH2)3-); c: 1,71 (−CH2-CH2-CAr); d: 2,80 (−CH2-CAr); f: 3,48 (−Si-CH2-CH2-CAr); e: 6,98 (H-CAr) |
| PEGSil CH | h: 0,09 (CH3-Si-O-); a: 0,91 (−CH2-CH3); b: 1,36 (−(CH2)3-); c: 1,71 (−CH2-CH2-CAr); d: 2,80 (−CH2-CAr); n: 3,38 (CH3-O-); m: 3,64 (−CH2-O-); l: 4,22 (CH2-CH2-C(=O)-); e: 6,98 (H-CAr) |
| Sample | ν (cm−1) Assignment |
|---|---|
| DodecSil CH | 794 [δ1(Si-(CH3)2), s2]; 1016 [νasym.(Si-O-Si), vs]; 1260 [δasym.(Si-CH3), s]; 1375 [δsym.(-CH2-), w]; 1456 [νasym(CAr = CAr), m]; 1511 [νsym(CAr = CAr), w]; 2855 [νsym.(C-H), s]; 2923 [νasym.(C-H), vs]; 2957 [νsym.(C-H), s]; 3056 [ν(CAr-H), w] |
| PEGSil CH | 795 [δ(Si-(CH3)2), s]; 1022 [νasym.(Si-O-Si), vs]; 1260 [δasym.(Si-CH3), s]; 1379 [δsym.(-CH2-), w]; 1456 [νasym(CAr = CAr), m]; 1508 [νsym(CAr = CAr), w]; 1735 [ν(C = O), w]; 2853 [νsym.(C-H), s]; 2926 [νasym.(C-H), vs]; 2952 [νsym.(C-H), s]; 3055 [ν(CAr-H), w] |
| Sample | PEGSil H | PEGSil CH | DodecSil H | DodecSil CH |
|---|---|---|---|---|
| RMS (5 × 5 μm), nm | 1.24 ± 0.20 | 1.21 ± 0.20 | 1.05 ± 0.10 | 1.06 ± 0.10 |
| Base Resistance, kΩ | 1087.5 ± 1.0 | 128.7 ± 1.0 | 18496.4 ± 1.0 | 113.3 ± 1.0 |
| Material | NO2 Concentration | Response | Operating Temperature | Reference |
|---|---|---|---|---|
| PEGSil H | 1 ppm | 1330% 605% 1980% | RT 50 °C 100 °C | Current work |
| rrP3HT | 5 ppm | 188% | 50 °C | [18] |
| P3HT | 5 ppm | 11% * | RT | [33] |
| Poly(methylsiloxane)-graft-poly(3-hexylthiophe)-graft-poly(ethylene glycol) | 5 ppm | 2300% | 50 °C | [18] |
| (P3HT) ZnO@GO hybrid | 5 ppm | 210% | 50 °C | [37] |
| P3HT/ZnO NS-NR composite | 4 ppm | 60% * | RT | [38] |
| RGO-P3HT composite | 4 ppm | 40% * | RT | [39] |
| P3HT:ZnO (ratio6:2) hybrid | 5 ppm | 20% * | RT | [40] |
| P3HT-SnO2composite | 5 ppm | 500% | 100 °C | [41] |
| Poly-(bisdodecylquaterthiophene) | 5 ppm | 300% * | RT | [26] |
| poly-(bisdodecylthioquaterthiophene) | 5 ppm | 450% * | RT | [26] |
| P3HT:ZnO-nanowire composite | 4 ppm | 30% * | RT | [42] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Procek, M.; Kepska, K.; Stolarczyk, A. A Study on the Impact of Poly(3-hexylthiophene) Chain Length and Other Applied Side-Chains on the NO2 Sensing Properties of Conducting Graft Copolymers. Sensors 2018, 18, 928. https://doi.org/10.3390/s18030928
Procek M, Kepska K, Stolarczyk A. A Study on the Impact of Poly(3-hexylthiophene) Chain Length and Other Applied Side-Chains on the NO2 Sensing Properties of Conducting Graft Copolymers. Sensors. 2018; 18(3):928. https://doi.org/10.3390/s18030928
Chicago/Turabian StyleProcek, Marcin, Kinga Kepska, and Agnieszka Stolarczyk. 2018. "A Study on the Impact of Poly(3-hexylthiophene) Chain Length and Other Applied Side-Chains on the NO2 Sensing Properties of Conducting Graft Copolymers" Sensors 18, no. 3: 928. https://doi.org/10.3390/s18030928