A Fast and Easily-Realized Concentration Sensor for Binary Gas Mixtures and Its Design Analysis
Abstract
:1. Introduction
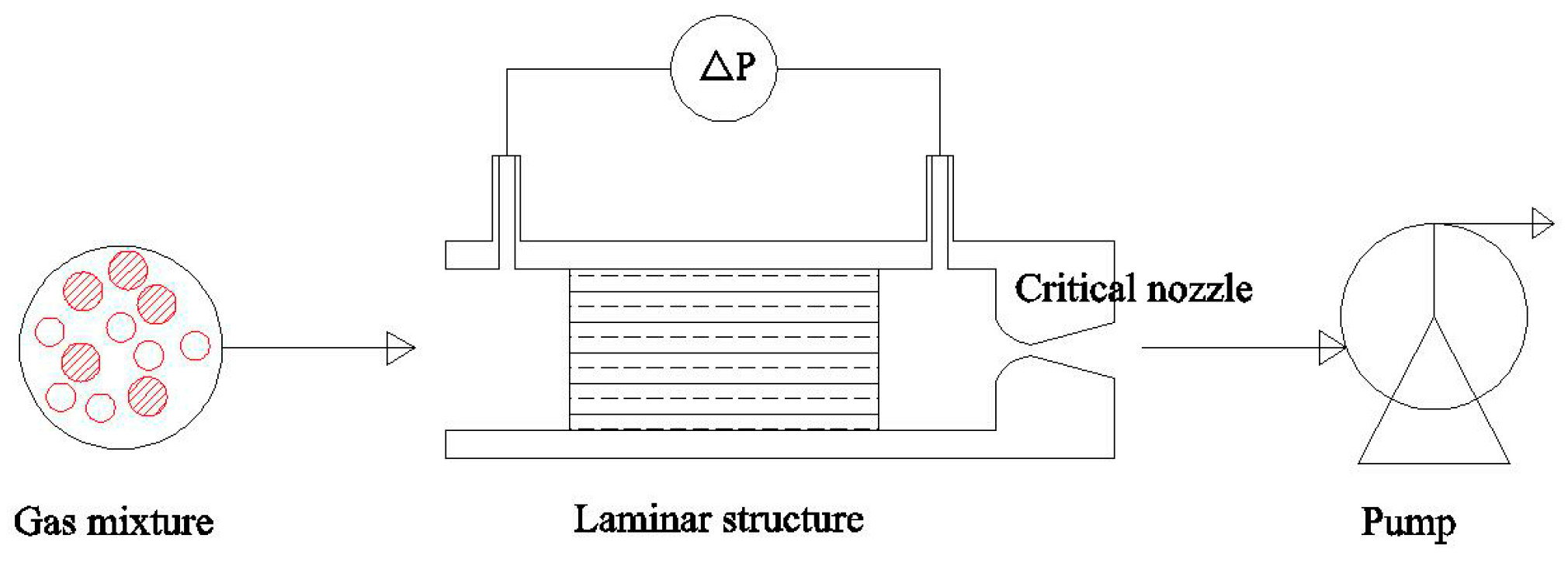
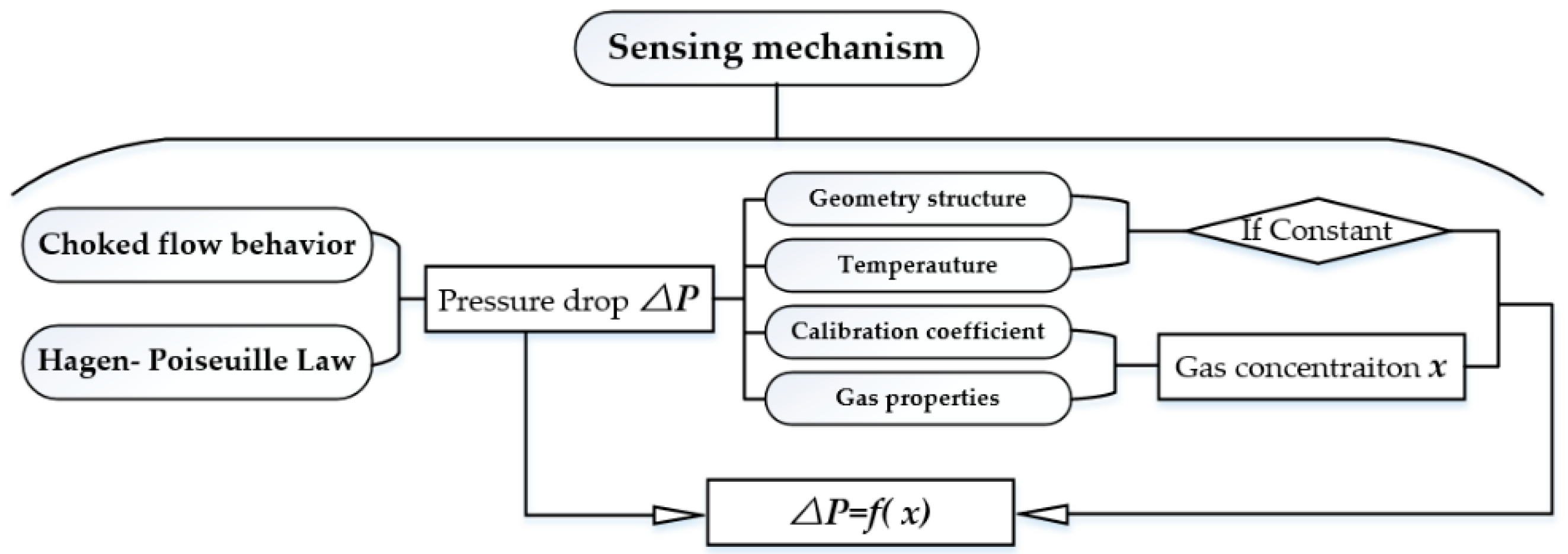
2. Sensing Mechanism
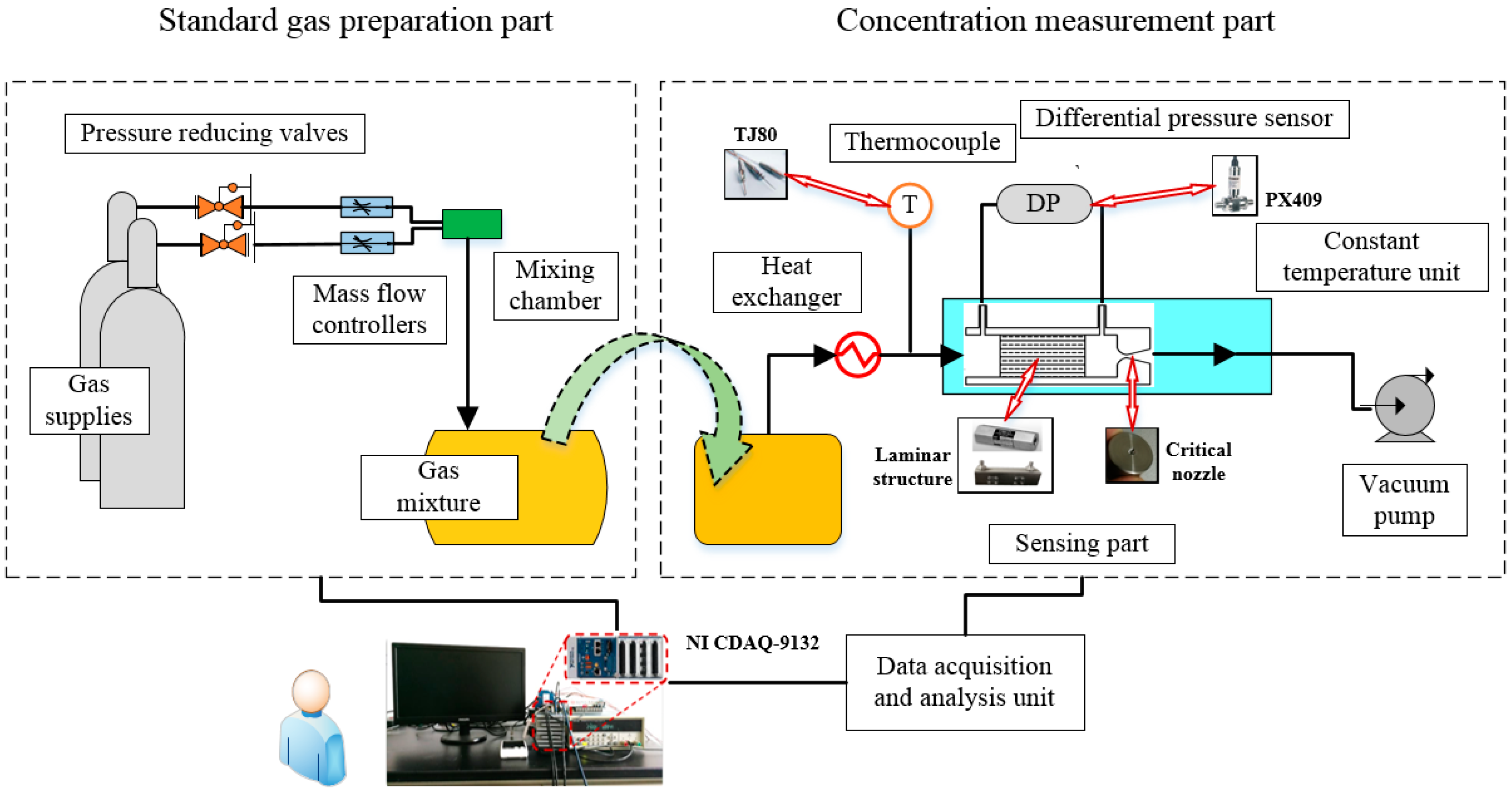
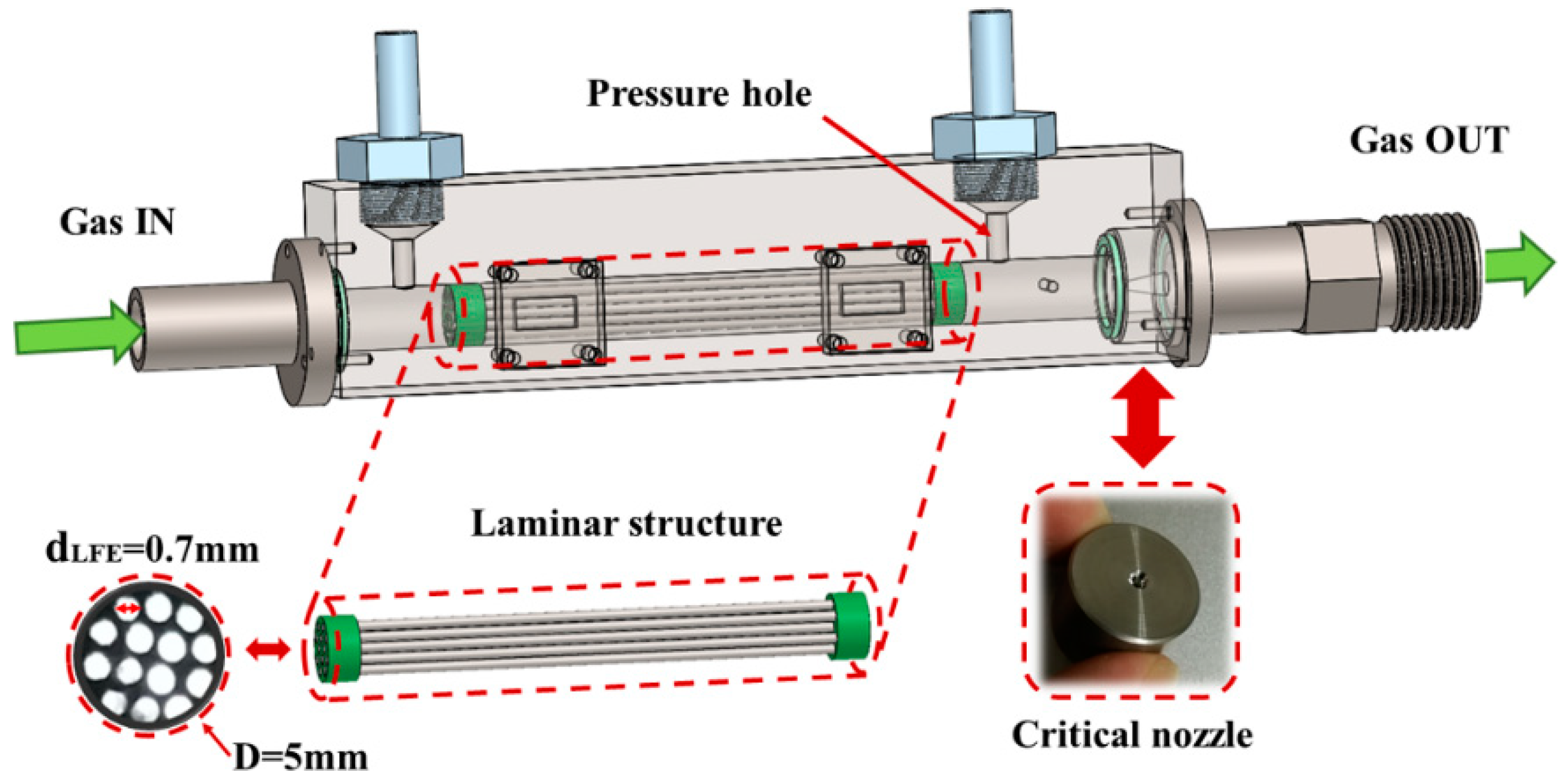
3. Design of Experimental Apparatus
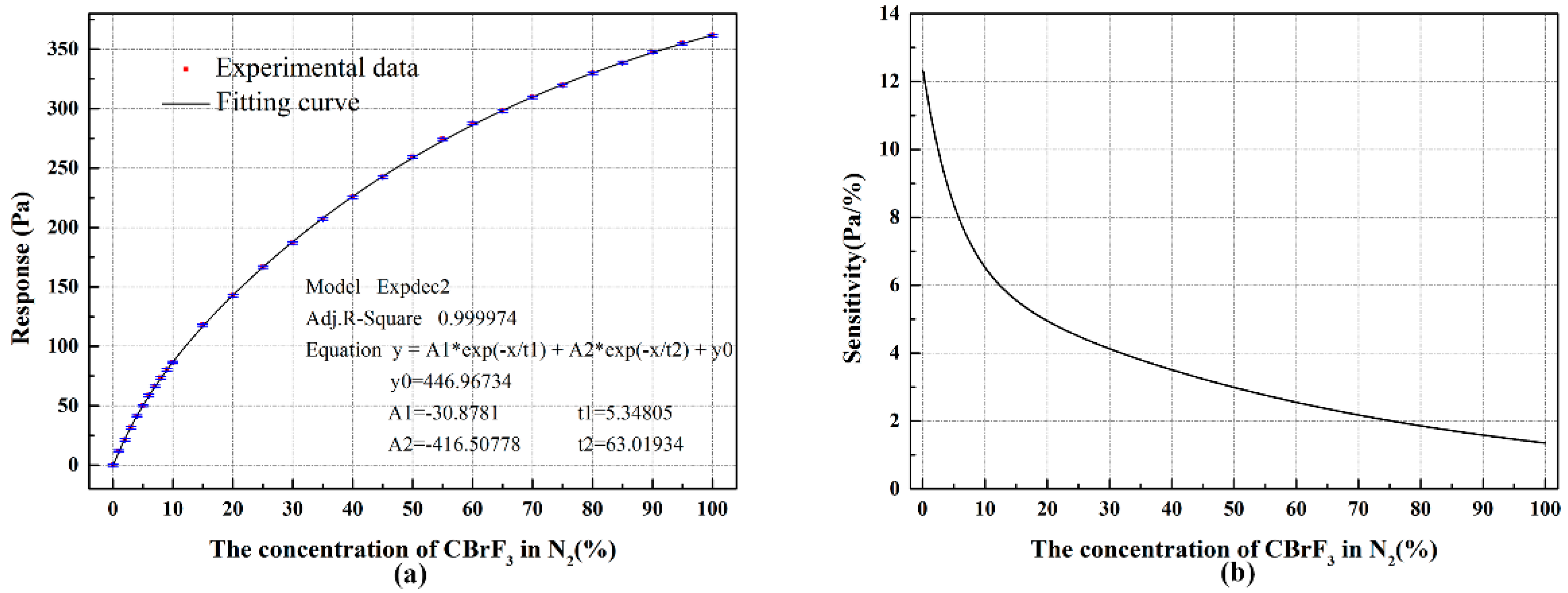
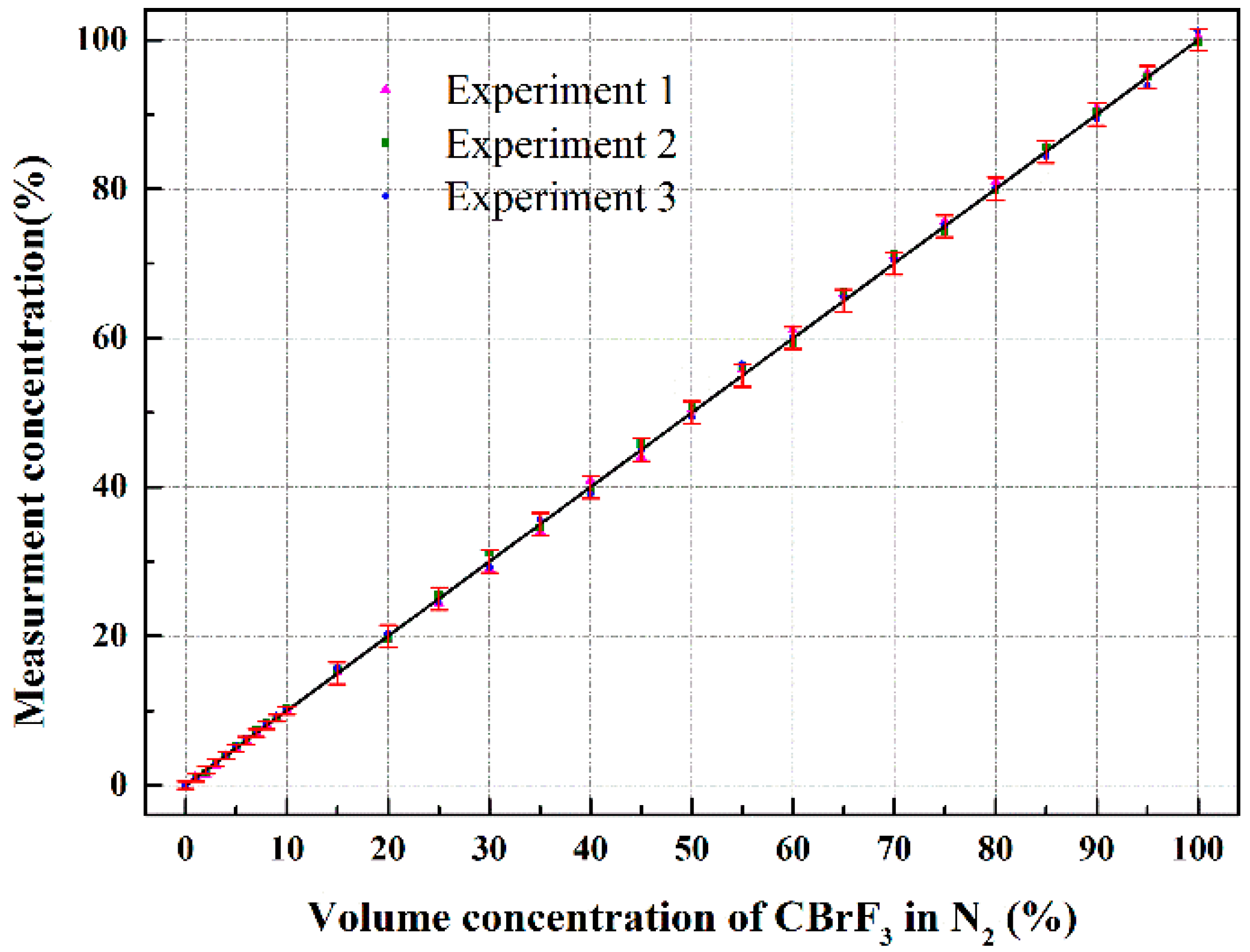
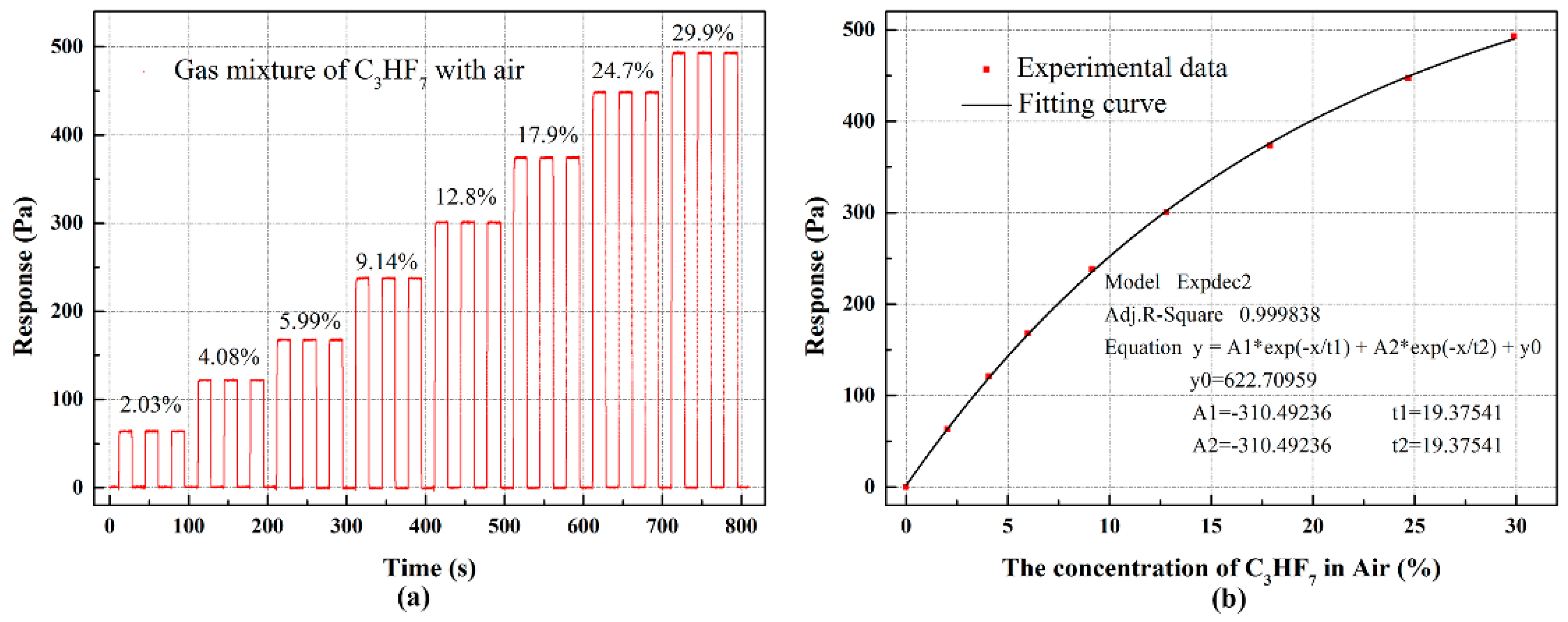
4. Results and Discussion
4.1. Experiment Results
- (a)
- Preparation of gas mixtures. The different concentration of gas mixtures is obtained by gas mixing equipment as described in standard gas preparation section.
- (b)
- Preheating the experimental apparatus. Heat the equipment to 70 °C and maintain relatively constant temperature.
- (c)
- Starting vacuum pump. The gases will enter the equipment, and the test begins when the temperature remains relatively constant in heat exchanger.
- (d)
- Data acquisition and calibration. The value of pressure drop with known gas concentration is recorded by a NI CompactDAQ controller. Based on the experimental data, the calibration curve is acquired, which can be used to confirm the concentration of unknown gas mixtures in the future.
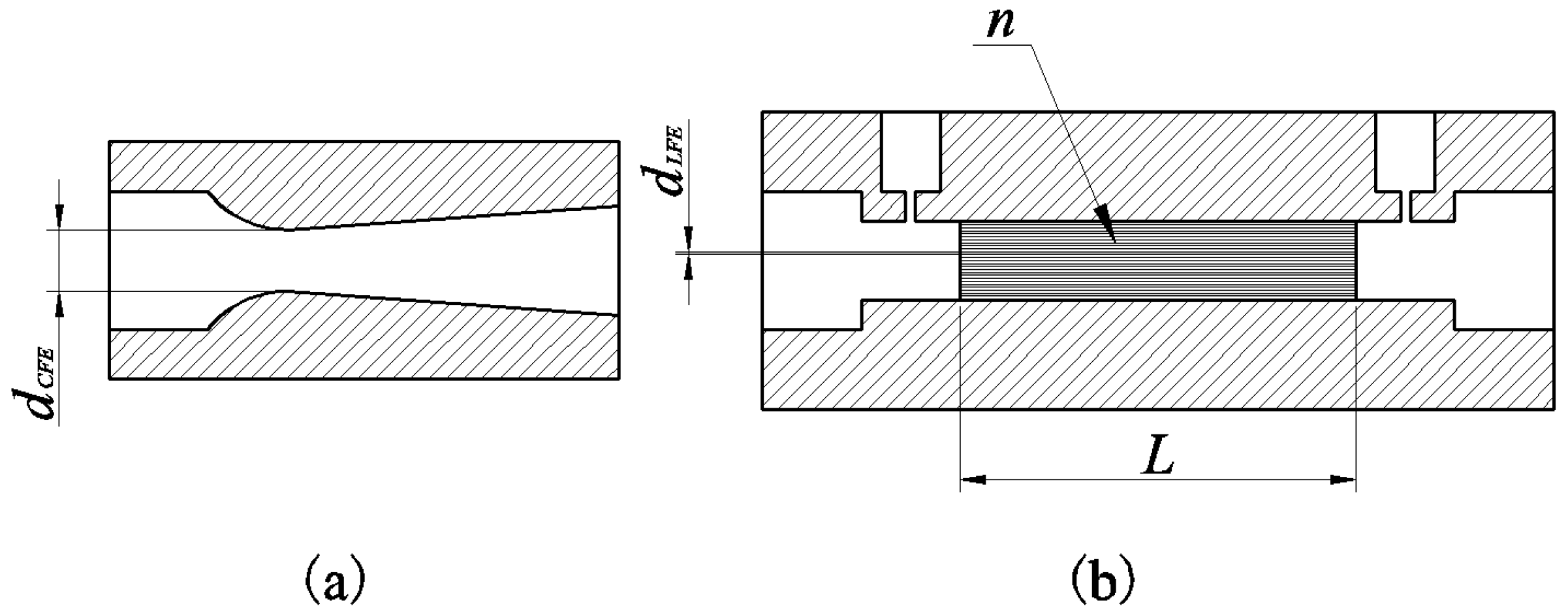
4.2. The Effect of Design Parameters on Sensing Performance
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hernandez-Ramirez, F.; Tarancon, A.; Casals, O.; Arbiol, J.; Romano-Rodriguez, A.; Morante, J.R. High response and stability in CO and humidity measures using a single SnO2 nanowire. Sens. Actuators B Chem. 2007, 121, 3–17. [Google Scholar] [CrossRef]
- Kida, T.; Kuroiwa, T.; Yuasa, M.; Shimanoe, K.; Yamazoe, N. Study on the response and recovery properties of semiconductor gas sensors using a high-speed gas-switching system. Sens. Actuators B Chem. 2008, 134, 928–933. [Google Scholar] [CrossRef]
- Yamazoe, N.; Shimanoe, K. Theoretical approach to the rate of response of semiconductor gas sensor. Sens. Actuators B Chem. 2010, 150, 132–140. [Google Scholar] [CrossRef]
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal oxide semi-conductor gas sensors in environmental monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Barritault, P.; Brun, M.; Gidon, S.; Nicoletti, S. Mid-IR source based on a free-standing microhotplate for autonomous CO2 sensing in indoor applications. Sens. Actuators A Phys. 2011, 172, 379–385. [Google Scholar] [CrossRef]
- Shemshad, J.; Aminossadati, S.M.; Kizil, M.S. A review of developments in near infrared methane detection based on tunable diode laser. Sens. Actuators B Chem. 2012, 171, 77–92. [Google Scholar] [CrossRef]
- Hu, Y.; Lu, S.; Guan, Y. Improvable method for Halon 1301 concentration measurement based on infrared absorption. Infrared Phys. Technol. 2015, 72, 122–126. [Google Scholar] [CrossRef]
- Kramer, A.; Paul, T.A. High-precision density sensor for concentration monitoring of binary gas mixtures. Sens. Actuators A Phys. 2013, 202, 52–56. [Google Scholar] [CrossRef]
- Sparks, D.; Smith, R.; Patel, J.; Najafi, N. A MEMS-based low pressure, light gas density and binary concentration sensor. Sens. Actuators A Phys. 2011, 171, 159–162. [Google Scholar] [CrossRef]
- Zhang, F.; Dong, F.; Tan, C. High GVF and low pressure gas–liquid two-phase flow measurement based on dual-cone flowmeter. Flow Meas. Instrum. 2010, 21, 410–417. [Google Scholar] [CrossRef]
- Van, W.; Kurth, D.B.D. Wet gas flow measurement with ultrasonic and differential pressure metering technology. In Proceedings of the International Symposium on Fluid Flow Measurement, Washington, DC, USA, 14–17 April 2015. [Google Scholar]
- He, D.; Bai, B.; Xu, Y.; Li, X. A new model for the V-Cone meter in low pressure wet gas metering. Meas. Sci. Technol. 2012, 23, 125305. [Google Scholar] [CrossRef]
- Zhao, L.M.; Ma, X.M.; Fu, J.J.; Li, D.D.; Qin, J. Improvable method of fire suppressant concentration measurement: An experimental validation. Flow Meas. Instrum. 2013, 30, 75–80. [Google Scholar] [CrossRef]
- Yamazaki, S.; Funaki, T.; Kawashima, K.; Kagawa, T. A concentration measurement system for binary gas mixtures using two flowmeters. Meas. Sci. Technol. 2007, 18, 2762–2768. [Google Scholar] [CrossRef]
- Youn, C.; Kawashima, K.; Kagawa, T. Concentration measurement systems with stable solutions for binary gas mixtures using two flowmeters. Meas. Sci. Technol. 2011, 22, 065401. [Google Scholar] [CrossRef]
- Potter, M.; Wiggert, D. Mechanics of Fluids, 3rd ed.; Cengage Learning: Stanford, CA, USA, 2010. [Google Scholar]
- Kim, H.D.; Kim, J.H.; Park, K.A.; Setoguchi, T.; Matsuo, S. Computational study of the gas flow through a critical nozzle. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2003, 217, 1179–1189. [Google Scholar] [CrossRef]
- Kim, H.D.; Kim, J.H.; Am Park, K. Study for the Gas Flow through a Critical Nozzle. J. Therm. Sci. 2003, 12, 250–259. [Google Scholar] [CrossRef]
- Measurement of Gas Flow by Means of Critical Flow Venturi Nozzles; International Organization for Standardization: Geneva, Switzerland, 2005.
- Sutera, S.P.; Skalak, R. The History of Poiseuille Law. Annu. Rev. Fluid Mech. 1993, 25, 1–19. [Google Scholar] [CrossRef]
- Wright, J.D. What Is the “Best” Transfer Standard for Gas Flow? In Proceedings of the FLOMEKO, Groningen, The Netherlands, 12–14 May 2003.
- Park, K.A.; Choi, Y.M.; Choi, H.M.; Cha, T.S.; Yoon, B.H. The evaluation of critical pressure ratios of sonic nozzles at low Reynolds numbers. Flow Meas. Instrum. 2001, 12, 37–41. [Google Scholar] [CrossRef]
- Sutton, G.P.; Biblarz, O. Rocket Propulsion Elements, 8th ed.; Wiley: Hoboken, NJ, USA, 2010; Volume xvi, 768p. [Google Scholar]
- Wilke, C.R. A Viscosity Equation for Gas Mixtures. J. Chem. Phys. 1950, 18, 517–519. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, Y.; Lu, S.; Zhang, D.; Hu, Y.; Yuan, W. A Fast and Easily-Realized Concentration Sensor for Binary Gas Mixtures and Its Design Analysis. Sensors 2018, 18, 1257. https://doi.org/10.3390/s18041257
Guan Y, Lu S, Zhang D, Hu Y, Yuan W. A Fast and Easily-Realized Concentration Sensor for Binary Gas Mixtures and Its Design Analysis. Sensors. 2018; 18(4):1257. https://doi.org/10.3390/s18041257
Chicago/Turabian StyleGuan, Yu, Song Lu, Dan Zhang, Yang Hu, and Wei Yuan. 2018. "A Fast and Easily-Realized Concentration Sensor for Binary Gas Mixtures and Its Design Analysis" Sensors 18, no. 4: 1257. https://doi.org/10.3390/s18041257




